Abstract
A better understanding of second-line drug (SLD) pharmacokinetics, including cavitary penetration, may help optimize SLD dosing. Patients with pulmonary multidrug-resistant tuberculosis (MDR-TB) undergoing adjunctive surgery were enrolled in Tbilisi, Georgia. Serum was obtained at 0, 1, 4, and 8 h and at the time of cavitary removal to measure levofloxacin concentrations. After surgery, microdialysis was performed using the ex vivo cavity, and levofloxacin concentrations in the collected dialysate fluid were measured. Noncompartmental analysis was performed, and a cavitary-to-serum levofloxacin concentration ratio was calculated. Twelve patients received levofloxacin for a median of 373 days before surgery (median dose, 11.8 mg/kg). The median levofloxacin concentration in serum (Cmax) was 6.5 μg/ml, and it was <2 μg/ml in 3 (25%) patients. Among 11 patients with complete data, the median cavitary concentration of levofloxacin was 4.36 μg/ml (range, 0.46 to 8.82). The median cavitary/serum levofloxacin ratio was 1.33 (range, 0.63 to 2.36), and 7 patients (64%) had a ratio of >1. There was a significant correlation between serum and cavitary concentrations (r = 0.71; P = 0.01). Levofloxacin had excellent penetration into chronic cavitary TB lesions, and there was a good correlation between serum and cavitary concentrations. Optimizing serum concentrations will help ensure optimal cavitary concentrations of levofloxacin, which may enhance treatment outcomes.
INTRODUCTION
The emergence of multidrug-resistant tuberculosis (MDR-TB) remains a major barrier to global TB control (1). The treatment course for MDR-TB (with resistance to isoniazid and rifampin) consists of 18 to 24 months of second-line antituberculosis drugs (SLDs), including a fluoroquinolone and an injectable agent (amikacin, kanamycin, or capreomycin). A 2009 meta-analysis of MDR-TB treatment outcomes found an overall success rate of 62% with a range of 36 to 79% (2). The most recent World Health Organization (WHO) global TB report reported an overall successful outcome rate of 48%, again with a wide range (3). These data show that we are far from the goal of a >75% successful outcome treatment rate among MDR-TB patients; however, the broad range of successful outcomes suggests that we may be able to more effectively maximize existing SLD regimens. A better knowledge of how to optimize available SLD regimens could improve outcomes and provide important principles for the efficacious use of new drugs.
The clinical pharmacology of SLDs has been a neglected area of research (4). Pharmacokinetic studies among patients with drug-susceptible TB demonstrate that low concentrations of first-line drugs are associated with poor clinical outcomes, and simulation studies have found that even with perfect adherence up to 1% of patients would develop further drug resistance during treatment due to variability in drug concentrations (5–7). A recent report on 25 patients with MDR-TB found that plasma SLD concentrations were frequently low and were associated with decreased drug activity ex vivo (8). These findings demonstrate the importance of performing clinical pharmacokinetic studies and highlight the potential negative consequences of low TB drug concentrations.
Even less is known about the ability of TB drugs to penetrate into diseased lung tissue, including cavitary lesions, which are a common manifestation of progressive pulmonary TB (4). There is only one published case report evaluating SLD concentrations inside human cavitary lesions (9). Reports of worse clinical outcomes among patients with cavitary disease offer compelling but indirect evidence for low intracavitary SLD concentrations (10–12).
Our study aims were to measure the cavitary concentration of levofloxacin and describe predictors of penetration among patients with MDR-TB undergoing adjunctive surgical resection in Tbilisi, Georgia. The country of Georgia is a high-burden-MDR-TB country as designated by the WHO (3), and as part of its National TB Treatment Guidelines, adjunctive surgical resection is frequently performed for patients with cavitary MDR-TB not responding to medical therapy (13). To evaluate target site penetration of levofloxacin, we utilized the technique of microdialysis (μD). The principle of microdialysis is based on the presence of a concentration gradient between two fluid compartments (cavity and μD catheter) across a semipermeable membrane (μD probe), with concentrations measured from the recovered dialysate fluid (14, 15). Microdialysis measures unbound (pharmacologically active) extracellular drug concentrations (15). The overall study goals were to provide novel data that may help guide optimal SLD dosing and also to develop a reliable and accurate method to measure tissue drug penetration for antituberculosis drugs.
(The findings of this study were presented at the 7th International Workshop on Clinical Pharmacology of Tuberculosis Drugs, Washington, DC, USA, 5 September 2014.)
MATERIALS AND METHODS
Study population.
Study participants were enrolled from the National Center for Tuberculosis Lung Diseases (NCTLD) in Tbilisi, Georgia. The NCTLD is the headquarters for the National TB Program and contains the National Reference Laboratory (NRL), Thoracic Surgery Center, and inpatient MDR-TB hospital. Patients with culture-confirmed MDR-TB who were receiving levofloxacin and scheduled to undergo adjunctive surgical resection were approached for enrollment during their preoperative hospital stay. Treatment regimens for all patients were individualized base on drug susceptibility testing (DST) results per WHO recommendations and given as directly observed therapy (DOT) (16). Patients weighing ≤75 kg received 750 mg levofloxacin, while those >75 kg received 1,000 mg; doses were given orally with a few milliliters of water (intravenous formulations were not available). The recommendation to perform adjunctive surgery was made by the NCTLD drug resistance committee. General criteria for surgical intervention included treatment failure, high likelihood of disease relapse based on DST results and radiological findings, localized cavitary lesion, and adequate cardiopulmonary function (13). All study participants provided informed consent. The Georgian NCTLD, Emory University, and University of Florida Institutional Review Boards (IRBs) approved the study.
Pharmacokinetics. (i) Serum.
Patients fasted overnight for a minimum of 8 h the day prior to surgery and received their daily oral dose of levofloxacin approximately 2 h before cavitary removal. Serum samples were collected immediately before and 1, 4, and 8 h after receiving levofloxacin. A serum sample was also collected at the time of cavity removal. Samples were kept in a −80°C freezer until they were shipped to the University of Florida Infectious Diseases Pharmacokinetics Laboratory, Gainesville, FL. Concentrations were measured using validated assays on a ThermoFinnigan P4000 high-pressure liquid chromatography (HPLC) pump with a model AS1000 fixed-volume autosampler, a model FL3000 fluorescence detector, a Gateway Series e computer, and a Chromquest HPLC data management system. The six-point standard curves ranged from 0.20 to 15 μg/ml, with linearity extending above and below this range. The recovery of the levofloxacin from human plasma was approximately 90%. The overall validation precision for levofloxacin quality control samples was 0.58 to 4.09% (17, 18).
(ii) μD.
Microdialysis (μD) was performed ex vivo immediately after surgical removal of the cavitary lesion. A semipermeable μD probe with a total length of 10 mm attached to a μD infusion pump (μ Dialysis AB, Stockholm, Sweden) was inserted into the center of the cavitary lesion using a slit cannula introducer. Subsequently, four different concentrations of levofloxacin (0.5, 2, 20, and 30 μg/ml) in Ringer's solution were each infused for a total of 35 min at flow rate of 1 μl/min, and the recovered fluid “dialysate” was collected into microvials. Approximately, 35 μl of dialysate was collected for each levofloxacin drug concentration infusion, which was then stored in an −80°C freezer until shipment to the United States. Levofloxacin concentrations in the dialysate fluid were measured using HPLC with mass spectroscopy detection (HPLC-MS/MS). The HPLC-MS/MS method was developed and validated on a Perkin-Elmer 200 series pump and autosampler (Perkin-Elmer, Waltham, MA, USA) coupled with an API 4000 mass spectrometer. Deuterated levofloxacin was used as the internal standard. The linearity was evaluated in the range of 5 to 200 ng/ml. The validation accuracy was in the range of 90.1 to 106.3%. The drug was stable under different tested conditions, including storage at −70°C and freeze-thaw cycles.
The no-net-flux method was utilized for calibration (14). The no-net-flux method requires fixed drug concentrations (as in our ex vivo tissue samples) and is considered the gold standard for μD calibration (19, 20). The underlying principle is that when infusing concentrations of drug higher than that in the surrounding extracellular fluid, net outflux of drug into the extracellular space occurs, and with infusing lower drug concentrations, net influx of drug from the extracellular space across the probe into the infusion fluid occurs. A regression line is then plotted, and the point at which no net flux occurs (x intercept) is the estimated levofloxacin concentration in the extracellular fluid from inside the cavitary lesion.
(iii) Tissue.
For the first three patients enrolled, an ∼0.4-gram sample of resected lung tissue, which included sections of the cavity wall and center, was homogenized and assayed with HPLC to quantify total levofloxacin concentrations. Results were reported as micrograms of levofloxacin per gram of tissue. The same method used for serum was adapted and partially validated for drug quantification.
Laboratory. (i) AFB testing.
All sputum and tissue examinations were performed at the NRL, which undergoes annual external quality assessment by the Antwerp WHO Supranational TB Reference Laboratory. Each patient had the following specimens collected: a preoperative sputum sample and five samples of resected lung tissue, including from healthy tissue, surrounding nodule, external and internal cavity wall, and caseous necrosis. Acid-fast bacillus (AFB) smear microscopy and cultures were performed using standard methodologies (21). Prior to culture, all tissue samples were first homogenized with a tissue grinder. Decontaminated sputum and tissue samples where inoculated on to Lowenstein-Jensen (LJ)-based solid medium. Positive cultures were confirmed to be Mycobacterium tuberculosis complex (MTBC) using the MTBDRplus assay along with colony morphology (21). DST for first- and second-line drugs was performed as previously described (21, 22).
(ii) Pathology.
Resected lung tissue not used for other purposes in 5 cases was formalin fixed and paraffin embedded. Sections measuring 4 μm were stained with hematoxylin and eosin and acid-fast stains. Microscopic study assessed the inflammatory response, size of granulomas (microscopic versus macroscopic), amount of necrosis in the cavity (abundant [multiple coalescing fields with necrosis] versus patchy), thickness of fibrosis, and amounts of vessels.
Data collection.
A standardized data collection form was used to abstract data from medical records and the NRL database. Information pertaining to socio-demographic characteristics, treatment regimens and course, medical history, and radiological results was collected. All data were entered into an online REDCap database (23).
Data analysis.
Data analyses were performed using SAS software (version 9.3) and, for noncompartmental pharmacokinetic analysis (NCA), Phoenix WinNonlin. The following pharmacokinetic parameters were determined: maximal serum concentration (Cmax), time at which Cmax occurred (Tmax), area under the serum concentration-versus-time curve (AUC), volume of distribution divided by bioavailability (V/F), clearance divided by bioavailability (CL/F), half-life (t1/2), and elimination constant (Ke). The fraction of the dose absorbed (F) was assumed to be 1 for data analysis. Free levofloxacin serum concentrations were calculated by multiplying the measured serum levofloxacin concentration by (100% − 25% [the published percent protein binding for levofloxacin]) (24). In comparing free serum and cavitary levofloxacin concentrations, the serum levofloxacin concentration from the time of surgical resection was used.
RESULTS
Study population.
Twelve patients undergoing surgical resection for MDR-TB were enrolled (Table 1). The median age was 34 years; most were male (92%) and had no history of prior TB treatment (75%). No patients had HIV infection; 2 (17%) had diabetes, and 5 (42%) were coinfected with hepatitis C virus. The median body mass index (BMI) was 22.6 kg/m2, while median creatinine clearance was 118 ml/min and albumin was 4.4 g/dl. All patients had unilateral cavitary disease with a median cavity diameter of 2.8 cm (range, 2.1 to 10.0) (an example is shown in Fig. 1). Patients had been receiving levofloxacin for a median of 373 days prior to surgery, with a median levofloxacin dose of 11.8 mg/kg. Only 1 patient (9%) had a tissue culture positive for M. tuberculosis, while 6 of 9 patients (67%) had ≥1 tissue specimen that was AFB smear positive. Further characteristics are shown in Table 1.
TABLE 1.
Study population characteristics for 12 patients with multidrug-resistant pulmonary tuberculosis
| Parameter | Valuea (n = 12) |
|---|---|
| Demographic characteristics | |
| Male sex | 11 (92) |
| Age, yr | 33.5 (17–54) |
| Georgian ethnicity | 10 (84) |
| Diabetes mellitus | 2 (17) |
| Hepatitis C | 5 (42) |
| Alcohol user | 1 (8) |
| Tobacco user | 5 (42) |
| Prior treatment for tuberculosis | 3 (25) |
| Wt, kg | 68 (49–100) |
| Body mass index, kg/m2 | 22.6 (17–33) |
| Cavity diam, cm | 2.8 (2.1–10.0) |
| Laboratory values | |
| Creatinine clearance,b ml/min | 117.7 (81–143) |
| Albumin level, g/dl | 4.4 (3.5–4.9) |
| Hemoglobin level, g/dl | 12.8 (12.1–13.5) |
| Alanine aminotransferase level, U/liter | 30.5 (10–125) |
| Treatment | |
| Type of surgery | |
| Segmentectomy | 6 (50) |
| Lobectomy | 6 (50) |
| Levofloxacin dose | |
| 750 mg | 10 (83) |
| 1,000 mg | 2 (17) |
| Levofloxacin, mg/kg | 11.8 (7.5–15.3) |
| Days receiving levofloxacin | 372 (15–810) |
Data are presented either as number (percentage) or as median value (range).
Using the Cockcroft-Gault equation.
FIG 1.
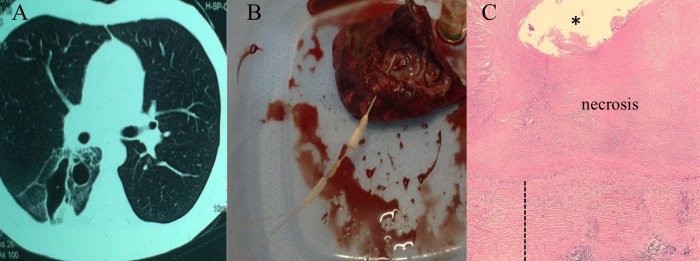
Representative pictures of a chest CT scan of a study patient showing a cavitary lesion (A), a resected cavitary lesion with insertion of microdialysis probe past cavity wall into center of lesion (the proper positioning of the probe was evaluated after microdialysis for each patient) (B), and pathological examination of a resected cavitary lesion stained with hematoxylin and eosin (C). Magnification, ×20. *, center of cavity; ┊, fibrous wall thickness.
Serum pharmacokinetics.
The serum concentrations versus time for all patients are shown in Fig. 2. The majority of patients (66%) had levofloxacin Cmax values below the recommended minimum Cmax of 8 μg/ml (range, 1.12 to 12.3), including three patients with Cmax of <2 μg/ml. Two patients had delayed absorption (Fig. 2). The median Ke (0.09 h−1), Tmax (2 h), and t1/2 (7.6 h) values were similar to those reported in the literature. Further NCA results are shown in Table 2. The median levofloxacin dose was 11.8 mg/kg (range, 7.5 to 15.3), and there was a significant correlation between dosage and Cmax, as shown in Fig. 3A.
FIG 2.
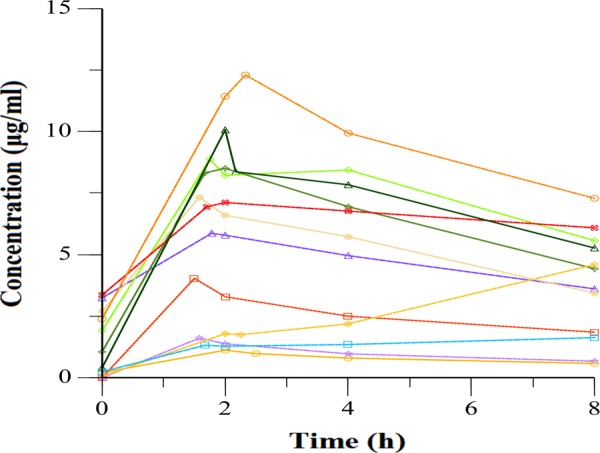
Serum concentrations of levofloxacin versus time after dosing in 12 adults with multidrug-resistant tuberculosis. Patients with delayed absorption are indicated with dashed lines.
TABLE 2.
Noncompartmental analysis of serum levofloxacin concentrations
| Parametera | Median (range) (n = 12) |
|---|---|
| Ke (h−1)b | 0.09 (0.03–0.12) |
| t1/2 (h)b | 7.6 (6.0–26.6) |
| Tmax (h) | 2.0 (1.5–8.0) |
| Cmax (μg/ml) | 6.5 (1.1–12.3) |
| AUClast (h · μg/ml) | 39.4 (6.0–70.8) |
| AUC0–∞ (h · μg/ml)b | 86.2 (12.2–283.8) |
| CL/F (liters/h)b | 10.1 (5.7–69.6) |
| V/F (liters)b | 140.5 (63.2–754) |
Ke, elimination constant; t1/2, half-life; Tmax, time to Cmax; Cmax, maximal serum concentration; AUClast, area under the curve; CL, clearance; V, volume of distribution; F, bioavailability (assumed to be 1 for purposes of analysis).
Two patients were excluded due to delayed absorption.
FIG 3.
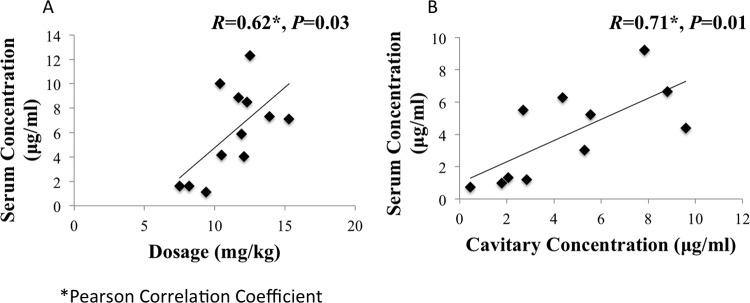
(A) Correlation between peak serum levofloxacin concentration and dosages; (B) correlation between free serum levofloxacin concentration at time of resection and cavitary levofloxacin concentration.
Cavitary drug concentrations.
Eleven patients had both serum and cavitary drug concentration results; one patient was excluded due to invalid microdialysis results. Placement of the microdialysis catheter inside the ex vivo cavitary lesion was successful for all 11 patients, and Fig. 1B depicts the typical placement of a microdialysis probe inside the resected cavitary lesions.
The median cavitary concentration of levofloxacin was 4.36 μg/ml, with a range of 0.46 to 8.82 μg/ml (Table 3). In comparison to the free serum concentration of levofloxacin at the time of surgical resection, the median cavitary/serum levofloxacin concentration ratio was 1.33, with a range of 0.63 to 2.36. The majority of patients (7 of 11; 64%) had a cavitary/serum levofloxacin concentration ratio >1. There was a significant correlation between serum and cavitary concentrations (r = 0.71; P = 0.01), as shown in Fig. 2B. Overall, a t test found no significant difference between serum and cavitary levofloxacin concentrations (P = 0.38). The maximal cavity diameter size was not associated with the cavity/serum levofloxacin ratio in regression analysis (0.20); however, the patient with the cavitary lesion with the largest diameter (10 cm) did have the lowest ratio (0.49). Three patients had both free cavitary and whole-tissue levofloxacin concentrations determined, with the results shown in Table 4.
TABLE 3.
Comparison of free serum and cavitary levofloxacin concentrations among patients with multidrug-resistant pulmonary tuberculosis
| Subjecta | Serum concn at time of resectionb (μg/ml) | Cavitary concn (μg/ml) | Cavitary/serum concn ratio |
|---|---|---|---|
| 1 | 0.74 | 0.46 | 0.63 |
| 2 | 4.40 | 9.59 | 2.18 |
| 3 | 3.03 | 5.31 | 1.75 |
| 4 | 6.66 | 8.82 | 1.32 |
| 5 | 1.21 | 2.85 | 2.36 |
| 7 | 1.31 | 2.07 | 1.58 |
| 8 | 5.50 | 2.70 | 0.49 |
| 9 | 5.21 | 5.55 | 1.07 |
| 10 | 9.23 | 7.83 | 0.85 |
| 11 | 6.28 | 4.36 | 0.69 |
| 12 | 0.99 | 1.78 | 1.80 |
| Median | 4.40 | 4.36 | 1.33 |
No cavitary concentration was available for subject 6.
Free serum concentration = measured levofloxacin concentration × 0.75.
TABLE 4.
Comparison of levofloxacin tissue concentrations measured by microdialysis versus whole-tissue homogenization
| Subject | Levofloxacin concn measured by: |
|
|---|---|---|
| Microdialysis (μg/ml) | Whole-tissue homogenization (μg/g) | |
| 1 | 0.46 | 5.30 |
| 2 | 9.59 | 40.25 |
| 3 | 5.31 | 12.85 |
Pathology.
Five patients had pathological examination of their resected tissue (an example is shown in Fig. 1C). The range of inflammation varied from well-formed granulomas (4/5) to lymphoid aggregates in areas of fibrosis (1/5). The granulomas were microscopic in three cases and macroscopic in another with abundant necrosis and patchy areas with abundant acid-fast bacilli. These cases had various levels of fibrous tissue around the granulomas and new vessel formation. The case without granulomas had abundant intra-alveolar hemorrhage.
DISCUSSION
In a cohort of patients with chronic pulmonary MDR-TB undergoing surgical resection, we found that in the majority of patients (64%), levofloxacin had excellent penetration into cavitary lesions. To our knowledge these are the first reported measurements of lung and specifically cavitary penetration of levofloxacin among patients with TB and the first time that microdialysis has been used to measure drug concentrations in human cavitary lung lesions. The relatively high target site penetration of levofloxacin into complex cavitary lesions may in part explain the effectiveness of the drug among patients receiving treatment for MDR-TB. Additionally, our results demonstrate that the method of microdialysis offers promise as a means to measure the cavitary penetration of other TB drugs, data that may help design optimal patient treatment regimens.
The cavitary lesion is a hallmark lesion of progressive pulmonary TB and is seen in up to 50% of patients with MDR-TB (25). Cavitary lesions are heterogeneous and characterized by various levels of fibrosis, necrosis, and vascularization, as demonstrated by the histopathology from our study, and they contain high numbers of M. tuberculosis organisms, many of which reside extracellularly in the caseous center. Clinically, studies have shown the presence of cavitary lesions to be associated with longer time to sputum culture conversion (26), high rates of relapse (27), the development of acquired dug resistance (12, 28), and worse treatment outcomes among patients with MDR-TB (29, 30). These clinical studies suggest that drug penetration into cavitary lesions may be hindered, possibly due to the physical and functional barriers of cavitary lesions. Studies performed in the 1980s and earlier found lower concentrations of isoniazid and rifampin in resected lung tissue than in serum (31, 32). These studies used various methods, each with limitations, to measure drug concentrations, and no subsequent human investigations (excluding case reports) have been reported since. The technical and ethical difficulties of gaining access to cavitary lesions among patients with TB have been a major barrier to measuring the penetrating abilities of antituberculosis drugs. In lieu of direct measurements, the lung tissue penetration of second-line drugs has been inferred from using serum concentrations to model tissue concentrations and from bronchial alveolar lining fluid (BAL fluid) or epithelial lining fluid (ELF) drug concentrations (33).
The technique of microdialysis was first used to measure drug pharmacokinetics in the 1990s, with much of the early work focused on the central nervous system (14). It was not until the last decade that the technique been used to measure the lung penetration of specific drugs. The main benefit of microdialysis is its ability to measure free drug concentrations at the site of action, where adequate drug concentrations are most vital. There have been eight lung microdialysis studies performed in humans and measuring the penetration of various antibiotics, including three studies evaluating levofloxacin.(34–36) In the levofloxacin studies, all patients were undergoing elective cardiothoracic surgery and had a temporary microdialysis catheter placed to measure the penetration into normal lung tissue over time. Our results are the first time microdialysis has been used in a patient with TB, in a cavitary lesion, and with ex vivo tissue. The successful implementation of the microdialysis method gives investigators an innovative method to further explore the tissue-penetrating capabilities of antituberculosis drugs in patients undergoing surgical resection. By further characterizing the tissue penetration of all TB drugs, it may be possible to tailor specific regimens for patients with extensive cavitary disease. We found that levofloxacin concentrations were similar inside cavitary lesions and in serum. Fluoroquinolones in general are well known to have good tissue penetration, and our results provide evidence that this holds true even in most cases of chronic pulmonary TB cavitary lesions. We purposefully inserted the microdialysis catheters through the cavitary wall into the center of the lesion to best approximate drug concentrations in the caseous portion of the lesion, an area with a high extracellular bacillus burden and decreased vascularization. Our encouraging data highlight the ability of levofloxacin to reach this central part of cavitary lesions and consequentially the importance of fluoroquinolones for severe pulmonary MDR-TB. The relatively high cavitary concentrations of levofloxacin may account for the low rate of positive tissue cultures for M. tuberculosis; however, there were several patients who had AFB smear-positive tissue samples, and the lack of growth may also have been secondary to using less sensitive solid culture methods, nonviable bacilli, technical error, or bacilli that were in a dormant state. Given our small cohort, we were unable to decipher why some patients had poor drug penetration, and further ongoing work will address this issue. The only other data on the tissue penetration of fluoroquinolones among patients with TB come from a case report of a 13-year-old girl with MDR-TB who had surgical resection performed. Whole-tissue moxifloxacin concentrations were found to range from 0.73 to 0.95 μg/g (9). We also determined whole-tissue drug concentrations in three patients and found concentrations to be much higher than those in this case report, with a range of 5.30 to 40.25 μg/g. Of note, these whole tissue concentrations include drug that is protein bound and unbound and drug that is intracellular and extracellular, and they are averages of concentrations across several tissue compartments (in our case the cavitary wall, necrotic center, and surrounding tissue). It is thus difficult to compare the concentrations obtained from whole-tissue homogenates and microdialysis, and the advantage of microdialysis is that it provides the amount of active drug at the site of disease.
Further work using the novel method of matrix-assisted laser desorption ionization (MALDI) mass spectrometry imaging (MSI) has characterized the distribution of moxifloxacin in necrotic granulomas obtained from rabbits. Using this method, which can produce a two-dimensional map of intra- and extracellular drug distribution, moxifloxacin was shown to accumulate at high levels in the cellular part of granulomas but not in the caseous center (37). The same investigators are using MALDI MSI to measure cavitary concentrations in resected lung from TB patients (NCT00816426). Techniques that allow for the investigation of drug distribution, such as MALDI, are complementary to the extracellular active drug concentrations obtained from microdialysis, and together these should provide a better understanding of drug tissue penetration.
A concerning finding from our study was the low serum Cmax and AUC values found for the majority of patients. In most patients, the Cmax values were below the minimal recommended value of 8 μg/ml (18), with three having extremely low Cmax values of <2 μg/ml. Our results are in contrast to prior data on the serum population pharmacokinetics of levofloxacin in adults with TB (18). One explanation may be a cohort selection bias given that subjects were chronic MDR-TB patients experiencing microbiological and/or clinical failure of their treatment and thus more likely to have suboptimal levofloxacin concentrations. Most samples were obtained while the patient was receiving anesthesia, which may have affected drug absorption. We are initiating a study to evaluate drug concentrations among nonsurgery patients to better understand SLD concentrations among patients with MDR-TB. Based on our findings of a strong correlation between serum and cavitary levofloxacin concentrations, ensuring optimal serum levofloxacin concentrations should lead to similar cavitary levofloxacin concentrations in most patients. This highlights the potential role of therapeutic drug monitoring (TDM) for levofloxacin and also emphasizes the need to determine the optimal levofloxacin dose for patients with MDR-TB. The significant correlation between the levofloxacin dose (mg per kg) and serum drug concentration suggests that weight-based dosing may be a way to optimize serum concentrations. A study (NCT01918397) evaluating levofloxacin doses up to 20 mg/kg among patients with MDR-TB is beginning enrollment and will provide data on the optimal dose of levofloxacin.
Our study is subject to certain limitations. These include the lack of chest computed tomography (CT) scan results and pathological examinations for all patients enrolled in the study. This precluded us from evaluating whether certain radiological or pathological characteristics were associated with cavitary penetration of levofloxacin. This information is being collected for a follow-up study evaluating the cavitary penetration of moxifloxacin and pyrazinamide. Another limitation was the measurement of the cavitary levofloxacin concentration at only one time point, which disallowed us from comparing the levofloxacin AUCs from the cavity and plasma. The timing of surgical resection was meant to capture cavitary levofloxacin concentrations near their peak, but it is not known if there is a delay of levofloxacin penetration into the cavity. Ethical and safety considerations prohibited us from measuring cavitary concentrations in vivo. Given the rapid absorption of levofloxacin, our sampling scheme may have prevented us from recording the actual Cmax in some patients.
In summary, in the first human study to measure cavitary drug concentrations among patients with TB, we found a correlation between the serum and cavitary concentrations of levofloxacin and that levofloxacin has good penetration into cavitary lesions. A key clinical implication of our study is that through optimizing serum levofloxacin concentrations, cavitary drug concentrations can be optimized in most patients. Further work is needed to better understand why penetration in some lesions is poor and to determine the lung penetration of other SLDs. This knowledge will ultimately help clinicians enhance individualized treatment regimens and improve clinical outcomes.
ACKNOWLEDGMENTS
This work was supported in part by the National Institutes of Health Fogarty International Center (D43TW007124), the National Institute of Allergy and Infectious Diseases (K23AI103044), the Atlanta Clinical and Translational Science Institute (UL1TR000454), and the Emory University Global Health Institute. A.B.B. thanks the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Brazil) for her Ph.D. scholarship.
We thank the chemists from the University of Florida Infectious Diseases Pharmacokinetics Laboratory, including Vaneska Mayor, Theodore Zagurski, Kyung Mee Kim, and Behrang Mahjoub, for their help with the analysis of clinical samples. We also thank Robert J. May for help with processing the tissue samples and Judith Johnson for help with the biosafety and shipping procedures.
No author has a commercial or other association that might pose a conflict of interest.
REFERENCES
- 1.Dheda K, Gumbo T, Gandhi NR, Murray M, Theron G, Udwadia Z, Migliori GB, Warren R. 2014. Global control of tuberculosis: from extensively drug-resistant to untreatable tuberculosis. Lancet Respir Med 2:321–338. doi: 10.1016/S2213-2600(14)70031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orenstein EW, Basu S, Shah NS, Andrews JR, Friedland GH, Moll AP, Gandhi NR, Galvani AP. 2009. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis 9:153–161. doi: 10.1016/S1473-3099(09)70041-6. [DOI] [PubMed] [Google Scholar]
- 3.WHO. 2013. Global tuberculosis report. WHO/HTM/20131. WHO, Geneva, Switzerland. [Google Scholar]
- 4.Dartois V, Barry CE. 2010. Clinical pharmacology and lesion penetrating properties of second- and third-line antituberculous agents used in the management of multidrug-resistant (MDR) and extensively-drug resistant (XDR) tuberculosis. Curr Clin Pharmacol 5:96–114. doi: 10.2174/157488410791110797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srivastava S, Pasipanodya JG, Meek C, Leff R, Gumbo T. 2011. Multidrug-resistant tuberculosis not due to noncompliance but to between-patient pharmacokinetic variability. J Infect Dis 204:1951–1959. doi: 10.1093/infdis/jir658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. 2013. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis 208:1464–1473. doi: 10.1093/infdis/jit352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chideya S, Winston CA, Peloquin CA, Bradford WZ, Hopewell PC, Wells CD, Reingold AL, Kenyon TA, Moeti TL, Tappero JW. 2009. Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis from Botswana. Clin Infect Dis 48:1685–1694. doi: 10.1086/599040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mpagama SG, Ndusilo N, Stroup S, Kumburu H, Peloquin CA, Gratz J, Houpt ER, Kibiki GS, Heysell SK. 2014. Plasma drug activity in patients on treatment for multidrug-resistant tuberculosis. Antimicrob Agents Chemother 58:782–788. doi: 10.1128/AAC.01549-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akkerman OW, van Altena R, Klinkenberg T, Brouwers AH, Bongaerts AH, van der Werf TS, Alffenaar JW. 2013. Drug concentration in lung tissue in multidrug-resistant tuberculosis. Eur Respir J 42:1750–1752. doi: 10.1183/09031936.00047413. [DOI] [PubMed] [Google Scholar]
- 10.Kempker RR, Vashakidze S, Solomonia N, Dzidzikashvili N, Blumberg HM. 2012. Surgical treatment of drug-resistant tuberculosis. Lancet Infect Dis 12:157–166. doi: 10.1016/S1473-3099(11)70244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu HB, Jiang RH, Li L. 2011. Pulmonary resection for patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. J Antimicrob Chemother 66:1687–1695. doi: 10.1093/jac/dkr210. [DOI] [PubMed] [Google Scholar]
- 12.Kempker RR, Rabin AS, Nikolaishvili K, Kalandadze I, Gogishvili S, Blumberg HM, Vashakidze S. 2012. Additional drug resistance in Mycobacterium tuberculosis isolates from resected cavities among patients with multidrug-resistant or extensively drug-resistant pulmonary tuberculosis. Clin Infect Dis 54:e51–54. doi: 10.1093/cid/cir904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vashakidze S, Gogishvili S, Nikolaishvili K, Dzidzikashvili N, Tukvadze N, Blumberg HM, Kempker RR. 2013. Favorable outcomes for multidrug and extensively drug resistant tuberculosis patients undergoing surgery. Ann Thorac Surg 95:1892–1898. doi: 10.1016/j.athoracsur.2013.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaurasia CS, Muller M, Bashaw ED, Benfeldt E, Bolinder J, Bullock R, Bungay PM, DeLange EC, Derendorf H, Elmquist WF, Hammarlund-Udenaes M, Joukhadar C, Kellogg DL Jr, Lunte CE, Nordstrom CH, Rollema H, Sawchuk RJ, Cheung BW, Shah VP, Stahle L, Ungerstedt U, Welty DF, Yeo H. 2007. AAPS-FDA Workshop white paper: microdialysis principles, application, and regulatory perspectives. J Clin Pharmacol 47:589–603. doi: 10.1177/0091270006299091. [DOI] [PubMed] [Google Scholar]
- 15.Azeredo FJ, Dalla Costa T, Derendorf H. 2014. Role of microdialysis in pharmacokinetics and pharmacodynamics: current status and future directions. Clin Pharmacokinet 53:205–212. doi: 10.1007/s40262-014-0131-8. [DOI] [PubMed] [Google Scholar]
- 16.WHO. 2014. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. WHO/HTM/TB/2014.11. WHO, Geneva, Switzerland. [PubMed] [Google Scholar]
- 17.Johnson JL, Hadad DJ, Boom WH, Daley CL, Peloquin CA, Eisenach KD, Jankus DD, Debanne SM, Charlebois ED, Maciel E, Palaci M, Dietze R. 2006. Early and extended early bactericidal activity of levofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis 10:605–612. [PubMed] [Google Scholar]
- 18.Peloquin CA, Hadad DJ, Molino LP, Palaci M, Boom WH, Dietze R, Johnson JL. 2008. Population pharmacokinetics of levofloxacin, gatifloxacin, and moxifloxacin in adults with pulmonary tuberculosis. Antimicrob Agents Chemother 52:852–857. doi: 10.1128/AAC.01036-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stenken JA. 1999. Methods and issues in microdialysis calibration. Anal Chim Acta 379:337–358. doi: 10.1016/S0003-2670(98)00598-4. [DOI] [Google Scholar]
- 20.Lonnroth P, Jansson PA, Smith U. 1987. A microdialysis method allowing characterization of intercellular water space in humans. Am J Physiol 253:E228–231. [DOI] [PubMed] [Google Scholar]
- 21.Tukvadze N, Kempker RR, Kalandadze I, Kurbatova E, Leonard MK, Apsindzelashvili R, Bablishvili N, Kipiani M, Blumberg HM. 2012. Use of a molecular diagnostic test in AFB smear positive tuberculosis suspects greatly reduces time to detection of multidrug resistant tuberculosis. PLoS One 7:e31563. doi: 10.1371/journal.pone.0031563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parsons LM, Somoskovi A, Gutierrez C, Lee E, Paramasivan CN, Abimiku A, Spector S, Roscigno G, Nkengasong J. 2011. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clin Microbiol Rev 24:314–350. doi: 10.1128/CMR.00059-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. 2009. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis SL, Neuhauser MM, McKinnon PS. 2005. Quinolones, p 337–366. In Yu VL, Edwards G, McKinnon PS, Peloquin C, Morse GD (ed), Antimicrobial chemotherapy and vaccines, 2nd ed, vol II Antimicrobial agents. Esun Technologies, LLC, Pittsburgh, PA. [Google Scholar]
- 25.Ahuja SD, Ashkin D, Avendano M, Banerjee R, Bauer M, Bayona JN, Becerra MC, Benedetti A, Burgos M, Centis R, Chan ED, Chiang CY, Cox H, D'Ambrosio L, DeRiemer K, Dung NH, Enarson D, Falzon D, Flanagan K, Flood J, Garcia-Garcia ML, Gandhi N, Granich RM, Hollm-Delgado MG, Holtz TH, Iseman MD, Jarlsberg LG, Keshavjee S, Kim HR, Koh WJ, Lancaster J, Lange C, de Lange WC, Leimane V, Leung CC, Li J, Menzies D, Migliori GB, Mishustin SP, Mitnick CD, Narita M, O'Riordan P, Pai M, Palmero D, Park SK, Pasvol G, Pena J, Perez-Guzman C, Quelapio MI, Ponce-de-Leon A, et al. . 2012. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med 9:e1001300. doi: 10.1371/journal.pmed.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holtz TH, Sternberg M, Kammerer S, Laserson KF, Riekstina V, Zarovska E, Skripconoka V, Wells CD, Leimane V. 2006. Time to sputum culture conversion in multidrug-resistant tuberculosis: predictors and relationship to treatment outcome. Ann Intern Med 144:650–659. doi: 10.7326/0003-4819-144-9-200605020-00008. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton CD, Stout JE, Goodman PC, Mosher A, Menzies R, Schluger NW, Khan A, Johnson JL, Vernon AN. 2008. The value of end-of-treatment chest radiograph in predicting pulmonary tuberculosis relapse. Int J Tuberc Lung Dis 12:1059–1064. [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan G, Post FA, Moreira AL, Wainwright H, Kreiswirth BN, Tanverdi M, Mathema B, Ramaswamy SV, Walther G, Steyn LM, Barry CE III, Bekker LG. 2003. Mycobacterium tuberculosis growth at the cavity surface: a microenvironment with failed immunity. Infect Immun 71:7099–7108. doi: 10.1128/IAI.71.12.7099-7108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim HR, Hwang SS, Kim HJ, Lee SM, Yoo CG, Kim YW, Han SK, Shim YS, Yim JJ. 2007. Impact of extensive drug resistance on treatment outcomes in non-HIV-infected patients with multidrug-resistant tuberculosis. Clin Infect Dis 45:1290–1295. doi: 10.1086/522537. [DOI] [PubMed] [Google Scholar]
- 30.Torun T, Tahaoglu K, Ozmen I, Sevim T, Atac G, Kir A, Gungor G, Bolukbasi Y, Maden E. 2007. The role of surgery and fluoroquinolones in the treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 11:979–985. [PubMed] [Google Scholar]
- 31.Kislitsyna NA. 1985. Comparative evaluation of rifampicin and isoniazid penetration into the pathological foci of the lungs in tuberculosis patients. Probl Tuberk 1985:55–57. (In Russian.) [PubMed] [Google Scholar]
- 32.Kislitsyna NA, Kotova NI. 1980. Rifampicin and isoniazid concentration in the blood and resected lungs in tuberculosis with combined use of the preparations. Probl Tuberk 1980:63–65. (In Russian.) [PubMed] [Google Scholar]
- 33.Chierakul N, Klomsawat D, Chulavatnatol S, Chindavijak B. 2001. Intrapulmonary pharmacokinetics of ofloxacin in drug-resistant tuberculosis. Int J Tuberc Lung Dis 5:278–282. [PubMed] [Google Scholar]
- 34.Dhanani J, Roberts JA, Chew M, Lipman J, Boots RJ, Paterson DL, Fraser JF. 2010. Antimicrobial chemotherapy and lung microdialysis: a review. Int J Antimicrob Agents 36:491–500. doi: 10.1016/j.ijantimicag.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Lindenmann J, Kugler SA, Matzi V, Porubsky C, Maier A, Dittrich P, Graninger W, Smolle-Juttner FM, Joukhadar C. 2011. High extracellular levels of cefpirome in unaffected and infected lung tissue of patients. J Antimicrob Chemother 66:160–164. doi: 10.1093/jac/dkq413. [DOI] [PubMed] [Google Scholar]
- 36.Matzi V, Lindenmann J, Porubsky C, Kugler SA, Maier A, Dittrich P, Smolle-Juttner FM, Joukhadar C. 2010. Extracellular concentrations of fosfomycin in lung tissue of septic patients. J Antimicrob Chemother 65:995–998. doi: 10.1093/jac/dkq070. [DOI] [PubMed] [Google Scholar]
- 37.Prideaux B, Dartois V, Staab D, Weiner DM, Goh A, Via LE, Barry CE III, Stoeckli M. 2011. High-sensitivity MALDI-MRM-MS imaging of moxifloxacin distribution in tuberculosis-infected rabbit lungs and granulomatous lesions. Anal Chem 83:2112–2118. doi: 10.1021/ac1029049. [DOI] [PMC free article] [PubMed] [Google Scholar]


