Abstract
Objectives
This study evaluated the maximum depth and percentage of irrigant penetration into dentinal tubules by passive ultrasonic irrigation (PUI).
Materials and Methods
Thirty extracted human teeth were instrumented and divided into three groups. According to final irrigation regimen, 5.25% sodium hypochlorite (Group A, NaOCl), 2% chlorhexidine (Group B, CHX) and saline solution (Group C, control group) were applied with Irrisafe 20 tips (Acteon) and PUI. Irrigant was mixed with 0.1% rhodamine B. Sections at 2 mm, 5 mm, and 8 mm from the apex were examined with confocal laser scanning microscopy (CLSM). The percentage and maximum depth of irrigant penetration were measured. Kruskal-Wallis test and Mann-Whitney test were performed for overall comparison between groups at each level and for pairwise comparison, respectively. Within a group, Wilcoxon test was performed among different levels. p values less than 0.05 were considered significant.
Results
In all groups, highest penetration depth and percentage of penetration were observed at the 8 mm level. At 2 mm level, Groups A and B had significantly greater depths and percentages in penetration than Group C (p < 0.05), but there were no significant differences between Groups A and B. At 5 mm level, penetration depths and percentage of penetration was not significantly different among the groups.
Conclusions
NaOCl and CHX applied by PUI showed similar depth and percentage of penetration at all evaluated levels.
Keywords: Chlorhexidine, Confocal laser scanning microscope, Dentinal penetration, Passive ultrasonic irrigation, Sodium hypochlorite
Introduction
Disinfection of the root canal system is a specific requirement for endodontic treatment success.1 Irrigant penetration in the canal system depends on the root canal anatomy, irrigant application techniques, solution volume, root canal instrumentation and irrigant's physic-chemical characteristics.2 Sodium hypochlorite (NaOCl) and chlorhexidine (CHX) are the most commonly used irrigants, and they are sometimes combined with ethylenediaminetetraacetic acid (EDTA) or other chelating agents.3 Different studies showed that the use of NaOCl between 2.5% and 5%, combined with 10 - 17% EDTA solutions, is particularly effective in the elimination of organic and inorganic debris.4
Passive ultrasonic irrigation (PUI) has showed to be more effective than conventional irrigation in cleaning and disinfecting root canals.5,6,7 PUI-activated irrigation produces acoustic microwaves, cavitation and heat generation, that helps the irrigant to access to the difficult-to-reach places, favouring the elimination of dentinal debris, opening tubules and maximizing the irrigant antibacterial effect, because it can spread better along the root canal system.8,9,10,11 There are some variants in this technique. Ultrasonic intermittent activation, used with three 20 seconds sequences, removed more dentinal debris than conventional syringe irrigation.12 Currently, an ultrasound oscillation frequency of 30 KHz, with displacement amplitude of 20 - 30 µm, is recommended.12 It seems that a volume increase does not significantly improve washing action and effectiveness in debris removal.13
PUI is recommended after biomechanical preparation, with smooth and fine wires (15 or 20 ISO sizes), so that they can oscillate easily without contact with walls in the widened canals. The use of NaOCI as the final irrigant agent, combined with ultrasound or a wave vibration system shows the greatest antibacterial effect.9 Previous studies analysed CHX as final PUI-applied irrigant related to its antimicrobial action and reported a prolonged antimicrobial action of CHX when applied with ultrasonic activation. In our knowledge there are no studies comparing the penetration of NaOCl and CHX into the dentinal tubules.14,15,16
Both, NaOCL and CHX, have properties that can be complementary in achieving endodontic therapy objectives. Dentinal penetration of both irrigants is important in order to improve their clinical effect. The aim of this study was to compare the maximum penetration depth into dentinal tubules and the percentage of penetration of 5.25% NaOCl and 2% CHX activated with PUI as final irrigants.
Materials and Methods
The Ethical Committee of the Universitat de València (Valencia, Spain) approved the study. Thirty recently extracted human mandibular premolar teeth, Wein type I (one root, one canal), radiographically verified, with straight mature roots, and without caries lesions or resorption, were used. Teeth were placed in 2.5% NaOCl solution for 5 minutes, stored in saline solution. The root was separated from the crown to obtain a root length of 15 mm, with bucco-lingual oriented cuts. A single operator instrumented all the canals. The working length was established by inserting a size 10 K-file (VDW, Munich, Germany) until it was just visible at the apical foramen and, then, 1 mm was subtracted. The canals were enlarged with the Mtwo rotary system (VDW) basic sequence (10/0.04, 15/0.05, 20/0.06, 25/0.06), followed by 30/0.04 and 40/0.04 rotary files. Irrigation with 2.5 mL of 5.25% NaOCI was performed between each file, using a 27G Miraject needle (Hager Werken, Duisburg-Grobenbaum, Germany) inserted 1 mm shorter than the working length. The root canals were rinsed for 2 minutes with 3 mL 17% EDTA (Tubuliclean, OGNA LAB, Muggiò, Italy) followed by 3 minutes rinse with 3 mL of saline solution. Canals were dried with paper points. Apex was covered with composite resin. A 40/0.04 gutta-percha point was placed at the working length in order to avoid composite intrusion and removed after resin curing.
Teeth were randomly divided into three groups (n = 10) according to final irrigation. For Group A, 5.25% NaOCl (prepared in the laboratory of the university) mixed with 0.1% of Rhodamine B (RB, Panreact Química SA, Barcelona, Spain) with Irrisafe 20 tips (Acteon, Merignac, France) and PUI. Three mL of irrigant were applied by intermittent flux (3 cycles × 20 seconds) activated through a 3 W and 30 kHz piezoelectric ultrasound Suprasson P5 Booster unit (Satelec Acteon, Merignac, France). Ultrasonic tip was placed 1 mm coronal to the working length and was used with 2 - 3 mm apical-coronal movements. For Group B, 2% chlorhexidine digluconate (CHX, prepared in the laboratory of the university) mixed with 0.1% RB with Irrisafe 20 tips applied as described in group A. For Group C (control), irrigation with saline solution mixed with 0.1% RB was applied with Irrisafe 20 tips as described before.
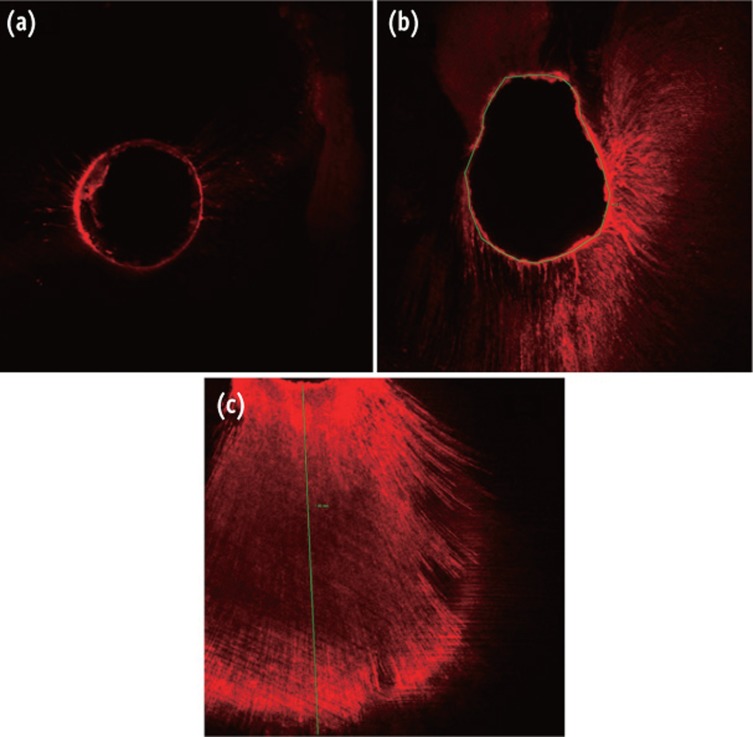
Roots were included in resin blocks and transversely sectioned to a thickness of 1 mm, using a slow-speed, water-cooled diamond saw (Isomet Low Speed Saw, Buehler, Lake Bluff, IL, USA) at 2 mm (apical), 5 mm (middle) and 8 mm (coronal) from the apex of the root. The samples were then bonded onto glass slides and ground with wet silicon carbide papers. The slides were examined with a confocal laser scanning microscope (CLSM, Leica TCS SP2, Leica microsystems, Barcelona, Spain) at ×40 under Ar/HeNe laser excitation with a wave length of 543 nm. The scanner makes 10 virtual sections of each sample at 4 µm, which were reconstructed providing a comprehensive image of the course of the dentinal tubules. Images were stored and measured by two blinded operators with the Leica Confocal Software (Leica TCS SP2, Leica microsystems). The point of deepest penetration was measured from the canal wall to the point of maximum dye penetration in µm. For each section, the circumference of the root canal was outlined and the portion of the root canal perimeter showing tubular penetration was outlined and measured. The percentage of the canal wall where the irrigant had penetrated was calculated. Figure 1a shows the image without dye penetration, Figure 1b shows how the perimeter was marked, and dentinal penetration measurement can be seen in Figure 1c.
Figure 1. Representative confocal laser scanning microscopic images. Only stained irrigant can be seen with this technique. (a) The image shows fluorescence around the canal walls without penetration into dentinal tubules; (b) Deep penetration is shown in part of the canal wall. The green line was used to mark and to measure the percentage of canal wall showing dentinal tubule penetration; (c) An intense irrigant-labelled penetration is shown. The maximum penetration into dentinal tubules was measured in each case (green line).
Data were analysed with SPSS 19.0 (Statistical Package for the Social Sciences, IBM, Armonk, NW, USA). Kruskal-Wallis test and Mann-Whitney test were performed for overall comparison between groups at each level and for pairwise comparison, respectively. Within a group, Wilcoxon test was performed among different levels. P values less than 0.05 were considered significant. P values less than 0.05 were considered significant. Examiners' reliability was assessed by intra-class correlation coefficient (ICC), in order to get concordance between two quantitative measurements.
Results
Inter-examiner agreement (ICC) was R = 0.88. Both examiners repeated 10% of the measurements in order to evaluate the internal consistence of their measurements, with an ICC of R = 0.90 and R = 0.89, respectively.
According to the maximum penetration depth into the dentinal tubules, groups were ranked in the order of NaOCl, CHX, and control groups. Mann-Whitney tests revealed that dye penetration was significantly higher in Groups A and B compared to the control group at 2 mm level (p < 0.05, Table 1), but there were no significant differences between Groups A and B. No significant differences were found among groups at the 5 mm level. At 8 mm level, Group A had a significantly greater penetration depth than Group C (p < 0.05), but it was not different from Group B. Wilcoxon signed rank sum test showed a significantly lower penetration at 2 mm level than at 8 mm level in all groups (p < 0.05, Table 1).
Table 1. Dye penetration depth (µm) into dentinal tubules according to the irrigant solutions and levels of section.
| Group | Depth of penetration | |||
|---|---|---|---|---|
| 2 mm | 5 mm | 8 mm | Total | |
| 5.25% NaOCl (A) | 496.11 ± 309.06Ba | 883.82 ± 501.99Aa | 1070.69 ± 329.75Bb | 816.87 ± 472.35 |
| 2% CHX (B) | 465.71 ± 163.33Ba | 594.76 ± 182.01Aa | 880.72 ± 266.34ABb | 647.06 ± 267.74 |
| Saline (C) | 162.65 ± 124.73Aa | 498.12 ± 252.79Ab | 723.81 ± 309.29Ab | 461.53 ± 231.59 |
Columns express differences between groups. Values with the same superscript uppercase letters within each column are not statiscally different.
Rows express differences between levels within each experimental group. Values with the same superscript lowercase letters within each row are not statiscally different.
NaOCl, Sodium hypochlorite; CHX, Chlorhexidine.
According to the Mann-Whitney test, the percentage of canal wall perimeter showing dye penetration were higher in Groups A and B than in Group C at 2 mm and 8 mm level (p < 0.05, Table 2). At 5 mm level, the percentages of dye penetration were the same in all groups. Wilcoxon signed rank test revealed that the maximum percentage of penetration was found at the 8 mm level with statistically significant differences with the 2 mm level in all groups (p < 0.05, Table 2).
Table 2. Percentage of dye penetration along the canal wall perimeter.
| Group | Penetrated perimeter | |||
|---|---|---|---|---|
| 2 mm | 5 mm | 8 mm | Total | |
| 5.25% NaOCl (A) | 49.23 ± 24.11Ba | 60.76 ± 32.39Aab | 80.08 ± 11.62Bb | 63.35 ± 29.55 |
| 2% CHX (B) | 54.03 ± 29.32Ba | 48.89 ± 21.97Aa | 74.24 ± 20.08Bb | 59.06 ± 25.80 |
| Saline (C) | 15.23 ± 10.79Aa | 39.76 ± 26.93Aab | 49.72 ± 16.17Ab | 34.91 ± 29.25 |
Columns express differences between groups. Values with the same superscript uppercase letters within each column are not statiscally different.
Rows express differences between levels within each experimental group. Values with the same superscript lowercase letters within each row are not statiscally different.
NaOCl, Sodium hypochlorite; CHX, Chlorhexidine.
Discussion
CLSM has been used in edodontics to assess the adaptation of filling materials and endodontic cement penetration into the dentinal tubules, as well as for visualizing intratubular bacteria.17,18,19,20,21 In this study it was used to show the irrigant penetration into dentinal tubules. Due to the tortuous path of the dentinal tubules, they are difficult to observe in full through a single cut.22 With CLSM the sample can be displayed not only in surface but also in depth, making multiple cuts and obtaining a three-dimensional image.22,23 CLSM provides a more accurate and informative structural correlation than the two dimensional analysis.24
PUI improves the efficacy of irrigation solutions in removing organic and inorganic debris from root canal walls.25,26 The term 'passive' was first introduced related to the 'noncutting' action of the ultrasonically activated file. The technique relies on the transmission of acoustic energy from an oscillating file or smooth wire to an irrigant in the canal space. The energy is transmitted by means of ultrasonic waves and can induce acoustic streaming of the irrigant.27 A possible explanation for the improved irrigant penetration into non-sclerotic tubules of the canal wall could be a better current flow and increased irrigant volume, associated with ultrasonic agitation.12
Higher penetration values into dentinal tubules can be associated with an increase in debris elimination and also to a better penetration of the endodontic cement. The mechanical interlocking of the sealer plug in the dentinal tubules has been suggested to improve retention of the root canal filling material.28,29 Sealer penetration is also considered beneficial for preventing reinfection of the root canal system, because the sealer would serve as a reasonable blocking agent that may prevent bacterial repopulation or an inactivator in the tubules if some level of leakage in the main body of the filled root canal occurs. In addition, if antibacterial endodontic sealer is used, the deeply penetrated cement can kill bacteria located inside the tubules.30,31
The present study showed that, in all groups, the maximum depth of the irrigant penetration and the percentage of canal wall penetration decreased from the coronal to the apical third of root canals (Tables 1 and 2), and these results are in agreement with previous studies.18,32,33 This may be due to a better removal of the smear layer in coronal than in apical third of root canal. As it has been already described in literature, there is not a completely effective procedure in dentinal debris removal and tubule opening, especially in the apical third.3,34 It must also be considered that there are more dentinal tubules with a larger diameter in the coronal area than in the apical one.34
Most of the researches in this field analyse cement penetration after different irrigation and activation techniques and obtain deeper penetration values than those from our study (Table 1).32,35,36 The association of irrigant and rhodamine B, instead of cement and fluorochrome, could explain these differences. Nevertheless, data from perimetric percentage of root canal walls with tubular penetration are similar to those from the previously mentioned authors, and there is no evidence that this association could alter the results.
The majority of the related studies evaluated NaOCl action as final irrigant.12,37,38 We have found two papers where CHX was evaluated as a final irrigant. Ferreira et al., found CHX to be less effective than NaOCl in removal debris, when applied ultrasonically in a continuous way.3 Nevertheless, de Vasconcelos et al. did not find differences in smear layer cleaning and tubule opening efficiency between NaOCl and CHX when used as a final irrigant after EDTA application.39 We did not directly observe the effect of irrigants on the removal of smear layer. CLSM analysis of penetration of the irrigant into the dentinal tubule was used because it can be a good indicator for evaluating the degree of smear layer removal in an in vitro tooth model. The results of the present study demonstrated that in all sections, final irrigation activated by PUI with NaOCl was as effective as CHX in terms of irrigant penetration (Table 1). No significant differences were found between the CHX and the NaOCl groups regarding penetration percentage.
As penetration percentage can be mainly associated with better root canal wall cleaning, it could be more clinically important to obtain root canal walls with higher percentage of penetrated dentinal tubules than getting deeper penetration into dentinal tubules, in order to obtain a better three dimensional seal of the root canal. These results seem interesting in order to use CHX as a final PUI irrigant, because of CHX substantivity and its capacity to inhibit bacterial adherence to dentin. Especially in necrotic pulps, according to Baca et al., CHX shows better anti-Enterococcus faecalis effect than NaOCl, when smear layer is eliminated after the root canal shaping. In addition, the erosive effect of NaOCl used after EDTA irrigation could be avoided.16,40
Conclusions
PUI showed similar penetration depth and percentage of penetration of NaOCl and CHX into dentinal tubules at all evaluated levels. The use of CHX with PUI application can be considered as an alternative for final irrigation in the clinical practice, as it can penetrate into the dentinal tubules as NaOCl does.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Bronnec F, Bouillaguet S, Machtou P. Ex vivo assessment of irrigant penetration and renewal during the final irrigation regimen. Int Endod J. 2010;43:663–672. doi: 10.1111/j.1365-2591.2010.01723.x. [DOI] [PubMed] [Google Scholar]
- 2.Vera J, Arias A, Romero M. Effect of maintaining apical patency on irrigant penetration into the apical third of root canals when using passive ultrasonic irrigation: an in vivo study. J Endod. 2011;37:1276–1278. doi: 10.1016/j.joen.2011.05.042. [DOI] [PubMed] [Google Scholar]
- 3.Ferreira RB, Marchesan MA, Silva-Sousa YT, Sousa-Neto M. Effectiveness of root canal debris removal Passive ultrasonic irrigation using passive ultrasound irrigation with chlorhexidine digluconate or sodium hypochlorite individually or in combination as irrigants. J Contemp Dent Pract. 2008;9:68–75. [PubMed] [Google Scholar]
- 4.Grande NM, Plotino G, Falanga A, Pomponi M, Somma F. Interaction between EDTA and sodium hypochlorite: a nuclear magnetic resonance analysis. J Endod. 2006;32:460–464. doi: 10.1016/j.joen.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Gu LS, Kim JR, Ling J, Choi KK, Pashley DH, Tay FR. Review of contemporary irrigant agitation techniques and devices. J Endod. 2009;35:791–804. doi: 10.1016/j.joen.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Paragliola R, Franco V, Fabiani C, Mazzoni A, Nato F, Tay FR, Breschi L, Grandini S. Final rinse optimization: influence of different agitation protocols. J Endod. 2010;36:282–285. doi: 10.1016/j.joen.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Castagna F, Rizzon P, da Rosa RA, Santini MF, Barreto MS, Duarte MA, Só MV. Effect of passive ultrassonic instrumentation as a final irrigation protocol on debris and smear layer removal-a SEM analysis. Microsc Res Tech. 2013;76:496–502. doi: 10.1002/jemt.22192. [DOI] [PubMed] [Google Scholar]
- 8.van der Sluis LW, Versluis M, Wu MK, Wesselink PR. Passive ultrasonic irrigation of the root canal: a review of the literature. Int Endod J. 2007;40:415–426. doi: 10.1111/j.1365-2591.2007.01243.x. [DOI] [PubMed] [Google Scholar]
- 9.Plotino G, Pameijer CH, Grande NM, Somma F. Ultrasonics in endodontics: a review of the literature. J Endod. 2007;33:81–95. doi: 10.1016/j.joen.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 10.van der Sluis LW, Vogels MP, Verhaagen B, Macedo R, Wesselink PR. Study on the influence of refreshment/activation cycles and irrigants on mechanical cleaning efficiency during ultrasonic activation of the irrigant. J Endod. 2010;36:737–740. doi: 10.1016/j.joen.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Zeltner M, Peters OA, Paqué F. Temperature changes during ultrasonic irrigation with different inserts and modes of activation. J Endod. 2009;35:573–577. doi: 10.1016/j.joen.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 12.de Gregorio C, Estevez R, Cisneros R, Paranjpe A, Cohenca N. Efficacy of different irrigation and activation systems on the penetration of sodium hypochlorite into simulated lateral canals and up to working length: an in vitro study. J Endod. 2010;36:1216–1221. doi: 10.1016/j.joen.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 13.van der Sluis LW, Gambarini G, Wu MK, Wesselink PR. The influence of volume, type of irrigant and flushing method on removing artificially placed dentine debris from the apical root canal during passive ultrasonic irrigation. Int Endod J. 2006;39:472–476. doi: 10.1111/j.1365-2591.2006.01108.x. [DOI] [PubMed] [Google Scholar]
- 14.Leonardo MR, Tanomaru Filho M, Silva LA, Nelson Filho P, Bonifácio KC, Ito IY. In vivo antimicrobial activity of 2% chlorhexidine used as root canal irrigating solution. J Endod. 1999;25:167–171. doi: 10.1016/s0099-2399(99)80135-6. [DOI] [PubMed] [Google Scholar]
- 15.Alves FR, Almeida BM, Neves MA, Moreno JO, Rôças IN, Siqueira JF., Jr Disinfecting oval-shaped root canals: effectiveness of different supplementary approaches. J Endod. 2011;37:496–501. doi: 10.1016/j.joen.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Baca P, Mendoza-Llamas ML, Arias-Moliz MT, González-Rodríguez MP, Ferrer-Luque CM. Residual effectiveness of final irrigation regimens on Enteroccus faecalis-infected root canals. J Endod. 2011;37:1121–1123. doi: 10.1016/j.joen.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Lin LM, Rosenberg PA, Lin J. Do procedural errors cause endodontic treatment failure? J Am Dent Assoc. 2005;136:187–193. doi: 10.14219/jada.archive.2005.0140. [DOI] [PubMed] [Google Scholar]
- 18.Kara Tuncer A, Tuncer S. Effect of different final irrigation solutions on dentinal tubule penetration depth and percentage of root canal sealer. J Endod. 2012;38:860–863. doi: 10.1016/j.joen.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Nagayoshi M, Kitamura C, Fukuizumi T, Nishihara T, Terashita M. Antimicrobial effect of ozonated water on bacteria invading dentinal tubules. J Endod. 2004;30:778–781. doi: 10.1097/00004770-200411000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Zapata RO, Bramante CM, de Moraes IG, Bernardineli N, Gasparoto TH, Graeff MS, Campanelli AP, Garcia RB. Confocal laser scanning microscopy is appropriate to detect viability of Enterococcus faecalis in infected dentin. J Endod. 2008;34:1198–1201. doi: 10.1016/j.joen.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Parmar D, Hauman CH, Leichter JW, McNaughton A, Tompkins GR. Bacterial localization and viability assessment in human ex vivo dentinal tubules by fluorescence confocal laser scanning microscopy. Int Endod J. 2011;44:644–651. doi: 10.1111/j.1365-2591.2011.01867.x. [DOI] [PubMed] [Google Scholar]
- 22.Love RM, Jenkinson HF. Invasion of dentinal tubules by oral bacteria. Crit Rev Oral Biol Med. 2002;13:171–183. doi: 10.1177/154411130201300207. [DOI] [PubMed] [Google Scholar]
- 23.DAlpino PH, Pereira JC, Svizero NR, Rueggeberg FA, Pashley DH. Use of fluorescent compounds in assessing bonded resin-based restorations: a literature review. J Dent. 2006;34:623–634. doi: 10.1016/j.jdent.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Diaspro A, Robello M. Two-photon excitation of fluorescence for three-dimensional optical imaging of biological structures. J Photochem Photobiol B. 2000;55:1–8. doi: 10.1016/s1011-1344(00)00028-2. [DOI] [PubMed] [Google Scholar]
- 25.Townsend C, Maki J. An in vitro comparison of new irrigation and agitation techniques to ultrasonic agitation in removing bacteria from a simulated root canal. J Endod. 2009;35:1040–1043. doi: 10.1016/j.joen.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Vinhorte MC, Suzuki EH, de Carvalho MS, Marques AA, Sponchiado Júnior EC, Garcia LFR. Effect of passive ultrasonic agitation during final irrigation on cleaning capacity of hybrid instrumentation. Restor Dent Endod. 2014;39:104–108. doi: 10.5395/rde.2014.39.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmad M, Pitt Ford TR, Crum LA, Walton AJ. Ultrasonic debridement of root canals: acoustic cavitation and its relevance. J Endod. 1988;14:486–493. doi: 10.1016/S0099-2399(88)80105-5. [DOI] [PubMed] [Google Scholar]
- 28.Shokouhinejad N, Sabeti M, Gorjestani H, Saghiri MA, Lotfi M, Hoseini A. Penetration of Epiphany, Epiphany self-etch, and AH Plus into dentinal tubules: a scanning electron microscopy study. J Endod. 2011;37:1316–1319. doi: 10.1016/j.joen.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 29.White RR, Goldman M, Lin PS. The influence of the smeared layer upon dentinal tubule penetration by plastic filling materials. J Endod. 1984;10:558–562. doi: 10.1016/S0099-2399(84)80100-4. [DOI] [PubMed] [Google Scholar]
- 30.Heling I, Chandler NP. The antimicrobial effect within dentinal tubules of four root canal sealers. J Endod. 1996;22:257–259. doi: 10.1016/s0099-2399(06)80144-5. [DOI] [PubMed] [Google Scholar]
- 31.Balguerie E, van der Sluis L, Vallaeys K, Gurgel-Georgelin M, Diemer F. Sealer penetration and adaptation in the dentinal tubules: a scanning electron microscopic study. J Endod. 2011;37:1576–1579. doi: 10.1016/j.joen.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Moon YM, Shon WJ, Baek SH, Bae KS, Kum KY, Lee W. Effect of final irrigation regimen on sealer penetration in curved root canals. J Endod. 2010;36:732–736. doi: 10.1016/j.joen.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Yoo YJ, Lee W, Kim HC, Shon WJ, Baek SH. Multivariate analysis of the cleaning efficacy of different final irrigation techniques in the canal and isthmus of mandibular posterior teeth. Restor Dent Endod. 2013;38:154–159. doi: 10.5395/rde.2013.38.3.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Jadaa A, Paqué F, Attin T, Zehnder M. Necrotic pulp dissolution by passive ultrasonic irrigation in simulated accessory canals: impact of canal location and angulation. Int Endod J. 2009;42:59–65. doi: 10.1111/j.1365-2591.2008.01497.x. [DOI] [PubMed] [Google Scholar]
- 35.Bolles JA, He J, Svoboda KK, Schneiderman E, Glickman GN. Comparison of Vibringe, EndoActivator, and needle irrigation on sealer penetration in extracted human teeth. J Endod. 2013;39:708–711. doi: 10.1016/j.joen.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Moon YM, Kim HC, Bae KS, Baek SH, Shon WJ, Lee W. Effect of laser-activated irrigation of 1320-nanometer Nd:YAG laser on sealer penetration in curved root canals. J Endod. 2012;38:531–535. doi: 10.1016/j.joen.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 37.De Moor RJ, Meire M, Goharkhay K, Moritz A, Vanobbergen J. Efficacy of ultrasonic versus laser-activated irrigation to remove artificially placed dentin debris plugs. J Endod. 2010;36:1580–1583. doi: 10.1016/j.joen.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Desai P, Himel V. Comparative safety of various intracanal irrigation systems. J Endod. 2009;35:545–549. doi: 10.1016/j.joen.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 39.de Vasconcelos BC, Luna-Cruz SM, de-Deus G, de Moraes IG, Maniglia-Ferreira C, Gurgel-Filho ED. Cleaning ability of chlorhexidine gel and sodium hypochlorite associated or not with EDTA as root canal irrigants: a scanning electron microscopy study. J Appl Oral Sci. 2007;15:387–391. doi: 10.1590/S1678-77572007000500003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niu W, Yoshioka T, Kobayashi, Suda H. A scanning electron microscopic study of dentinal erosion by final irrigation with EDTA and NaOCl solutions. Int Endod J. 2002;35:934–939. doi: 10.1046/j.1365-2591.2002.00594.x. [DOI] [PubMed] [Google Scholar]