Abstract
Dopaminergic activity is both associated with sociosexual exposure and modulated by sexual experience and hormonal state across vertebrate taxa. Mature leopard geckos, a reptile with temperature-dependent sex determination, have dopaminoceptive nuclei that are influenced by their embryonic environment and sensitive to adult hormonal manipulation. In this study, we exposed hormonally manipulated male leopard geckos from different incubation temperatures to conspecifics and measured their sociosexual investigation, as well as phosphorylated DARPP-32 at Threonine 34 (pDARPP-32) immunoreactivity as a marker for D1 dopamine receptor activity in the nucleus accumbens, striatum, and preoptic area. Social investigation time by males of different incubation temperatures was modulated in opposite directions by exogenous androgen treatment. Males exposed to novel stimuli spent a greater proportion of time investigating females of different incubation temperatures. The time spent investigating females was positively correlated to pDARPP-32 immunoreactivity in the preoptic area. This is the first study quantifying pDARPP-32 in a lizard species, and suggests the protein as a potential marker to measure differences in the dopaminergic pathway in a social setting with consideration of embryonic environment and hormonal state.
Behaviors that lead to the individual repeating the action, such as sexual behavior, can activate dopaminergic pathways. Dopamine is generated and released during sexual exposure (Hull et al., ’95), and is affected by sexual experience (Mitchell and Stewart ’89) as well as androgen manipulation (Alderson and Baum, ’81; Du and Hull, ’99). The mesolimbic pathway includes the basolateral amygdala, nucleus accumbens, ventral pallidum, hippocampus, septum, striatum, and ventral tegmental area (VTA) (O’Connell and Hofmann, 2011). The projection of dopamine from the VTA to the dopaminoceptive nucleus accumbens is conserved across taxa and is involved in appetitive male-typical sexual behaviors and influenced by sociosexual exposure (Smeets, ’88; Gonzalez et al., ’90; O’Connell and Hofmann, 2011). In the nigrostriatal pathway, dopamine neurons in the substantia nigra project to the striatum, and are involved in motor coordination including copulation in male rats (Hull et al., 2004). The reptile striatum has fewer distinct subnuclei than in mammals and receives input primarily from the substantial nigra but also input from the VTA (Smeets et al., ’86, 2001; Gonzalez et al., ’90; Smeets and Medina, ’95); catecholamine synthesis in this pathway is associated with male sexual vigor in the whiptail lizard (Cnemidophorus inornatus) (Woolley et al., 2004).
Dopaminergic pathways have been determined by studying ligand binding and receptor distributions in brain nuclei as well as afferent and efferent connections of the nuclei. Dopamine D1 receptors, when activated, can modulate cellular activity through the dopamine-and cAMP-regulated phosphoprotein (DARPP-32) via phosphorylation of Threonine-34 (pDARPP-32) (Hemmings et al., ’84; Ouimet et al., ’84; Fienberg et al., ’98). Immunochemistry studies involving pDARPP-32 in the mesolimbic nuclei focus on dopamine activity under drug and alcohol manipulation (Maldve et al., 2002; Nairn et al., 2004; Zachariou et al., 2005). In both male and female rodents dopaminoceptive hypothalamic nuclei show pDARPP-32 differences during steroid hormone treatment and sexual behavior (Mani et al., 2000; Auger et al., 2001 McHenry et al., 2012) of particular interest is that D1 dopamine antagonist treatment reduces copulatory behavior in male rats (McHenry et al., 2012). Further, D1 receptor agonist treatment increases copulatory behavior male whiptail lizard (Cnemidophorus inornatus) (Woolley et al., 2001). While behaviors associated with pDARPP-32 in reptiles have not been described, DARPP-32 immunopositive cells in the striatum and NAcc have been described in the Tokay gecko (Gekko gecko) (Smeets et al., 2001).
In addition to the mesolimbic pathway, dopaminergic activity in the preoptic area of the hypothalamus (POA) is associated with both appetitive and consummatory male-typical copulatory behavior in mammal and bird species (Pehek et al., ’88; Balthazart et al., ’98; Dominguez and Hull, 2005). This association is influenced by steroid hormones across vertebrate taxa (Crews and Silver, ’85; Balthazart et al., ’98; Ball and Balthazart, 2004; Woolley et al., 2004).
The POA and dopaminergic pathways are of interest in the leopard gecko (Eublepharis macularius), a lizard species with temperature-dependent sex determination. In both sexes the brain and sociosexual behavior are affected by incubation temperature. In contrast to the sexually dimorphic nucleus of the POA (SDN-POA) of mammalian and avian species (Gorski et al., ’78; Viglietti-Panzica et al., ’86), POA differences in the leopard gecko are greater between incubation temperatures than between individuals of the same gonadal sex. For example, the POA is larger and has greater metabolic activity in males and females from a male-biased (32.5°C) incubation temperature (IncT) compared to males and females from a female-biased (30°C) IncT (Coomber et al., ’97; Crews et al., ’98). In addition, the volume and metabolic activity of the POA are androgen sensitive in male leopard gecko from a male-biased IncT (Crews et al., ’96; Rhen and Crews, 2001). The mesolimbic pathway also shows differences in the adult brain according to incubation temperature; males from a female-biased IncT have greater dopamine levels in the nucleus accumbens than do males from a male-biased IncT (Dias et al., 2007).
In order to understand how sociosexual behavior and the dopaminergic system are influenced by embryonic experience, adult sexual experience, and circulating androgens, sexually experienced male leopard geckos of different incubation temperatures were hormonally manipulated and exposed to conspecific stimuli. The pDARPP-32 immunoreactivity was used as a marker for D1 dopamine receptor activation in the following nuclei: nucleus accumbens, striatum, and POA. We expected that investigation time of conspecifics would be dependent on IncT, which is modulated by different social stimuli (Putz and Crews, 2006). We also expected that IncT-dependent conspecific investigation and pDARPP-32 immunoreactivity in dopaminoceptive nuclei would be responsive to androgen, as previous studies indicated IncT-specific sensitivities to androgen manipulation (Rhen and Crews, ’99; Dias et al., 2007), as well as social stimulus-dependent differences in sociosexual behaviors (Rhen and Crews, 2000; Sakata et al., 2002). If dopaminoceptive neuronal activity in male leopard geckos is similar to male rats (Hull et al., ’95; McHenry et al., 2012), we predict that pDARPP-32 immunoreactivity would positively correlate to stimulus female exposure.
METHODS
Animals
Animals were hatched from eggs obtained from controlled matings in the laboratory (Tousignant and Crews, ’95). Eggs were incubated individually in covered plastic caps containing a water/vermiculite ratio of 1:1 in a monitored incubation chamber (Precision, IL, USA) with a HOBO temperature logger (Onset Computer Corp, Pocasset, MA, USA). Hatchlings were fed live crickets every other day for 10 weeks and received water ad libitum. As juveniles and adults their diet changed to vitamin-dusted mealworms in a 14:10 day: night cycle. Animals (n = 37) were cared and treated under the guidance of IACUC protocol number AUP-2011-00135.
Castration and Hormone Treatment
Virgin adult males from different incubation temperatures (3–8 years of age) were placed in a tank with 2–4 adult females to gain sexual experience. This lasted for 4–12 weeks and ended 1–2 weeks before males were castrated; at the time of castration males were implanted with a single 20 mm Silastic capsule containing dihydrotestosterone (DHT) (Steraloids, Rhode Island, USA) or empty or a blank Silastic capsule (BLANK). Previous studies on leopard geckos demonstrate that androgens influence male-typical sociosexual behavior, and males were of particular interest as they initiate copulatory behavior (Crews et al., ’98; Holmes et al., 2005). Castration was performed using hypothermia anesthesia in accordance with IACUC protocol.
Experimental males were from a female-biased IncT (30°C), a male-biased IncT (32.5°C), and a high IncT (34°C) to generate temperature morphs; the high IncT males received Silastic capsules containing DHT (henceforth 34-DHT). The treatment groups were aged matched. There were not enough animals to generate a 34-BLANK group as the percentage of males is 5% at this incubation temperature. Females were unavailable for this study.
Social Y Maze Testing
Four weeks following castration and hormonal manipulation, experimental males were given a sequence of social exposures in a Plexiglas Y maze (Putz and Crews, 2006; Huang and Crews, 2012). The first social exposure Y maze test used an unfamiliar male and female from the male-biased IncT as stimulus animals in the short arms of the Y maze, followed by a Y maze test using a stimulus male and female from the female-biased incubation temperature. For the social Y maze tests, experimental males were exposed to the same stimulus male. However, the stimulus females varied to ensure receptivity as indicated by vitellogenic (yolking) follicles; this state was verified by observing vascularized follicles seen through their translucent abdomens. The first two tests were conducted 2–8 days apart, and the third test was conducted 12 weeks later to reduce any effects of social exposure experience from the different temperature morphs. Each test lasted 15 min; halfway through the test the stimulus animals were switched to the opposite short arm of the Y maze to control for possible side bias.
Euthanasia and Brain Removal
Twenty minutes after the last Y maze test, experimental males were given an overdose of sodium pentobarbital (Euthasol, Virbac Animal Health, Fort Worth, TX, USA). After expressing no reaction from a hard digit-pinch, males were then perfused with a saline solution and then with cold 4% paraformaldehyde (PFA). After perfusion, animals were decapitated, heads placed in 4% PFA overnight, and the brains extracted and cryoprotected in 20% sucrose before being frozen in −80°C. Frozen brains were then cryosectioned at 40 m on Superfrost Plus Gold microscope slides in a 1:6 series, where sections on one slide were six sections apart.
Immunohistochemisty
Slides were first placed in 4% PFA for 10 min, washed twice in PBS for 10 min and placed in 3% hydrogen peroxide to quench endogenous peroxidase activity for 20 min. After 10 min of PBS washing, the slides were placed in 1% NaBH4 for 20 min for antigen retrieval and washed for 10 min in PBS. Slides were then blocked with 4% normal goat serum for 60 min before being incubated with a primary antibody overnight with a dilution ratio of 1:300 rabbit polyclonal anti-pDARPP-32 (sc21601-R, Santa Cruz, Dallas, TX, USA). Sections incubated without primary antibodies served as negative controls. An additional negative control for pDARPP-32 included the antibody preadsorbed with 5× peptide concentration (sc21601 P, Santa Cruz). Neither control showed immunopositive staining (Fig. 1). On the second day, slides were washed for 20 min in PBS, incubated with 1:200 biotinylated goat anti-rabbit secondary antibody for pDARPP-32. Slides were washed in PBS for 20 min before incubating with Avidin Biotin complex (Vector Laboratories Inc., Burlingame, CA, USA) for 60 min. After washing for 20 min, immunoreactivity was detected with diaminobenzidine (DAB). Slides were washed with water, dried, and coverslipped for cell quantification.
Figure 1.
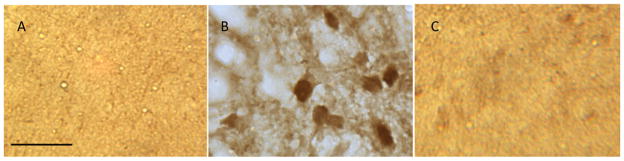
Antibody validation of pDARPP-32 on nucleus accumbens of sectioned brain tissue. Immunohistochemistry was conducted on cryosectioned leopard gecko (Eublepharis macularius) brain tissue using a rabbit polyconal antibody against pDARPP-32, and DAB staining was used to visualize pDARPP-32 immunoreactive cells. Bars indicate 50 μm. (A) Without pDARPP-32 antibody, no cells were detected. (B) pDARPP-32 neuronal staining was detected when tissue was incubated with antibody. (C) pDARPP-32 neuronal staining was not detected when antibody was preadsorbed with 5× pDARPP-32 peptide prior to incubation.
Immunoreactive Cell Quantification
For an unbiased estimation of immunopositive cell density and cell numbers of pDARPP-32, the optical fractionator module of StereoInvestigator software was used (Microbrightfield, Williston, VT, USA). The nucleus accumbens and striatum were outlined in three brain sections, and the POA was outlined in two sections. The nuclei were delineated according to descriptions and brain maps of previous gecko studies (Smeets and Steinbusch, ’89; Coomber et al., ’97). Within each outline, the optical disector of 50 × 50 × 10 m3 of x-y-z dimensions was placed in a 100 × 100 m2 grid to ensure standard and random sampling. Cells with immunopositive cytoplasmic staining for pDARPP-32 were counted in the sampling frame for each dopaminoceptive nucleus within the outlined region using the 40× objective on a Zeiss microscope. While pDARPP-32 immunoreactivity has been detected in the neuropil and the nucleus (Rex et al., 2008; Stipanovich et al., 2008), cytoplasmic staining only was quantified. Total cell number was calculated with a formula from West et al. (’91). Because individuals varied in the nuclei volume that was sampled, immunopositive neuronal densities were estimated in the nuclei using the formula derived from Mayhew and Gundersen (’96).
Statistics
Analysis of IncT or hormone implant effects on behavior and brain immunocytochemistry employed the statistical program R (R, a Language and Environment for Statistical Computing, Vienna, Austria 2010). A generalized linear mixed model analysis with Poisson distribution was used to compare conspecific investigation time in the Y maze among treatment groups. Further, the proportion of time spent with female conspecifics was calculated from the total investigation time per maze. Considering only the social investigators, paired t-tests were used to compare differences within each treatment group in proportion of investigation time with the two female temperature morphs of the two social Y maze tests. ANOVA or Kruskal–Wallis tests were used to detect differences among treatment groups for pDARPP-32 immunoreactive neuronal density in the nucleus accumbens, striatum, and the POA. To determine if pDARPP-32 immunoreactivity was correlated to social exposure in the last Y maze test, Pearson product correlation tests were conducted to relate pDARPP-32 immunoreactive neuronal density and colocalization in the aforementioned nuclei to total time spent in the last Y maze investigating conspecifics from a female-biased IncT.
RESULTS
Social Y Maze Tests
For each test, the total amount of time spent at the short arms of the Y maze with the stimulus conspecific was measured, as well as the proportion of investigation time spent with stimulus females from a male- and a female-biased IncT. When exposed to conspecifics from a male-biased IncT in the Y maze, no differences in total investigation time were detected within any treatment groups (Fig. 2A). When exposed to conspecifics from a female-biased IncT, there was an implant effect and IncT X implant interaction effect on total time spent with conspecifics (Fig. 2B, P = 0.021 and 0.024, respectively).
Figure 2.
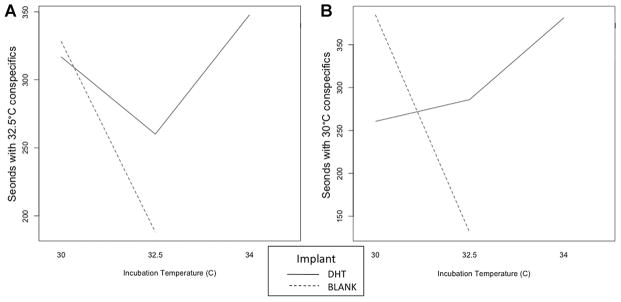
Manipulation effects on total time males spent with male and female conspecifics from a (A) male-biased incubation temperature (IncT) and (B) female-biased IncT. Generalized linear mixed model with Poisson distribution was performed on the total time spent investigating conspecifics in two Y maze tests. IncT, Implant, and IncT × Implant effects were not detected in total time spent with conspecifics from a male-biased IncT (P > 0.05). In the Y maze with conspecifics from a female-biased IncT, implant effect (P = 0.022) and incubation implant effect (P = 0.024) were detected. However, incubation temperature effect was not detected (P > 0.05).
Within each treatment group, those males that investigated conspecifics did not differ in the proportion of investigation time with stimulus females from a male- or a female-biased IncT, with the exception of males from the high IncT treated with DHT (34-DHT) (Fig. 3). High temperature males (34-DHT) spent a significantly greater percentage of time investigating females from a male-biased versus a female-biased IncT (t = −2.801, df = 6, P = 0.031).
Figure 3.
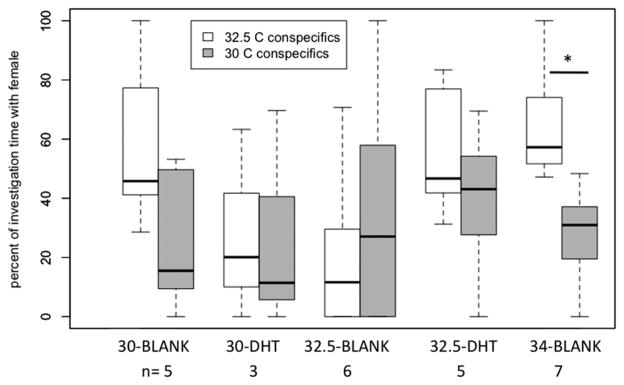
In male leopard geckos that investigated conspecifics in both Y mazes, percent of total conspecific investigation time spent with a stimulus female from a male-biased incubation temperature (IncT) and a stimulus female from a female-biased IncT. Box-and-whisker plots (median, 1st and 3rd quartile ± 1.5 inter-quartile range) indicated per treatment group. In the treatment group 34-DHT, males spent a greater percentage of investigative time with females in the Y maze with conspecifics from a male-biased compared to a female-biased IncT (P = 0.031). Individuals that did not investigate a conspecific in one Y maze were excluded from the respective test.
pDARPP-32 Immunoreactivity in Dopaminoceptive Nuclei
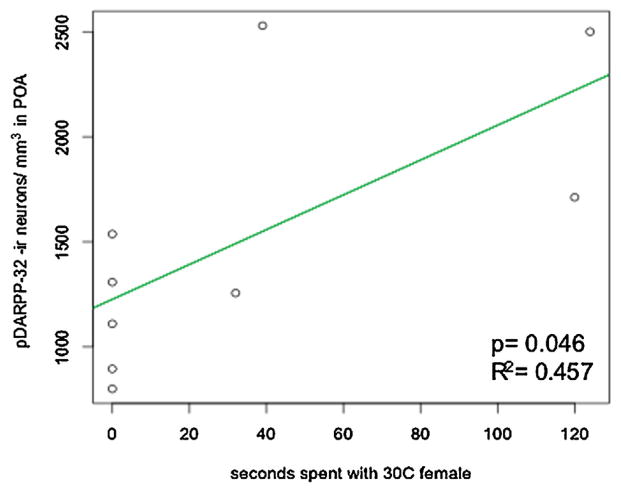
No differences were detected among treatment groups for pDARPP-32 immunoreactive neuronal density in the nucleus accumbens, striatum, or the POA (Table 1). Though pDARPP-32 immunoreactivity was detected in other cellular regions, only cytoplasmic staining was considered (Rex et al., 2008; Stipanovich et al., 2008). When relating exposure to females to density of pDARPP-32 immunoreactive neurons in the POA, males with BLANK treatment from male-biased IncT showed a positive correlation with time spent visiting stimulus females from a female-biased IncT (t = 2.426, P = 0.046, R2 = 0.457 [Fig. 4]). No other significant correlation between pDARPP-32 neuronal density and time investigating conspecifics was detected within treatment groups in the nucleus accumbens, striatum, or POA.
Table 1.
Density (#/mm3) of pDARPP-32 cells (mean ± SE) in dopaminoceptive nuclei of male leopard geckos from different incubation temperatures (30, 32.5, or 34°C) and hormone treatment (BLANK or DHT).
| Gecko group | Nucleus accumbens | Striatum | Preoptic area |
|---|---|---|---|
| 30-BLANK | 1,765 ± 69 | 1,356 ± 439 | 1,726 ± 272 |
| 30-DHT | 1,171 ± 259 | 676 ± 119 | 1,423 ± 371 |
| 32.5-BLANK | 1,431 ± 195 | 864 ± 143 | 1,516 ± 211 |
| 32.5-DHT | 1,608 ± 139 | 863 ± 115 | 1,707 ± 312 |
| 34-DHT | 1,580 ± 184 | 803 ± 85 | 1,329 ± 146 |
Figure 4.
Castrated plus BLANK treated male leopard geckos from a male-biased incubation temperature (IncT) show a positive correlation between pDARPP-32 immunoreactive neuronal density in the POA and time spent with stimulus females from a female-biased IncT.
DISCUSSION
The purpose of this study was to determine if sociosexual investigation differed among sexually experienced animals in relation to incubation temperature or hormonal manipulation, and if social exposure or manipulated factors affected the dopaminergic pathway. After measuring time spent with conspecifics of different incubation temperatures and in a Y maze behavioral design, pDARPP-32 immunoreactivity was used as a proxy for quantifying D1 dopamine receptor activation in dopaminoceptive nuclei. We found that total investigation time with stimulus conspecifics from a female-biased IncT was influenced by hormone implant in opposite directions for males from a female-biased versus a male-biased IncT. We also found that within the 34-DHT treatment group those males that exhibited social investigation spent a greater proportion of time with stimulus females (vs. males from a male-biased IncT) than with stimulus females (vs. males from a female-biased IncT). Dopaminoceptive nuclei pDARPP-32 immunoreactivity did not differ among treatment groups; however, in the POA of males treated with BLANK implants and from a male-biased IncT, pDARPP-32 immunoreactive neuronal density was positively correlated with the time these males spent in the Y maze arm with females from a female-biased IncT.
The treatment groups differed in their reaction to conspecifics from a female-biased IncT but not to conspecifics from a male-biased IncT. While this is the first study employing stimulus conspecifics from a male-biased IncT in the Y maze, previous work with this species has demonstrated that stimulus females of different incubation temperature elicit varying investigation time dependent on their incubation temperature (Putz and Crews, 2006). Although the subjects from this study had different sexual experience and stimulus conspecific exposure than animals in previous experiments, this result is consistent with previous findings that male-biased IncT morphs differ in total social investigation time (Huang and Crews, 2012). Notably, this is the first study to use the high temperature males treated with DHT; at this incubation temperature, males are relatively rare.
It is of particular interest that, in the absence of gonadal hormones, pDARPP-32 neuronal density in the POA of males from a male-biased IncT correlated positively with time spent investigating females from a female-biased IncT, while other treatment groups showed no such correlation. Because males of female-biased IncT have reduced preference for stimulus females from their own IncT (Putz and Crews, 2006), such animals might, in turn, have less dopaminergic sensitivity to this exposure. Previous studies of males from a male-biased IncT and treated with DHT have shown increased aggression toward other males (Rhen and Crews, 2000; Sakata and Crews, 2003). While aggressive behaviors were not detected in the Y maze tests in the present study, it is possible that the exposure to other males could override dopaminergic changes associated with exposure to females.
It was expected that dopamine in the nucleus accumbens and POA would increase in relation to female exposure, even in the absence of gonadal hormones, if the mesolimbic pathway is similar to rodents (Damsma et al., ’92; Hull et al. ’95). Alternatively, dopamine could increase sexual behavior by modulating POA steroid hormone metabolism as indicated in the Japanese quail (Balthazart et al., ’97, 2002; Cornil et al., 2002). However, any positive influence of female exposure on neuronal pDARPP-32 in the nucleus accumbens could have been dampened by time spent with stimulus males. Incubation temperature and hormone manipulation did not affect pDARPP-32-ir in the striatum. A possible explanation is that even though the males were sexually experienced, visual, and chemical cues—but lack of tactile investigation allowed in the Y maze—were not sufficient to elicit a change in dopamine activity in the striatum that is related to motor coordination in sexual behavior (Damsma et al., ’92).
These results are comparable to studies with sexually experienced male rats that, combined with recent sexual behavior, experience greater D1 dopamine receptor activity in the POA than males with sexual experience but no recent sexual behavior (McHenry et al., 2012). While no appetitive sexual behavior was observed in the Y maze tests, the time spent investigating females correlated positively to pDARPP-32-ir in the POA. The sensitivity to sociosexual exposure in castrated male leopard geckos is of particular interest, as previous studies in female rodent POA show that steroid hormones modulate phosphorylated DARPP-32 protein expression (Mani et al., 2000; Auger et al., 2001). pDARPP-32 immunoreactivity in the POA of female rats can also be affected by vaginal stimulation in the absence of progesterone (Meredith et al., ’98). The absence of incubation temperature or hormone implant effect on pDARPP-32 immunoreactivity neuronal density in dopaminoceptive nuclei might have also been influenced by the variation of social investigation time within each treatment group.
Because the Y maze included both male and female stimulus conspecifics that would elicit different behaviors, this mixed sex exposure potentially yielded different reactions in the dopaminergic system. In future experiments relating behavior to pDARPP-32, a single sex exposure design would be appropriate to delineate sex-specific exposure effects in these nuclei. Alternatively, more controlled exposure time and perhaps tactile investigation of the conspecific would generate a more defined relationship between neural protein expression and sociosexual behavior.
In conclusion, males from different IncT differ in hormone sensitivity in social investigation of conspecifics of female-biased IncT. Specific treatment groups were unique in reaction to stimulus females of male-compared to female-biased IncT and in POA pDARPP-32 immunoreactivity in relation to female exposure. As pDARPP-32 has been primarily used in studies of drug addiction and rodent sexual behavior, it is worth noting that the use of pDARPP-32 as a potential immunoreactive marker for studying D1 dopamine receptor activation in the limbic system of a reptile species in a sociosexual context is novel. This permitted us to demonstrate that the time male geckos spend investigating females correlates to pDARPP-32 immunoreactivity in the preoptic area.
Acknowledgments
Grant sponsor: NSF IOS; grant number: 0750938; grant sponsor: NSF GRFP.
We thank Molly Cummings, Hans Hofmann, for reading. Erika Hale for statistical feedback, Mai Vu and Sunny Patel for quantifying behavior and help with immunohistochemistry, Van B. Christopher for slide labeling, and Andrea Gore and Michelle Naugle for immunohistochemistry image feedback and help. This research supported by NSF NSF IOS 0750938 to DC and NSF GRFP to VH.
Abbreviations
- pDARPP-32
phosphorylated DARPP-32 at threonine 34
- DAB
diaminobenzadine
- TH
tyrosine hydroxylase
- DHT
dihydrotestosterone implant
- BLANK
blank implant
- POA
preoptic area
- NAcc
nucleus accumbens
- IncT
incubation temperature
Footnotes
Conflicts of interest: None.
LITERATURE CITED
- Alderson LM, Baum MJ. Differential effects of gonadal steroids on dopamine metabolism in mesolimbic and nigro-striatal pathways of male rat brain. Brain Res. 1981;218:189–206. doi: 10.1016/0006-8993(81)91300-7. [DOI] [PubMed] [Google Scholar]
- Auger AP, Meredith JM, Snyder GL, Blaustein JD. Oestradiol increases phosphorylation of a dopamine- and cyclic AMP-regulated phosphoprotein (DARPP-32) in female rat Brain. J Neuroendocrinol. 2001;13:761–768. doi: 10.1046/j.1365-2826.2001.00700.x. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Hormonal regulation of brain circuits mediating male sexual behavior in birds. Physiol Behav. 2004;83:329–346. doi: 10.1016/j.physbeh.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Castagna C, Ball GF. Differential effects of D1 and D2 dopamine-receptor agonists and antagonists on appetitive and consummatory aspects of male sexual behavior in Japanese quail. Physiol Behav. 1997;62:571–580. doi: 10.1016/s0031-9384(97)00163-7. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Absil P, Gérard M, Appeltants D, Ball GF. Appetitive and consummatory male sexual behavior in Japanese quail are differentially regulated by subregions of the preoptic medial nucleus. J Neurosci. 1998;18:6512–6527. doi: 10.1523/JNEUROSCI.18-16-06512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF. Interactions between aromatase (estrogen synthase) and dopamine in the control of male sexual behavior in quail. Comp Biochem Physiol B. 2002;132:37–55. doi: 10.1016/s1096-4959(01)00531-0. [DOI] [PubMed] [Google Scholar]
- Coomber P, Crews D, Gonzalez-Lima F. Independent effects of incubation temperature and gonadal sex on the volume and metabolic capacity of brain nuclei in the leopard gecko (Eublepharis macularius), a lizard with temperature-dependent sex determination. J Comp Neurol. 1997;380:409–421. doi: 10.1002/(sici)1096-9861(19970414)380:3<409::aid-cne9>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Balthazart J, Motte P, Massotte L, Seutin V. Dopamine activates noradrenergic receptors in the preoptic area. J Neurosci. 2002;22:9320–9330. doi: 10.1523/JNEUROSCI.22-21-09320.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Silver R. Reproductive physiology and behavior interactions in nonmammalian vertebrate. In: Adler N, Pfaff D, Goy RW, editors. Handbook of behavioral neurobiology. New York: Plenum Press; 1985. pp. 101–182. [Google Scholar]
- Crews D, Coomber P, Baldwin R, Azad N, Gonzalez-Lima F. Brain organization in a reptile lacking sex chromosomes: effects of gonadectomy and exogenous testosterone. Horm Behav. 1996;30:474–486. doi: 10.1006/hbeh.1996.0051. [DOI] [PubMed] [Google Scholar]
- Crews D, Sakata JT, Rhen T. Developmental effects on intersexual and intrasexual variation in growth and reproduction in a lizard with temperature-dependent sex determination. Comp Biochem Physiol C. 1998;119:229–241. doi: 10.1016/s0742-8413(98)00012-7. [DOI] [PubMed] [Google Scholar]
- Damsma G, Pfaus JG, Wenkstern D, Phillips AG, Fibiger HC. Sexual behavior increases dopamine transmission in the nucleus accumbens and striatum of male rats: comparison with novelty and locomotion. Behav Neurosci. 1992;106:181–191. doi: 10.1037//0735-7044.106.1.181. [DOI] [PubMed] [Google Scholar]
- Dias BG, Ataya RS, Rushworth D, Zhao J, Crews D. Effect of incubation temperature and androgens on dopaminergic activity in the leopard gecko, Eublepharis macularius. Dev Neurobiol. 2007;67:630–636. doi: 10.1002/dneu.20382. [DOI] [PubMed] [Google Scholar]
- Dominguez JM, Hull EM. Dopamine, the medial preoptic area, and male sexual behavior. Physiol Behav. 2005;86:356–368. doi: 10.1016/j.physbeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Du J, Hull EM. Effects of testosterone on neuronal nitric oxide synthase and tyrosine hydroxylase. Brain Res. 1999;836:90–98. doi: 10.1016/s0006-8993(99)01618-2. [DOI] [PubMed] [Google Scholar]
- Fienberg AA, Hiroi N, Mermelstein PG, et al. DARPP-32: regulator of the efficacy of dopaminergic neurotransmission. Science. 1998;281:838–842. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Russchen FT, Lohman AHM. Afferent connections of the striatum and the nucleus accumbens in the lizard Gecko gekko. Brain Behav Evol. 1990;36:39–58. doi: 10.1159/000115296. [DOI] [PubMed] [Google Scholar]
- Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;148:333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Greengard P, Tung HYL, Cohen P. DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature. 1984;310:503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- Holmes MM, Putz O, Crews D, Wade J. Normally occurring pseudohermaphroditism and testosterone-induced plasticity in the copulatory system of adult leopard geckos. Horm Behav. 2005;47:439–445. doi: 10.1016/j.yhbeh.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Huang V, Crews D. Differences induced by incubation temperature, versus androgen manipulation, in male leopard geckos (Eublepharis macularius) Physiol Behav. 2012;107:121–124. doi: 10.1016/j.physbeh.2012.06.014. [DOI] [PubMed] [Google Scholar]
- Hull EM, Du J, Lorrain DS, Matuszewich L. Extracellular dopamine in the medial preoptic area: implications for sexual motivation and hormonal control of copulation. J Neurosci. 1995;15:7465–7471. doi: 10.1523/JNEUROSCI.15-11-07465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Muschamp JW, Sato S. Dopamine and serotonin: influences on male sexual behavior. Phys Behav. 2004;83:291–307. doi: 10.1016/j.physbeh.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Maldve RE, Zhang TA, Ferrani-Kile K, et al. DARPP-32 and regulation of the ethanol sensitivity of NMDA receptors in the nucleus accumbens. Nat Neurosci. 2002;5:641–648. doi: 10.1038/nn877. [DOI] [PubMed] [Google Scholar]
- Mani SK, Fienberg AA, O’Callaghan JP, et al. Requirement for DARPP-32 in progesterone-facilitated sexual receptivity in female rats and mice. Science. 2000;287:1053–1056. doi: 10.1126/science.287.5455.1053. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Gundersen HJ. “If you assume, you can make an ass out of u and me”: a decade of the disector for stereological counting of particles in 3D space. J Anat. 1996;188:1–15. [PMC free article] [PubMed] [Google Scholar]
- McHenry JA, Bell GA, Parrish BP, Hull EM. Dopamine D1 receptors and phosphorylation of dopamine- and cyclic AMP-regulated phosphoprotein-32 in the medial preoptic area are involved in experience-induced enhancement of male sexual behavior in rats. Behav Neurosci. 2012;126:523–529. doi: 10.1037/a0028707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith JM, Moffatt CA, Auger AP, et al. Mating-related stimulation induces phosphorylation of dopamine- and cyclic AMP-regulated phosphoprotein-32 in progestin receptor-containing areas in the female rat brain. J Neurosci. 1998;18:10189–10195. doi: 10.1523/JNEUROSCI.18-23-10189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JB, Stewart J. Effects of castration, steroid replacement, and sexual experience on mesolimbic dopamine and sexual behaviors in the male rat. Brain Res. 1989;491:116–127. doi: 10.1016/0006-8993(89)90093-0. [DOI] [PubMed] [Google Scholar]
- Nairn AC, Svenningsson P, Nishi A, et al. The role of DARPP-32 in the actions of drugs of abuse. Neuropharmacology. 2004;47:14–23. doi: 10.1016/j.neuropharm.2004.05.010. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol. 2011;519:3599–3639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- Ouimet CC, Miller PE, Hemmings HC, Walaas SI, Greengard P. DARPP-32, a dopamine- and adenosine 3′:5-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. III. Immunocytochemical localization. J Neurosci. 1984;4:111–124. doi: 10.1523/JNEUROSCI.04-01-00111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehek EA, Warner RK, Bazzett T, et al. Microinjection of cis-flupenthixol, a dopamine antagonist into the medial preoptic area impairs sexual behavior of male rats. Brain Res. 1988;443:70–76. doi: 10.1016/0006-8993(88)91599-5. [DOI] [PubMed] [Google Scholar]
- Putz O, Crews D. Embryonic origin of mate choice in a lizard with temperature-dependent sex determination. Dev Psychobiol. 2006;48:29–38. doi: 10.1002/dev.20109. [DOI] [PubMed] [Google Scholar]
- Rex EB, Rankin ML, Ariano MA, Sibley DR. Ethanol regulation of D1 dopamine receptor signaling is mediated by protein kinase C in an isozyme-specific manner. Neuropsychopharmacology. 2008;33:2900–2911. doi: 10.1038/npp.2008.16. [DOI] [PubMed] [Google Scholar]
- Rhen T, Crews D. Embryonic temperature and gonadal sex organize male-typical sexual and aggressive behavior in a lizard with temperature-dependent sex determination. Endocrinology. 1999;140:4501–4508. doi: 10.1210/endo.140.10.7083. [DOI] [PubMed] [Google Scholar]
- Rhen T, Crews C. Organization and activation of sexual and agonistic behavior in the leopard gecko, Eublepharis macularius. Neuroendocrinology. 2000;71:252–261. doi: 10.1159/000054543. [DOI] [PubMed] [Google Scholar]
- Rhen T, Crews C. Distribution of androgen and estrogen receptor mRNA in the brain and reproductive tissues of the leopard gecko, Eublepharis macularius. J Comp Neurol. 2001;437:385–397. doi: 10.1002/cne.1290. [DOI] [PubMed] [Google Scholar]
- Sakata JT, Crews D. Embryonic temperature shapes behavioural change following social experience in male leopard geckos, Eublepharis macularius. Anim Behav. 2003;66:839–846. [Google Scholar]
- Sakata JT, Gupta A, Chuang C-P, Crews D. Social experience affects territorial and reproductive behaviours in male leopard geckos, Eublepharis macularius. Animal Behaviour. 2002;63:487–493. [Google Scholar]
- Smeets WJAJ. Distribution of dopamine immunoreactivity in the forebrain and midbrain of the snake Python regius: a study with antibodies against dopamine. J Comp Neurol. 1988;271:115–129. doi: 10.1002/cne.902710112. [DOI] [PubMed] [Google Scholar]
- Smeets WJAJ, Medina L. The efferent connections of the nucleus accumbens in the lizard Gekko gecko. Anat Embryol. 1995;191:73–81. doi: 10.1007/BF00215299. [DOI] [PubMed] [Google Scholar]
- Smeets WJAJ, Steinbusch HWM. Distribution of noradrenaline immunoreactivity in the forebrain and midbrain of the lizard Gekko gecko. J Comp Neurol. 1989;285:453–466. doi: 10.1002/cne.902850404. [DOI] [PubMed] [Google Scholar]
- Smeets WJAJ, Hoogland PV, Voorn P. The distribution of dopamine immunoreactivity in the forebrain and midbrain of the lizard Gekko gecko: an immunohistochemical study with antibodies against dopamine. J Comp Neurol. 1986;253:46–60. doi: 10.1002/cne.902530105. [DOI] [PubMed] [Google Scholar]
- Smeets WJAJ, Lopez JM, González A. Immunohistochemical localization of DARPP-32 in the brain of the lizard, Gekko gecko: co-occurrence with tyrosine hydroxylase. J Comp Neurol. 2001;435:194–210. doi: 10.1002/cne.1202. [DOI] [PubMed] [Google Scholar]
- Stipanovich A, Valjent E, Matamales M, et al. A phosphatease cascade by which rewarding stimuli control nucleosomal response. Nature. 2008;453:879–885. doi: 10.1038/nature06994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tousignant A, Crews D. Incubation temperature and gonadal sex affect growth and physiology in the leopard gecko (Eublepharis macularius), a lizard with temperature-dependent sex determination. J Morphol. 1995;224:159–170. doi: 10.1002/jmor.1052240205. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJG. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Woolley SC, Sakata JT, Gupta A, Crews D. Evolutionary changes in dopaminergic modulation of courtship behavior in Cnemidophorus whiptail lizards. Horm Behav. 2001;40:483–489. doi: 10.1006/hbeh.2001.1713. [DOI] [PubMed] [Google Scholar]
- Woolley SC, Sakata JT, Crews D. Tyrosine hydroxylase expression is affected by sexual vigor and social environment in male Cnemidophorus inornatus. J Comp Neurol. 2004;476:429–439. doi: 10.1002/cne.20236. [DOI] [PubMed] [Google Scholar]
- Viglietti-Panzica C, Panzica GC, Fiori MG, et al. Sexually dimorphic nucleus in the quail preoptic area. Neurosci Lett. 1986;64:129–134. doi: 10.1016/0304-3940(86)90087-x. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Sgambato-Faure V, Sasaki T, et al. Phosphorylation of DARPP-32 at threonine-34 is required for cocaine action. Neuropsychopharmacology. 2005;31:555–562. doi: 10.1038/sj.npp.1300832. [DOI] [PubMed] [Google Scholar]