Abstract
Objectives
This study was performed to investigate the effects of different intracanal medicaments on chemical structure and microhardness of dentin.
Materials and Methods
Fifty human dentin discs were obtained from intact third molars and randomly assigned into two control groups and three treatment groups. The first control group received no treatment. The second control group (no medicament group) was irrigated with sodium hypochlorite (NaOCl), stored in humid environment for four weeks and then irrigated with ethylenediaminetetraacetic acid (EDTA). The three treatment groups were irrigated with NaOCl, treated for four weeks with either 1 g/mL triple antibiotic paste (TAP), 1 mg/mL methylcellulose-based triple antibiotic paste (DTAP), or calcium hydroxide [Ca(OH)2] and finally irrigated with EDTA. After treatment, one half of each dentin disc was subjected to Vickers microhardness (n = 10 per group) and the other half was used to evaluate the chemical structure (phosphate/amide I ratio) of treated dentin utilizing attenuated total reflection Fourier transform infrared spectroscopy (n = 5 per group). One-way ANOVA followed by Fisher's least significant difference were used for statistical analyses.
Results
Dentin discs treated with different intracanal medicaments and those treated with NaOCl + EDTA showed significant reduction in microhardness (p < 0.0001) and phosphate/amide I ratio (p < 0.05) compared to no treatment control dentin. Furthermore, dentin discs treated with TAP had significantly lower microhardness (p < 0.0001) and phosphate/amide I ratio (p < 0.0001) compared to all other groups.
Conclusions
The use of DTAP or Ca(OH)2 medicaments during endodontic regeneration may cause significantly less microhardness reduction and superficial demineralization of dentin compared to the use of TAP.
Keywords: Calcium hydroxide, EDTA, Endodontic regeneration, FTIR, Microhardness, Triple antibiotic paste
Introduction
The endodontic management of necrotic immature teeth is one of the most challenging treatments due to their thin fragile roots and blunderbuss apices. Calcium hydroxide [Ca(OH)2] apexification or artificial apical plug application using mineral trioxide aggregate (MTA) has been traditionally used to treat necrotic teeth with incomplete apices.1,2 However, these approaches require a long time to complete treatment and do not reinforce the thin and weak roots of immature teeth.3 Indeed, it has been suggested that the long term use of Ca(OH)2 during apexification procedures significantly reduces fracture resistance of the root.4,5 Endodontic regeneration (ER, pulp revascularization) has been used as a treatment option to manage necrotic immature teeth.6 In a recent study, ER was proposed to significantly increase the thickness and length of immature roots compared to teeth treated with Ca(OH)2 apexification or MTA.7 However, some cases treated with ER have been associated with suboptimal clinical outcomes where there is an apical closure with no increase in immature root thickness.8,9
The clinical ER procedure suggested by the American Association of Endodontists (AAE) recommends the irrigation of the necrotic immature root canal with 1.5% sodium hypochlorite (NaOCl), followed by application of intracanal medicament for 1 - 4 weeks during the first visit.10 In the next visit and upon the confirmation of the absence of clinical signs and symptoms, irrigation with 17% ethylenediaminetetraacetic acid (EDTA) is recommended to release dentin endogenous proteins followed by induction of bleeding to produce natural scaffold and deliver stem cells into the canal.11,12,13 The final treatment steps include application of MTA for the coronal root canal seal and completion of the final coronal restoration.
Root canal irrigants and intracanal medicaments used during ER procedure may negatively affect the physical and mechanical properties of radicular dentin. The use of NaOCl, EDTA, triple antibiotic paste (TAP), or Ca(OH)2 was found to significantly reduce dentin flexure strength, microhardness and root resistance to fracture.5,14,15,16,17,18 Furthermore, previously mentioned irrigation solutions and medicaments were also found to affect the chemical structure of dentin.19,20,21 Exploring the combined effect of these irrigants and medicaments on dentin could be more representative of the actual clinical scenario and essential to understand which one of these chemicals has the most detrimental effect. No previous studies have explored the effect of intracanal medicaments and irrigants within the context of ER on mechanical and chemical properties of dentin. The aim of this study was to investigate the effect of various intracanal medicaments used during ER on microhardness and chemical integrity of human dentin.
Materials and Methods
Sample preparation
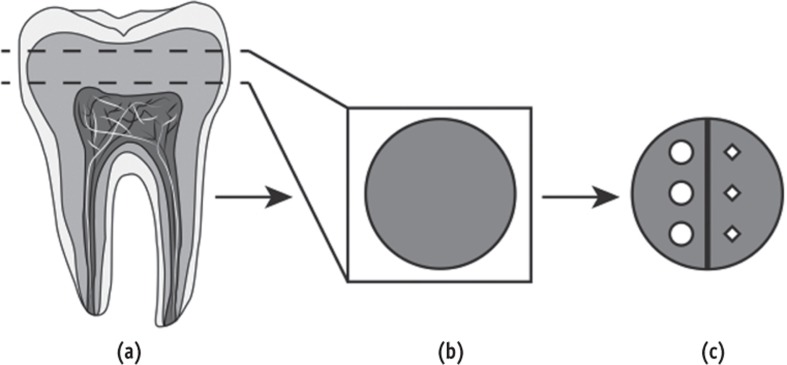
Fifty caries-free human third molars were selected for this study after obtaining Indiana University Institutional Review Board approval. The molars were stored at 4℃ in 0.1% thymol and used within six months after extraction. A 1.5 mm coronal dentin disc was cross-sectioned from each molar using a low-speed saw (IsoMet, Buehler, Lake Bluff, IL, USA) with a diamond disc (15LC Diamond Wafering Blade, Buehler) under deionized running water (Figure 1). The non-pulpal outer sides of the dentin discs were flattened to a uniform thickness using an automatic polishing machine (Struers Rotopol 31, Struers, Cleveland, OH, USA) with 500 grit silicon carbide grinding paper (Struers). The deep pulpal side of each dentin disc was polished with 1,200, 2,400 and 4,000 grit papers (Struers) and finally using a 1 µm diamond polishing suspension (Struers). As a final cleaning step, the polished specimens were sonicated in de-ionized water for 3 minutes.
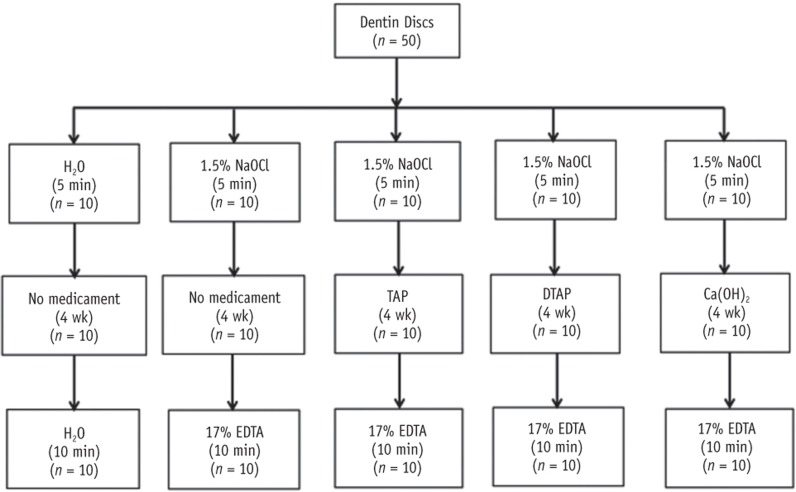
Figure 1. Schematic illustration of the experimental groups used in the study and the type of medicaments and/or irrigations used in each group.
NaOCl, sodium hypochlorite; TAP, triple antibiotic paste; DTAP, methylcellulose-based triple antibiotic paste; Ca(OH)2, calcium hydroxide; EDTA, ethylenediaminetetraacetic acid.
Preparation of medicaments used in the study
To prepare the clinically used concentration of TAP (1 g/mL), 1 g of United States Pharmacopeia grade antibiotic powders compounded of equal portions of metronidazole, ciprofloxacin and minocycline (Champs Pharmacy, San Antonio, TX, USA) was mixed with 1 mL of sterile water.22 To prepare a diluted pasty consistency of methylcellulose-based triple antibiotic paste (DTAP), 100 mg of United States Pharmacopeia grade antibiotic powders compounded of equal portions of metronidazole, ciprofloxacin and minocycline was dissolved in 100 mL of sterile water. Then, 8 g of methyl cellulose powder (Methocel 60 HG, Sigma-Aldrich, St. Louis, MO, USA) was added to the 100 mL of 1 mg/mL solution of TAP and mixed for 30 minutes using a magnetic stir bar to obtain a homogenous DTAP with 1 mg/mL concentration of TAP. A commercial Ca(OH)2 intracanal medicament (UltraCal XS, Ultradent, South Jordan, UT, USA) was also used in this study.
Treatment procedure
The dentin discs were randomized into three treatment groups and two control groups (Figure 1). For the first control group, the pulpal side of each dentin disc was irrigated with 20 mL of sterile water for 5 minutes using a 27 gauge needle and 5 mL syringes. Then, each sample was stored for four weeks at 37℃ in a sealed 2 mL conical sample cup (Fisher Scientific, Pittsburgh, PA, USA) containing a cotton pellet saturated with distilled water. After four weeks, the pulpal side of each dentin disc was irrigated with 20 mL of sterile water for 10 minutes. For the second control group (no medicament), the pulpal side of each dentin disc was slowly irrigated with 20 mL of 1.5% NaOCl for 5 minutes. Then, each sample was stored for four weeks at 37℃ in a sealed 2 mL conical sample cup containing a cotton pellet saturated with distilled water. After that, the pulpal side of each dentin disc was irrigated with 20 mL of 17% EDTA for 10 minutes. For each of the three treatment groups, the pulpal side of each dentin disc was slowly irrigated with 20 mL of 1.5% NaOCl for 5 minutes. Then, 0.1 mL of TAP, DTAP, or Ca(OH)2 paste was applied on the pulpal side of each specimen using a disposable syringe (BD, Franklin Lakes, NJ, USA). The treated dentin disks were kept for four weeks at 37℃ in a sealed 2 mL conical sample cup containing a cotton pellet saturated with distilled water. After four weeks, the treated side of each dentin specimen in all treatment groups was irrigated with 20 mL of 17% EDTA for 10 minutes. The amount of intracanal medicaments applied in the three treatment groups (0.1 mL) was just enough to cover the pulpal surface of dentin discs with the medicament to avoid application of excessive amount of paste. The four weeks of intracanal medication and the time and volume of both EDTA and NaOCl irrigation used in this study were based on the current ER protocol recommended by AAE.10
Microhardness measurement
The treatment side of each dentin disc was bisected by an imaginary line into two halves and labeled (Figure 2). One half of each treatment side was randomly selected and subjected to microhardness measurements using a Vickers microhardness tester (LM247, Leco, St. Joseph, MI, USA). Three indentations, spaced 200 µm apart, were made on each specimen using a 50 g load and a 10 second dwell time. The indentations were precisely measured using an optical microscope equipped with a digital camera and image analysis software. The representative hardness value for each specimen was obtained as the mean of the results from the three indentations.
Figure 2. Illustration of the method used to obtain dentin discs and the approach used to perform both microhardness and FTIR measurement on the same sample. (a) Dentin discs were cross-sectioned from caries free third molars; (b) The deep pulpal side of each dentin disc was subsequently ground and polished; (c) Half side of each treated dentin surface was used for microhardness measurement and the other half was used for FTIR measurement. FTIR, Fourier transform infrared spectrophotometer.
Fourier transform infrared spectroscopy measurement
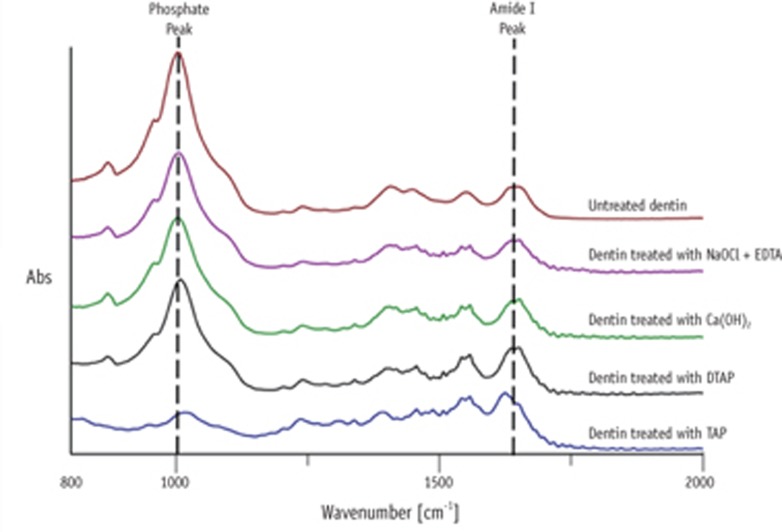
The chemical integrity of treated dentin was evaluated using Fourier transform infrared (FTIR) spectrophotometer (Jasco Inc., Tokyo, Japan) with a diamond attenuated total reflectance accessory. Five dentin disc samples were randomly selected from each group for FTIR measurement. Three different areas were selected from the other half, not subjected to microhardness testing (Figure 2). FTIR spectra were then collected from the three selected areas in each sample between 800 and 2,000 cm-1 at 4 cm-1 resolution using 100 scans. Each obtained spectrum was processed by smoothing, baseline correction and normalization to the amide I peak using Spectra manager software (Jasco Inc.). The ratio of integrated areas of the phosphate v1 and v3 peaks to the amide I peak was quantified to calculate the relative contents of these inorganic and organic components. The representative final ratio allocated for each dentin sample was obtained as the mean of the ratios obtained from the three single scans. Larger phosphate/amide I ratios compared to untreated dentin samples corresponded to a higher collagen deproteinization, whereas smaller phosphate/amide I ratios compared to untreated dentin samples corresponded to a higher dentin demineralization.
Scanning electron microscopy
Two dentin samples were randomly selected from each group for scanning electron microscopic (SEM) analysis to detect any morphological changes in the treated dentin side after experimental treatments. Each selected specimen was irrigated with 10 mL of de-ionized water, sonicated for 5 min in de-ionized water, and air dried for 48 hours using low vacuum pressure desiccator (Bel-Art, Wayne, NJ, USA) and silica gel crystals. Then, samples were sputter coated for 70 seconds with gold/palladium using a sputter coater (POLARON Sputter Coating System, Energy Beam Sciences, Agawam, MA, USA) and images were taken from the treated side of the dentin samples with an SEM (JEOL 7800F, JEOL, Peabody, MA, USA) in secondary electron imaging mode.
Statistical analyses
All data were checked for normality using the Kolmogorov-Smirnov test and the normality assumptions were satisfied. The differences in microhardness and phosphate/amide I ratios among experimental groups were examined using one-way ANOVA followed by Fisher's least significant difference. Pearson correlation coefficients overall and by group were calculated to determine the correlations between phosphate/amide I ratios and microhardness measurements. A 5% level of statistical significance was applied for all analyses.
Results
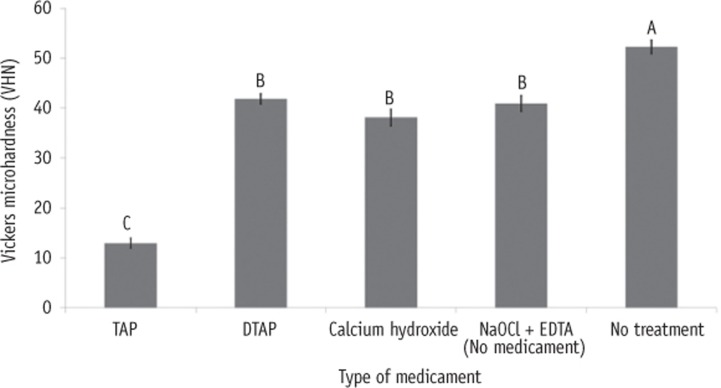
Figure 3 shows a significant reduction in mean microhardness values in NaOCl + EDTA treated dentin as well as dentin treated with TAP, DTAP and Ca(OH)2 compared to untreated dentin (p < 0.0001). Furthermore, dentin treated with TAP had lowest microhardness values compared to the other groups (p < 0.0001). However, no significant difference in mean microhardness values was found between NaOCl + EDTA, DTAP and Ca(OH)2 treated dentin.
Figure 3. Bar graphs showing the Vickers microhardness of dentin treated with various intracanal medicaments and that of the control group. Different upper-case letters indicate significant differences (p < 0.05).
TAP, triple antibiotic paste; DTAP, methylcellulose-based triple antibiotic paste; NaOCl, sodium hypochlorite; EDTA, ethylenediaminetetraacetic acid.
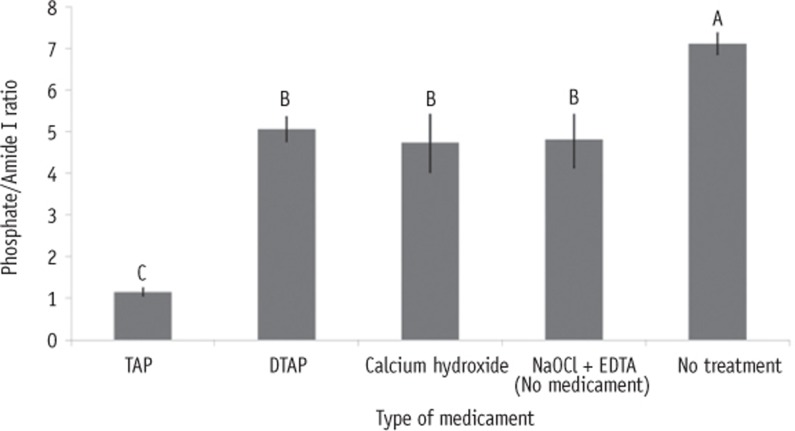
Figure 4 illustrates that untreated dentin had significantly higher mean phosphate/amide I ratio compared to dentin treated with NaOCl + EDTA (p = 0.003), TAP (p < 0.0001), DTAP (p = 0.006) and Ca(OH)2 (p = 0.002). Additionally, dentin treated with NaOCl + EDTA, DTAP, and Ca(OH)2 had significantly higher mean phosphate/amide I ratios compared to dentin treated with TAP (p < 0.0001). However, no significant differences in mean phosphate/amide I ratios were found between dentin treated with NaOCl + EDTA, DTAP and Ca(OH)2 (Figure 5).
Figure 4. Bar graphs showing the phosphate/Amide I ratios of dentin treated with various intracanal medicaments and that of the control group. Different upper-case letter indicates significant differences (p < 0.05).TAP, triple antibiotic paste; DTAP, methylcellulose-based triple antibiotic paste; NaOCl, sodium hypochlorite; EDTA, ethylenediaminetetraacetic acid.
Figure 5. Representative attenuated total reflectance (ATR) spectra of each group. NaOCl, sodium hypochlorite; EDTA, ethylenediaminetetraacetic acid; Ca(OH)2, calcium hydroxide; DTAP, methylcellulose-based triple antibiotic paste; TAP, triple antibiotic paste.
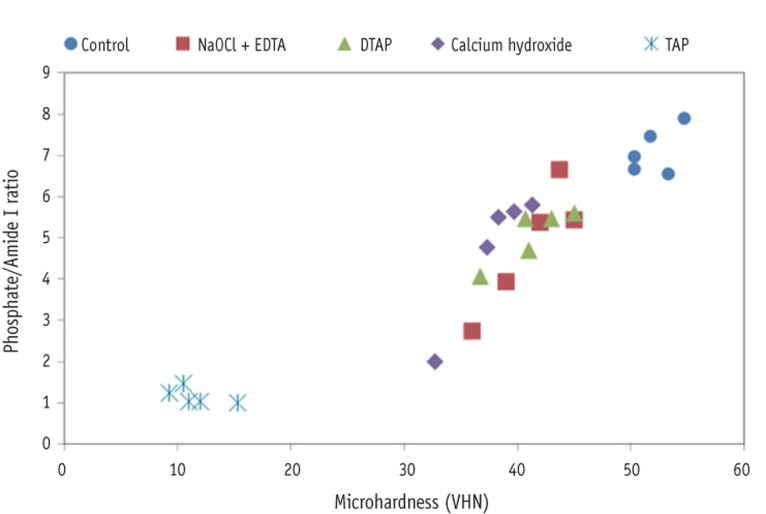
The overall correlation analysis showed that phosphate/amide I ratios and microhardness values of dentin were highly correlated (r = 0.94, p < 0.0001, Figure 6). When analyzed by group, even with the small sample sizes, a high correlation was found between phosphate/amide I ratios and microhardness values in dentin treated with NaOCl + EDTA (r = 0.91, p = 0.031), DTAP (r = 0.87, p = 0.053), and Ca(OH)2 (r = 0.96, p = 0.010). However, the correlation was only moderate in untreated dentin group (r = 0.53, p = 0.36) and negative in dentin treated with TAP (r = -0.59, p = 0.29).
Figure 6. Scatter correlation plots of phosphate/amide I ratios and microhardness measurements of various treatment groups and control group. The overall correlation analysis was high (r = 0.94, p < 0.0001). NaOCl, sodium hypochlorite; EDTA, ethylenediaminetetraacetic acid; DTAP, methylcellulose-based triple antibiotic paste; TAP, triple antibiotic paste.
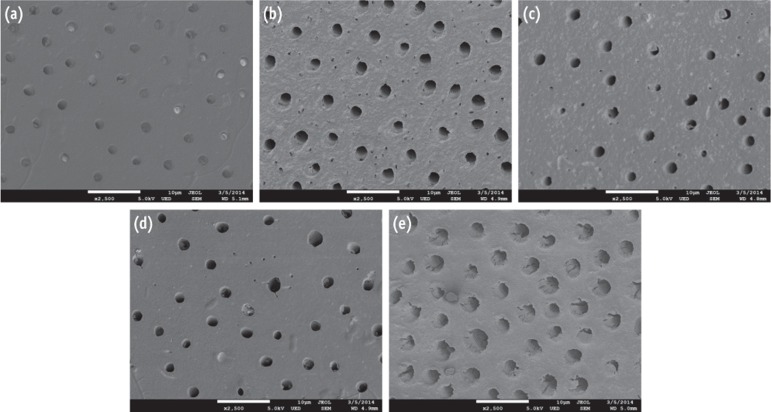
Representative dentin surfaces of each group are presented in Figure 7. All groups showed the absence of smear layer and intracanal medicament residues, which indicates the effectiveness of final EDTA irrigation step on removing residual paste. Dentin erosion was observed among NaOCl + EDTA treated dentin (Figure 7b) and TAP treated dentin (Figure 7e).
Figure 7. Representative scanning electron microscope (SEM) images from dentin treated with various intracanal medicaments and from an untreated control dentin. (a) Untreated control dentin; (b) Dentin treated with NaOCl + EDTA; (c) Dentin treated with Ca(OH)2; (d) Dentin treated with DTAP; (e) Dentin treated with TAP.
Discussion
ER has gained popularity in the last 15 years.6 However, it is still questionable to get optimal treatment outcomes such as continued root development with normal pulp-dentin complex.23 Therefore, it is essential to preserve the mechanical, physical and chemical properties of root dentin from any negative effects of chemical agents used in ER.
In this study, dentin treated with NaOCl + EDTA, DTAP, and Ca(OH)2 caused significant reduction in microhardness ranging from 20% to 27% compared to untreated control dentin. However, dentin treated with TAP caused significant reduction in microhardness compared to all other groups. Furthermore, dentin treated with TAP caused 75% reduction in microhardness compared to untreated control dentin. This could be explained by the strong demineralization effect of TAP reported in a previous study as well as the negative effect of both NaOCl and EDTA irrigants on microhardness of dentin.21,24 Hardness testing is not usually used to predict root fracture. However, the main advantage of performing a hardness test on dentin is that it is a well standardized indentation test that might be related to other mechanical properties such as tensile strength, compressive strength and modulus of elasticity.25 A recent study found that a significant decrease in microhardness of roots treated with TAP for one month was followed by significant reduction in root fracture resistance after three months treatment with TAP.18
Our study also showed no significant differences in microhardness between dentin treated with NaOCl + EDTA, DTAP, and Ca(OH)2. This indicates that both DTAP and Ca(OH)2 medicaments can be used in ER without causing any additional reduction in microhardness compared to the use of the essential irrigation solutions alone. However, additional mechanical tests should be performed in future studies to confirm the findings of this study. It is also noteworthy to mention that both Ca(OH)2 and lower concentrations of TAP (0.1 - 1 mg/mL) has been recommended in the recent ER literatures to avoid the toxic effect of the currently used 1,000 mg/mL TAP on stem cells of the apical papillae.22,26 Additionally, these low concentrations of TAP were also found to be effective against endodontic pathogens.27 However, the recommended low concentrations of TAP are usually in a liquid form, which makes its use challenging. Therefore, DTAP used in the current study was mixed with a methyl cellulose vehicle to create a clinically applicable intracanal medicament.
The chemical integrity of dentin after various ER protocols was also investigated in this study. A significant reduction in phosphate/amide I ratio was found in dentin treated with NaOCl + EDTA, TAP, DTAP, and Ca(OH)2 compared to untreated control dentin, which indicates a net superficial demineralization effect of medicaments and/or irrigation solutions tested in this study. Furthermore, dentin treated with TAP caused significant reduction in phosphate/amide I ratio compared to all other groups. This could be explained by the strong acidic nature of TAP (pH = 2.9) as well as the chelating effect of both EDTA and minocycline present in TAP.14,21,28 One of the limitations of studying the chemical structure of dentin with FTIR approach is that the depth of penetration of infrared radiation in the FTIR technique is limited to a few microns. Therefore, the spectral data and the phosphate/amide I ratios reported in our study may only represent the net chemical change of superficial dentin after various treatment protocols rather than the whole chemical change across the total thickness of the dentin specimens. The overall high correlation between phosphate/amide I ratios and microhardness values of dentin implies that the reported reduction in dentin microhardness among all treatment groups could be explained by the superficial demineralization effect following the ER protocols (reduction in phosphate/amide I ratios). These findings generally agree with previous studies, which also suggested that dentin microhardness depends on mineral concentration.25,29
In the current study, TAP treated dentin showed advanced superficial erosion in SEM analyses, which could be explained by the strong acidic nature of TAP. This might be the consequences of demineralization process accelerated by acidic TAP and calcium-chelating EDTA, resulting in the significant reduction in microhardness of TAP treated dentin.21 The final irrigation step with EDTA is expected to wash out the residual medicaments in case of Ca(OH)2 and DTAP treated dentin instead of causing excessive dentin erosion. Therefore, no prominent erosion was observed in our study among dentine treated with Ca(OH)2 or DTAP. On the other hand, the observed dentin erosion in NaOCl + EDTA treated dentin could be explained by the direct contact of EDTA with dentin surface in the absence of any intracanal medicament. It is worth noting that the 10 minutes irrigation with 20 mL of EDTA used in this study has been recommended by AAE during ER procedures.10 This relatively long EDTA irrigation time was proposed to wash out the remaining intracanal medicaments, open dentinal tubules and expose various growth factors during the ER procedure.23 However, prolonged EDTA irrigation for 10 minutes was suggested to cause excessive dentin erosion and shorter EDTA irrigation time is usually recommended during routine endodontic therapy.30,31
In this study, deep coronal dentin were used instead of radicular dentin to guarantee enough dentin surface area to perform both microhardness and FTIR measurement on the same samples and obtain a valid correlation analyses between the two outcomes. A recent report suggested no significant differences in tubular density and tubular cross section area between deep coronal and radicular dentin, regardless of the acidic challenge used.32
Conclusions
Within the limitations of this in vitro study, the use of TAP in ER caused significantly higher dentin demineralization and reduction in dentin microhardness compared to DTAP or Ca(OH)2. The use of DTAP or Ca(OH)2 rather than TAP as intracanal medicaments during ER may minimize the reduction in dentin microhardness and the change in chemical structure of superficial dentin.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Yassen GH, Chin J, Mohammedsharif AG, Alsoufy SS, Othman SS, Eckert G. The effect of frequency of calcium hydroxide dressing change and various pre- and inter-operative factors on the endodontic treatment of traumatized immature permanent incisors. Dent Traumatol. 2012;28:296–301. doi: 10.1111/j.1600-9657.2011.01089.x. [DOI] [PubMed] [Google Scholar]
- 2.El-Meligy OA, Avery DR. Comparison of apexification with mineral trioxide aggregate and calcium hydroxide. Pediatr Dent. 2006;28:248–253. [PubMed] [Google Scholar]
- 3.Trope M. Treatment of the immature tooth with a non-vital pulp and apical periodontitis. Dent Clin North Am. 2010;54:313–324. doi: 10.1016/j.cden.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Andreasen JO, Munksgaard EC, Bakland LK. Comparison of fracture resistance in root canals of immature sheep teeth after filling with calcium hydroxide or MTA. Dent Traumatol. 2006;22:154–156. doi: 10.1111/j.1600-9657.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 5.Yassen GH, Platt JA. The effect of nonsetting calcium hydroxide on root fracture and mechanical properties of radicular dentine: a systematic review. Int Endod J. 2013;46:112–118. doi: 10.1111/j.1365-2591.2012.02121.x. [DOI] [PubMed] [Google Scholar]
- 6.Diogenes A, Henry MA, Teixeira FB, Hargreaves KM. An update on clinical regenerative endodontics. Endod Top. 2013;28:2–23. [Google Scholar]
- 7.Jeeruphan T, Jantarat J, Yanpiset K, Suwannapan L, Khewsawai P, Hargreaves KM. Mahidol study 1: comparison of radiographic and survival outcomes of immature teeth treated with either regenerative endodontic or apexification methods: a retrospective study. J Endod. 2012;38:1330–1336. doi: 10.1016/j.joen.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 8.Nosrat A, Homayounfar N, Oloomi K. Drawbacks and unfavorable outcomes of regenerative endodontic treatments of necrotic immature teeth: a literature review and report of a case. J Endod. 2012;38:1428–1434. doi: 10.1016/j.joen.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 9.Nosrat A, Li KL, Vir K, Hicks ML, Fouad AF. Is pulp regeneration necessary for root maturation? J Endod. 2013;39:1291–1295. doi: 10.1016/j.joen.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 10.American Association of Endodontists: Considerations for Regenerative Procedures. [updated 2014 Aug 12]. Available from: http://www.aae.org/clinical-resources/regenerative-endodontics/considerations-for-regenerative-procedures.aspx.
- 11.Galler KM, D'Souza RN, Federlin M, Cavender AC, Hartgerink JD, Hecker S, Schmalz G. Dentin conditioning codetermines cell fate in regenerative endodontics. J Endod. 2011;37:1536–1541. doi: 10.1016/j.joen.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 12.Schmalz G, Smith AJ. Pulp development, repair, and regeneration: challenges of the transition from traditional dentistry to biologically based therapies. J Endod. 2014;40(Supplement 4):S2–S5. doi: 10.1016/j.joen.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 13.Lovelace TW, Henry MA, Hargreaves KM, Diogenes A. Evaluation of the delivery of mesenchymal stem cells into the root canal space of necrotic immature teeth after clinical regenerative endodontic procedure. J Endod. 2011;37:133–138. doi: 10.1016/j.joen.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Zhang K, Kim YK, Cadenaro M, Bryan TE, Sidow SJ, Loushine RJ, Ling JQ, Pashley DH, Tay FR. Effects of different exposure times and concentrations of sodium hypochlorite/ethylenediaminetetraacetic acid on the structural integrity of mineralized dentin. J Endod. 2010;36:105–109. doi: 10.1016/j.joen.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Grigoratos D, Knowles J, Ng YL, Gulabivala K. Effect of exposing dentine to sodium hypochlorite and calcium hydroxide on its flexural strength and elastic modulus. Int Endod J. 2001;34:113–119. doi: 10.1046/j.1365-2591.2001.00356.x. [DOI] [PubMed] [Google Scholar]
- 16.Eldeniz AU, Erdemir A, Belli S. Effect of EDTA and citric acid solutions on the microhardness and the roughness of human root canal dentin. J Endod. 2005;31:107–110. doi: 10.1097/01.don.0000136212.53475.ad. [DOI] [PubMed] [Google Scholar]
- 17.De-Deus G, Paciornik S, Mauricio MH. Evaluation of the effect of EDTA, EDTAC and citric acid on the microhardness of root dentine. Int Endod J. 2006;39:401–407. doi: 10.1111/j.1365-2591.2006.01094.x. [DOI] [PubMed] [Google Scholar]
- 18.Yassen GH, Vail MM, Chu TG, Platt JA. The effect of medicaments used in endodontic regeneration on root fracture and microhardness of radicular dentine. Int Endod J. 2013;46:688–695. doi: 10.1111/iej.12046. [DOI] [PubMed] [Google Scholar]
- 19.Hu X, Peng Y, Sum CP, Ling J. Effects of concentrations and exposure times of sodium hypochlorite on dentin deproteination: attenuated total reflection Fourier transform infrared spectroscopy study. J Endod. 2010;36:2008–2011. doi: 10.1016/j.joen.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 20.Zhang K, Tay FR, Kim YK, Mitchell JK, Kim JR, Carrilho M, Pashley DH, Ling JQ. The effect of initial irrigation with two different sodium hypochlorite concentrations on the erosion of instrumented radicular dentin. Dent Mater. 2010;26:514–523. doi: 10.1016/j.dental.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Yassen GH, Chu TM, Eckert G, Platt JA. Effect of medicaments used in endodontic regeneration technique on the chemical structure of human immature radicular dentin: an in vitro study. J Endod. 2013;39:269–273. doi: 10.1016/j.joen.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Ruparel NB, Teixeira FB, Ferraz CC, Diogenes A. Direct effect of intracanal medicaments on survival of stem cells of the apical papilla. J Endod. 2012;38:1372–1375. doi: 10.1016/j.joen.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 23.Geisler TM. Clinical considerations for regenerative endodontic procedures. Dent Clin North Am. 2012;56:603–626. doi: 10.1016/j.cden.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Ari H, Erdemir A, Belli S. Evaluation of the effect of endodontic irrigation solutions on the microhardness and the roughness of root canal dentin. J Endod. 2004;30:792–795. doi: 10.1097/01.don.0000128747.89857.59. [DOI] [PubMed] [Google Scholar]
- 25.Kinney JH, Marshall SJ, Marshall GW. The mechanical properties of human dentin: a critical review and reevaluation of the dental literature. Crit Rev Oral Biol Med. 2003;14:13–29. doi: 10.1177/154411130301400103. [DOI] [PubMed] [Google Scholar]
- 26.Althumairy RI, Teixeira FB, Diogenes A. Effect of dentin conditioning with intracanal medicaments on survival of stem cells of apical papilla. J Endod. 2014;40:521–525. doi: 10.1016/j.joen.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Sabrah AH, Yassen GH, Gregory RL. Effectiveness of antibiotic medicaments against biofilm formation of Enterococcus faecalis and Porphyromonas gingivalis. J Endod. 2013;39:1385–1389. doi: 10.1016/j.joen.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Minabe M, Takeuchi K, Kumada H, Umemoto T. The effect of root conditioning with minocycline HCl in removing endotoxin from the roots of periodontallyinvolved teeth. J Periodontol. 1994;65:387–392. doi: 10.1902/jop.1994.65.5.387. [DOI] [PubMed] [Google Scholar]
- 29.Featherstone JD, ten Cate JM, Shariati M, Arends J. Comparison of artificial caries-like lesions by quantitative microradiography and microhardness profiles. Caries Res. 1983;17:385–391. doi: 10.1159/000260692. [DOI] [PubMed] [Google Scholar]
- 30.Ozdemir HO, Buzoglu HD, Calt S, Cehreli ZC, Varol E, Temel A. Chemical and ultramorphologic effects of ethylenediaminetetraacetic acid and sodium hypochlorite in young and old root canal dentin. J Endod. 2012;38:204–208. doi: 10.1016/j.joen.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 31.Zehnder M. Root canal irrigants. J Endod. 2006;32:389–398. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 32.Caiado AC, de Goes MF, de Souza-Filho FJ, Rueggeberg FA. The effect of acid etchant type and dentin location on tubular density and dimension. J Prosthet Dent. 2010;103:352–361. doi: 10.1016/S0022-3913(10)60076-5. [DOI] [PubMed] [Google Scholar]