Abstract
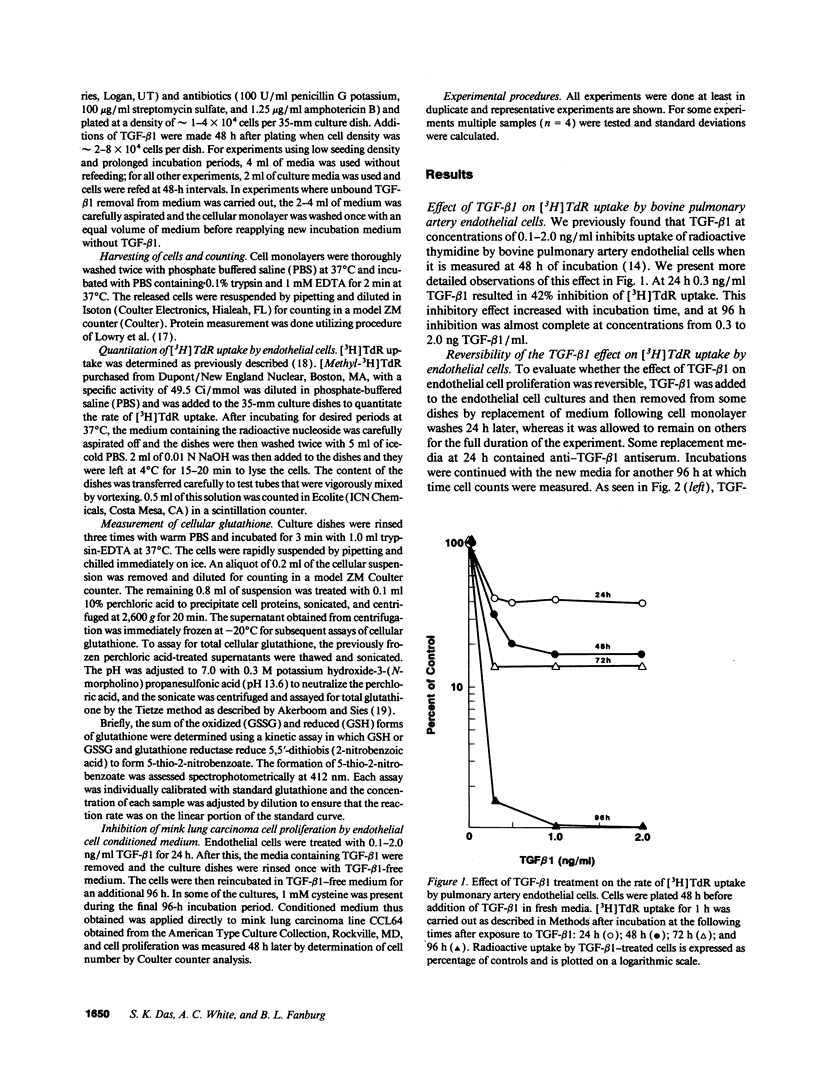
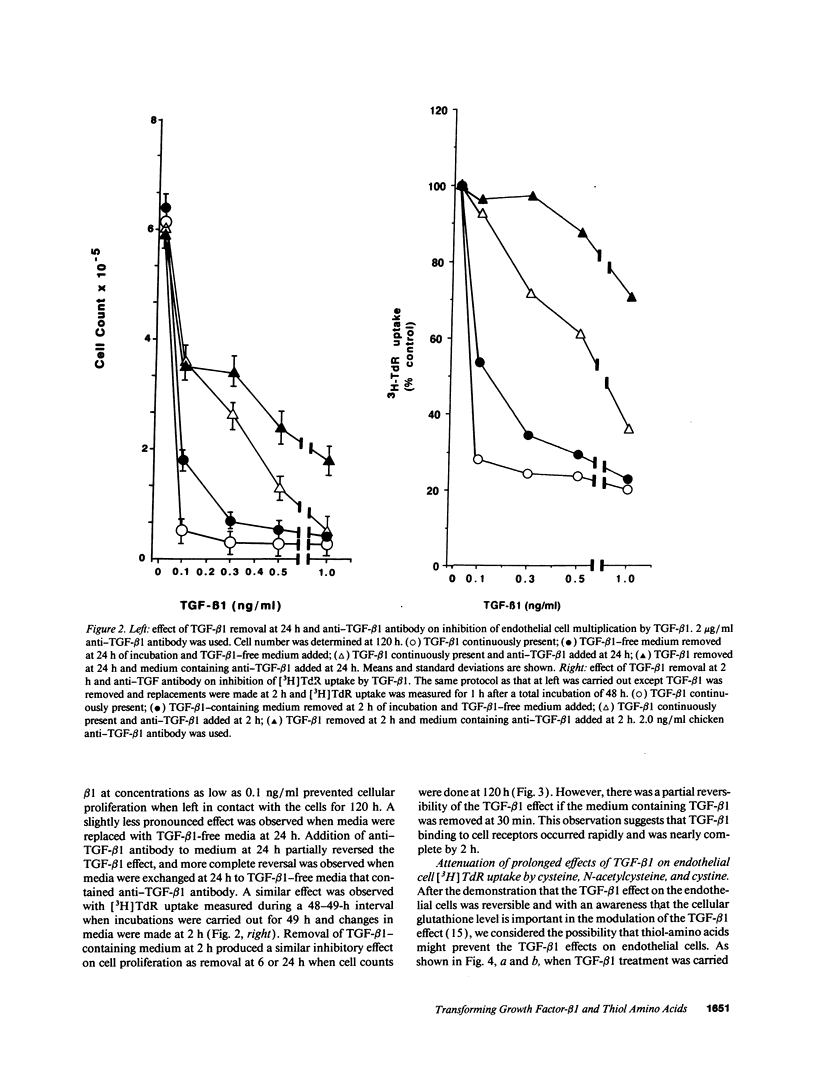
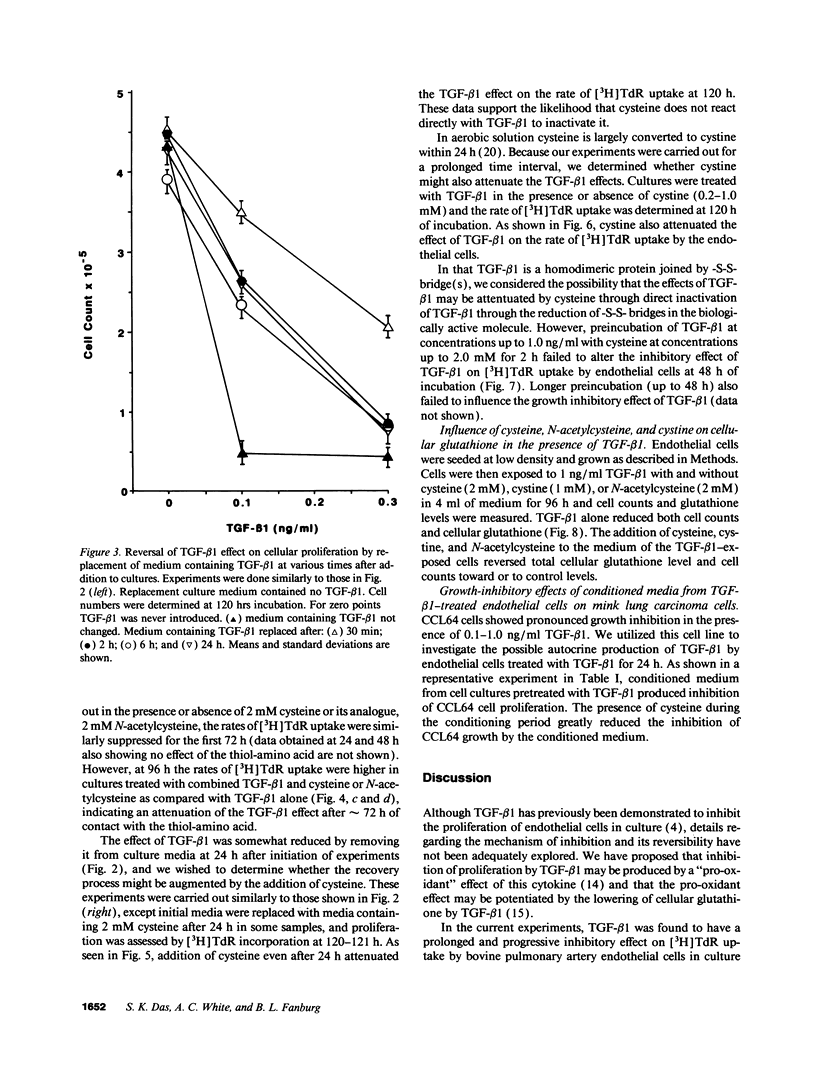
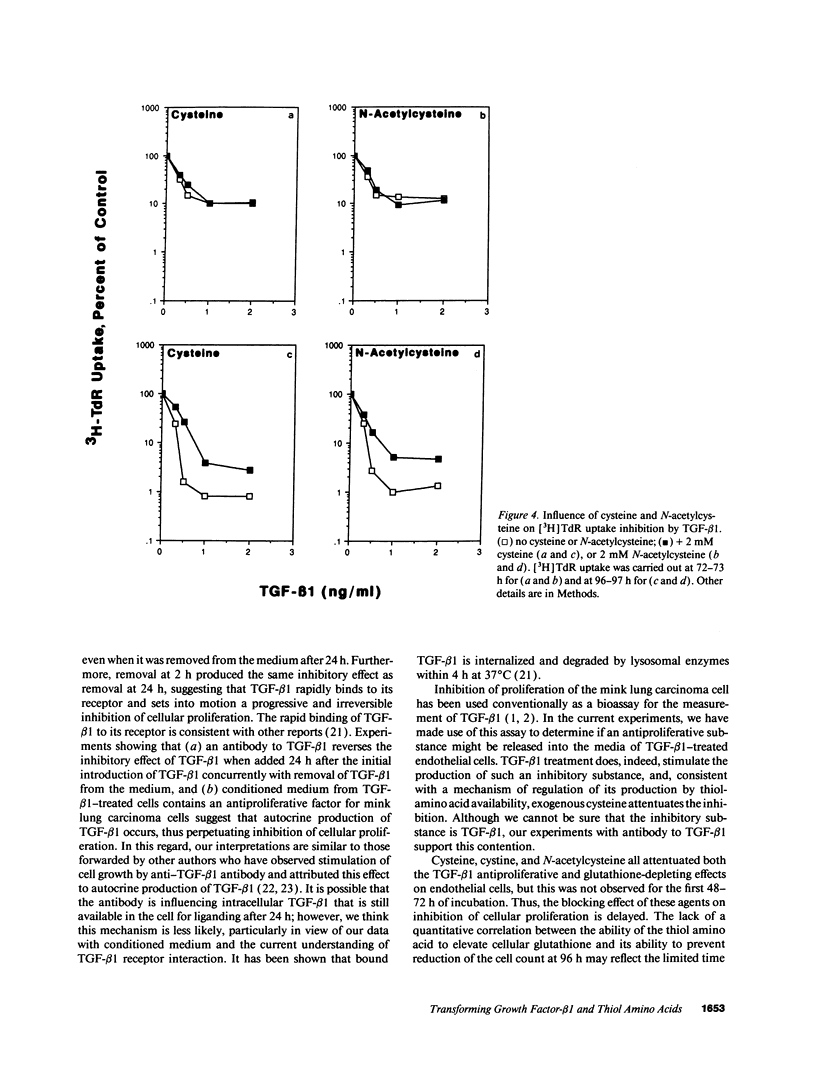
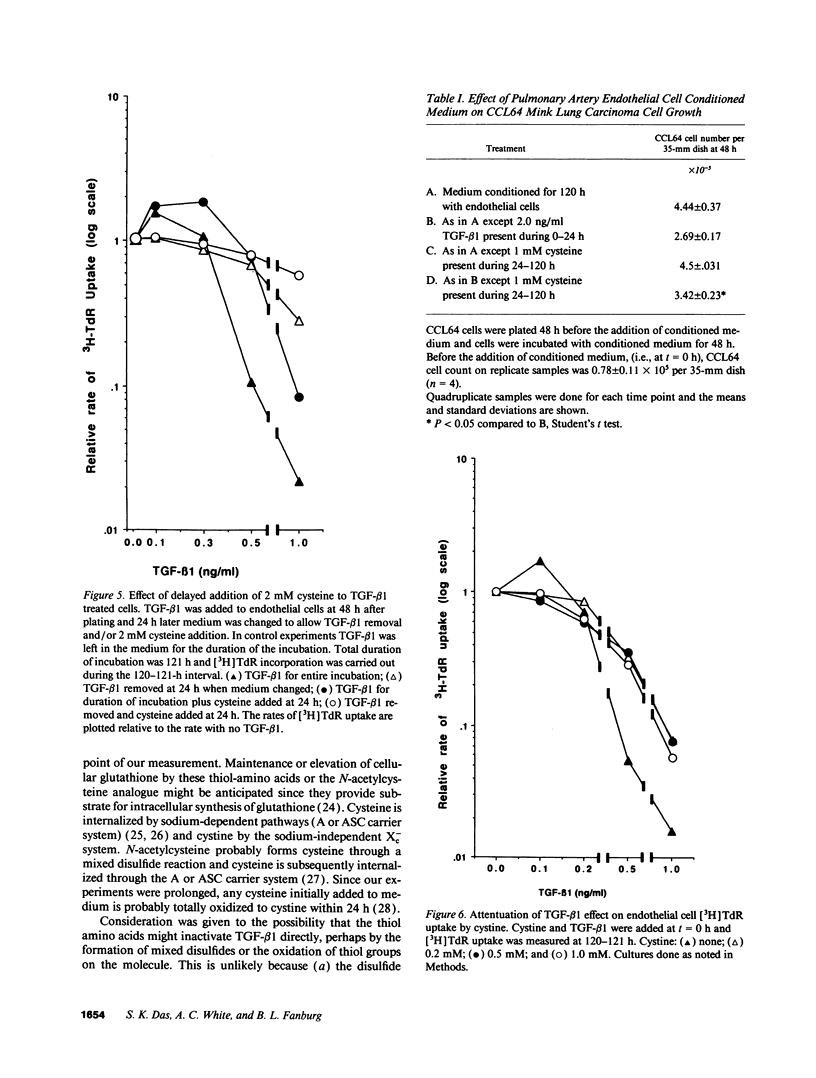
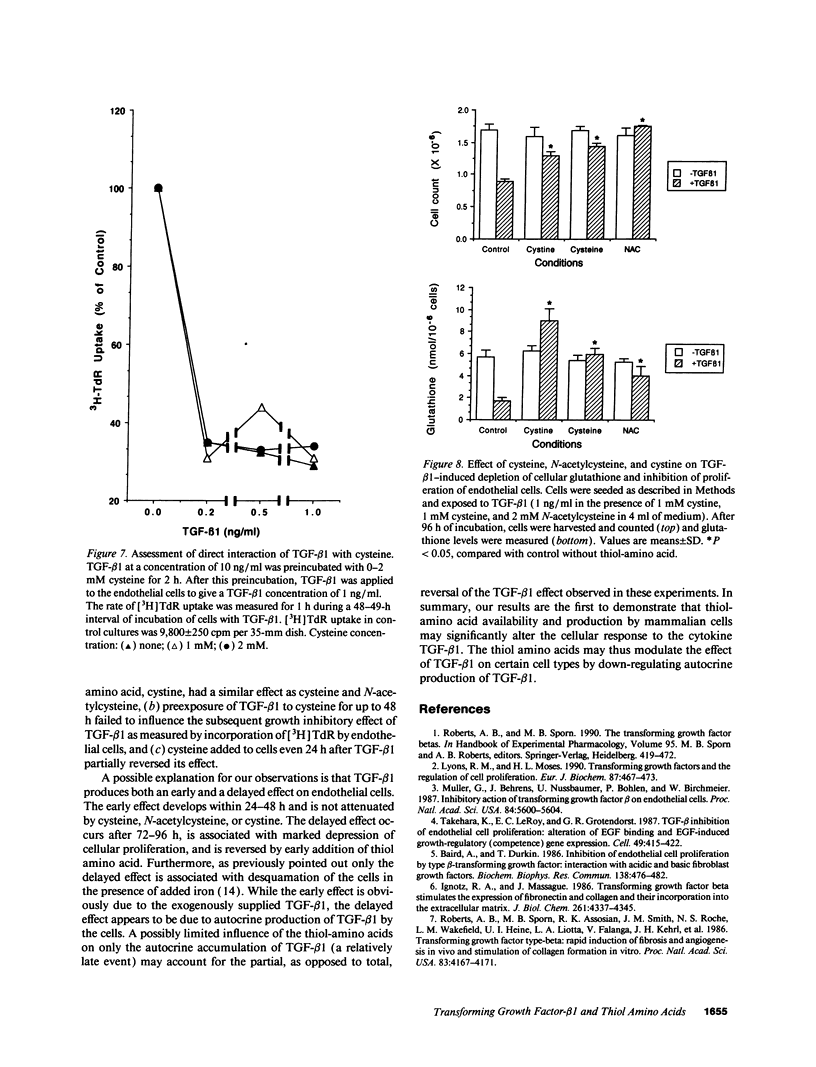
Early passaged bovine pulmonary artery endothelial cells exposed to 0.1-2.0 ng/ml transforming growth factor-beta 1 (TGF-beta 1) showed concentration-dependent growth inhibition, as assessed by [3H]thymidine labeling and cell counts, over a 96-h interval. Most of the inhibition of [3H]thymidine labeling measured at 96 h persisted when the medium was replaced with TGF-beta 1-free medium after 24 h, but the inhibition of labeling was prevented by the presence of anti-TGF-beta 1 antibody in the replacement medium. Additions of 2 mM cysteine, 1 mM cystine, or 2 mM N-acetylcysteine at the time of the initial addition of TGF-beta 1 blocked the inhibitory effect of TGF-beta 1 on [3H]-thymidine labeling when this was assessed after 72-96 h, but not at earlier times. Prevention of the inhibitory effect on cellular proliferation produced by cysteine, cystine and N-acetylcysteine was associated with elevation of cellular glutathione that was present at 48-96 h. There was no evidence for direct inactivation of TGF-beta 1 by the thiol-amino acids. Conditioned medium from TGF-beta 1-treated endothelial cells inhibited proliferation of mink lung carcinoma (CCL64) cells, supporting a previously reported concept of autocrine production of TGF-beta 1 by the endothelial cells. The inhibitory action of the conditioned medium was partially prevented when 1 mM cysteine was added during conditioning. Thus, TGF-beta 1 treatment of endothelial cells appears to set off autocrine production by these cells of TGF-beta 1 that perpetuates the inhibition of cellular proliferation. Replenishment of cellular glutathione with thiol-amino acids counteracts the growth-inhibitory effect of TGF-beta 1 through a currently undefined mechanism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerboom T. P., Sies H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981;77:373–382. doi: 10.1016/s0076-6879(81)77050-2. [DOI] [PubMed] [Google Scholar]
- Baird A., Durkin T. Inhibition of endothelial cell proliferation by type beta-transforming growth factor: interactions with acidic and basic fibroblast growth factors. Biochem Biophys Res Commun. 1986 Jul 16;138(1):476–482. doi: 10.1016/0006-291x(86)90305-0. [DOI] [PubMed] [Google Scholar]
- Bannai S. Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J Biol Chem. 1986 Feb 15;261(5):2256–2263. [PubMed] [Google Scholar]
- Bannai S., Tateishi N. Role of membrane transport in metabolism and function of glutathione in mammals. J Membr Biol. 1986;89(1):1–8. doi: 10.1007/BF01870891. [DOI] [PubMed] [Google Scholar]
- Christensen H. N. Exploiting amino acid structure to learn about membrane transport. Adv Enzymol Relat Areas Mol Biol. 1979;49:41–101. doi: 10.1002/9780470122945.ch2. [DOI] [PubMed] [Google Scholar]
- Das S. K. Activation of a well-behaved cell cycle in araC-treated V79 cells by caffeine. Mutat Res. 1988 Mar-Apr;207(3-4):171–177. doi: 10.1016/0165-7992(88)90083-8. [DOI] [PubMed] [Google Scholar]
- Das S. K., Fanburg B. L. TGF-beta 1 produces a "prooxidant" effect on bovine pulmonary artery endothelial cells in culture. Am J Physiol. 1991 Oct;261(4 Pt 1):L249–L254. doi: 10.1152/ajplung.1991.261.4.L249. [DOI] [PubMed] [Google Scholar]
- Deneke S. M., Gershoff S. N., Fanburg B. L. Potentiation of oxygen toxicity in rats by dietary protein or amino acid deficiency. J Appl Physiol Respir Environ Exerc Physiol. 1983 Jan;54(1):147–151. doi: 10.1152/jappl.1983.54.1.147. [DOI] [PubMed] [Google Scholar]
- Fedorcsák I., Harms-Ringdahl M., Ehrenberg L. Prevention of sulfhydryl autoxidation by a polypeptide from red kidney beans, described to be a stimulator of RNA synthesis. Exp Cell Res. 1977 Sep;108(2):331–339. doi: 10.1016/s0014-4827(77)80040-2. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Kehrl J. H., Roberts A. B., Wakefield L. M., Jakowlew S., Sporn M. B., Fauci A. S. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol. 1986 Dec 15;137(12):3855–3860. [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keski-Oja J., Lyons R. M., Moses H. L. Immunodetection and modulation of cellular growth with antibodies against native transforming growth factor-beta 1. Cancer Res. 1987 Dec 15;47(24 Pt 1):6451–6458. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lyons R. M., Moses H. L. Transforming growth factors and the regulation of cell proliferation. Eur J Biochem. 1990 Feb 14;187(3):467–473. doi: 10.1111/j.1432-1033.1990.tb15327.x. [DOI] [PubMed] [Google Scholar]
- Müller G., Behrens J., Nussbaumer U., Böhlen P., Birchmeier W. Inhibitory action of transforming growth factor beta on endothelial cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5600–5604. doi: 10.1073/pnas.84.16.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps D. T., Deneke S. M., Daley D. L., Fanburg B. L. Elevation of glutathione levels in bovine pulmonary artery endothelial cells by N-acetylcysteine. Am J Respir Cell Mol Biol. 1992 Sep;7(3):293–299. doi: 10.1165/ajrcmb/7.3.293. [DOI] [PubMed] [Google Scholar]
- Pierce G. F., Vande Berg J., Rudolph R., Tarpley J., Mustoe T. A. Platelet-derived growth factor-BB and transforming growth factor beta 1 selectively modulate glycosaminoglycans, collagen, and myofibroblasts in excisional wounds. Am J Pathol. 1991 Mar;138(3):629–646. [PMC free article] [PubMed] [Google Scholar]
- Poli G., Kinter A. L., Justement J. S., Bressler P., Kehrl J. H., Fauci A. S. Transforming growth factor beta suppresses human immunodeficiency virus expression and replication in infected cells of the monocyte/macrophage lineage. J Exp Med. 1991 Mar 1;173(3):589–597. doi: 10.1084/jem.173.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey P. G., Young M. F., Flanders K. C., Roche N. S., Kondaiah P., Reddi A. H., Termine J. D., Sporn M. B., Roberts A. B. Osteoblasts synthesize and respond to transforming growth factor-type beta (TGF-beta) in vitro. J Cell Biol. 1987 Jul;105(1):457–463. doi: 10.1083/jcb.105.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Gill P. J., Silbert J. E., Douglas W. H., Fanburg B. L. Involvement of cell surface heparin sulfate in the binding of lipoprotein lipase to cultured bovine endothelial cells. J Clin Invest. 1981 Oct;68(4):995–1002. doi: 10.1172/JCI110354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara K., LeRoy E. C., Grotendorst G. R. TGF-beta inhibition of endothelial cell proliferation: alteration of EGF binding and EGF-induced growth-regulatory (competence) gene expression. Cell. 1987 May 8;49(3):415–422. doi: 10.1016/0092-8674(87)90294-7. [DOI] [PubMed] [Google Scholar]
- Van Obberghen-Schilling E., Roche N. S., Flanders K. C., Sporn M. B., Roberts A. B. Transforming growth factor beta 1 positively regulates its own expression in normal and transformed cells. J Biol Chem. 1988 Jun 5;263(16):7741–7746. [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield L. M., Smith D. M., Masui T., Harris C. C., Sporn M. B. Distribution and modulation of the cellular receptor for transforming growth factor-beta. J Cell Biol. 1987 Aug;105(2):965–975. doi: 10.1083/jcb.105.2.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A. C., Das S. K., Fanburg B. L. Reduction of glutathione is associated with growth restriction and enlargement of bovine pulmonary artery endothelial cells produced by transforming growth factor-beta 1. Am J Respir Cell Mol Biol. 1992 Apr;6(4):364–368. doi: 10.1165/ajrcmb/6.4.364. [DOI] [PubMed] [Google Scholar]


