Background: Alg44 regulates the production of alginate in Pseudomonas aeruginosa via c-di-GMP binding.
Results: The structure of the PilZ domain of Alg44 in complex with c-di-GMP reveals residues that control c-di-GMP/Alg44 stoichiometry.
Conclusion: Binding of dimeric c-di-GMP is required for alginate biosynthesis.
Significance: This is the first example of a receptor requiring a specific form of c-di-GMP for activation.
Keywords: biofilm, crystal structure, cyclic di-GMP (c-di-GMP), isothermal titration calorimetry (ITC), Pseudomonas aeruginosa (P. aeruginosa), PilZ, alginate, exopolysaccharide
Abstract
Pseudomonas aeruginosa is an opportunistic human pathogen that secretes the exopolysaccharide alginate during infection of the respiratory tract of individuals afflicted with cystic fibrosis and chronic obstructive pulmonary disease. Among the proteins required for alginate production, Alg44 has been identified as an inner membrane protein whose bis-(3′,5′)-cyclic dimeric guanosine monophosphate (c-di-GMP) binding activity post-translationally regulates alginate secretion. In this study, we report the 1.8 Å crystal structure of the cytoplasmic region of Alg44 in complex with dimeric self-intercalated c-di-GMP and characterize its dinucleotide-binding site using mutational analysis. The structure shows that the c-di-GMP binding region of Alg44 adopts a PilZ domain fold with a dimerization mode not previously observed for this family of proteins. Calorimetric binding analysis of residues in the c-di-GMP binding site demonstrate that mutation of Arg-17 and Arg-95 alters the binding stoichiometry between c-di-GMP and Alg44 from 2:1 to 1:1. Introduction of these mutant alleles on the P. aeruginosa chromosome show that the residues required for binding of dimeric c-di-GMP in vitro are also required for efficient alginate production in vivo. These results suggest that the dimeric form of c-di-GMP represents the biologically active signaling molecule needed for the secretion of an important virulence factor produced by P. aeruginosa.
Introduction
Bis-(3′,5′)-cyclic dimeric guanosine monophosphate (c-di-GMP)6 is a second messenger that controls a wide range of biological processes in bacteria. High levels of c-di-GMP often correlate with the activation of cellular pathways that promote the transition from a planktonic, highly motile state to a multicellular, biofilm-embedded community (1, 2). The opportunistic pathogen, Pseudomonas aeruginosa, is the leading cause of morbidity and mortality among cystic fibrosis patients partly due to its ability to adopt a biofilm mode of growth (3). Biofilms, of which secreted polysaccharides comprise a major constituent, protect pathogenic bacteria from the immune response of their host as well as increase their tolerance to administered antibiotics (4). P. aeruginosa is capable of producing at least three exopolysaccharides that have been implicated in biofilm formation: alginate and the Pel and Psl polysaccharides (5). The majority of P. aeruginosa isolates are non-mucoid and utilize the Pel and/or Psl polysaccharides as the primary structural component of the biofilm matrix (6, 7). However, during chronic cystic fibrosis lung infections, P. aeruginosa converts to a mucoid biofilm phenotype characterized by the secretion of alginate (8). In mucoid biofilms, the Pel and/or Psl polysaccharides serve as the structural scaffold of the biofilm, whereas alginate is layered on top of this foundation to serve as a protective barrier (9). The production of alginate by P. aeruginosa is post-translationally regulated by intracellular c-di-GMP concentrations, demonstrating a direct role for this signaling dinucleotide in facilitating biofilm-related infection in the cystic fibrosis lung (10).
c-di-GMP synthesis is carried out by GGDEF domain-containing diguanylate cyclases, whereas c-di-GMP degradation is catalyzed by either EAL or HD-GYP domains found in c-di-GMP phosphodiesterases. Generally speaking, the activities of these enzymes are modulated to either increase or decrease the intracellular concentration of c-di-GMP through signaling cascades that are activated in response to extracellular stimuli. Although there has been a fairly extensive characterization of the biosynthesis and degradation of c-di-GMP, the scale and diversity of the downstream effector proteins responsible for the phenotypic response are continually expanding. Examples of c-di-GMP receptors identified to date include a wide range of protein domains, such as the AAA σ54 interaction domain of FleQ from P. aeruginosa (11), the non-canonical receiver (REC) domain of VpsT from Vibrio cholerae (12) and the degenerate (non-catalytic) EAL domains of P. aeruginosa FimX (13) and Pseudomonas fluorescens LapD (14), the degenerate GGDEF domain of P. aeruginosa PelD (15, 16), and the inner membrane PgaC/PgaD poly-β-1,6-N-acetylglucosamine synthase complex from Escherichia coli (17). In addition to the above-mentioned domains, the most widespread c-di-GMP receptor identified to date is the PilZ domain. Originally identified through a bioinformatics approach (18), PilZ domain-containing proteins from a variety of bacterial species have been shown to interact directly with c-di-GMP (19–21). For example, Vibrio cholerae VCA0042, Pseudomonas putida PP4397, and P. aeruginosa PA4608 are PilZ domain-containing proteins of unknown function capable of binding c-di-GMP (21–23). Mechanistic insight into how c-di-GMP-PilZ interactions post-translationally activate exopolysaccharide production was revealed by the structural characterization of the BcsA-BcsB cellulose synthase complex in its apo- and c-di-GMP bound forms (24, 25). c-di-GMP binding to the C-terminal PilZ domain of the cellulose synthase subunit BcsA results in a conformational change that causes the glycosyltransferase domain of this enzyme to transition from an autoinhibited to an active state, allowing for the polymerization and translocation of cellulose polymers.
An outstanding question regarding many c-di-GMP receptors is the biological significance of the differing c-di-GMP/protein stoichiometries that have been observed. Of the aforementioned c-di-GMP-binding proteins, some have been shown to bind a single molecule of the dinucleotide, whereas others are able to bind a dimeric self-intercalated form of the molecule (23, 26). For example, VCA0042 binds monomeric c-di-GMP, whereas PP4397 binds dimeric c-di-GMP although both proteins share the same overall fold (23, 26). The molecular basis for the stoichiometry between c-di-GMP and its receptor has been identified in some PilZ domains; however, the phenotypic consequences of these differences have not yet been examined.
In this study, we examined the effect of modulating c-di-GMP-receptor stoichiometry on secretion of the exopolysaccharide alginate, an important virulence factor produced by P. aeruginosa. Among the proteins required for alginate biosynthesis and secretion, Alg44 has been identified as a PilZ-containing inner membrane protein that post-translationally regulates alginate production (10). We have determined the x-ray crystal structure of the PilZ domain of Alg44 (Alg44PilZ) in complex with c-di-GMP and subsequently, when probing the ligand binding site in vitro, identified site-specific mutants that alter the binding stoichiometry between c-di-GMP and Alg44PilZ from 2:1 to 1:1. The effect of this altered c-di-GMP binding stoichiometry on the ability of Alg44 to activate alginate biosynthesis was subsequently examined in P. aeruginosa.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
The nucleotide sequence for the alg44 gene from P. aeruginosa PAO1 was obtained from the Pseudomonas Genome Database (27) and used to design gene-specific primers. For crystallization, the region of alg44 encoding amino acid residues 14–122 was generated by PCR amplification from genomic DNA and TA-cloned into the ChampionTM pET SUMO (Invitrogen) expression vector as per the manufacturer's instructions. The L69M (for phasing) and R95A mutations were generated using the QuikChange® Lightning site-directed mutagenesis kit (Agilent Technologies). The resulting expression plasmids encode the following fusion proteins: an N-terminal His6 tag, the Saccharomyces cerevisiae Smt3 protein (SUMO), a S. cerevisiae Ulp1 protease (SUMO protease) cleavage site, and Alg44(14–122) L69M or Alg44(14–122) L69M/R95A. For ITC experiments, the region of alg44 encoding amino acid residues 1–122 was PCR-amplified and cloned into the pET24a (Novagen) expression vector via its NdeI and XhoI restriction sites. This expression plasmid encodes Alg44(1–122) followed by a non-cleavable C-terminal His6 tag. The Q16L, R17A, R21A, D44A, S46A, R87A, and R95A mutants were generated using the QuikChange® Lightning site-directed mutagenesis kit (Agilent Technologies).
For structure determination, the Alg44(14–122) L69M construct was transformed into E. coli B834 Met− cells (Novagen) in minimal medium supplemented with l-selenomethionine and expressed as per the protocol of Lee et al. (28). All other constructs were transformed into E. coli BL21 CodonPlus (DE3) cells (Stratagene) and grown in lysogeny broth (LB) containing 50 μg ml−1 kanamycin at 37 °C. Upon reaching an A600 of 0.6, protein expression was induced by the addition of 1.0 mm isopropyl β-d-1-thiogalactopyranoside (IPTG) at a temperature of 25 °C for 16 h. The cell cultures were subsequently harvested by centrifugation at 2392 × g for 20 min, flash-frozen, and stored at −20 °C until purification.
For purification, frozen cell pellets of all constructs were thawed and resuspended in 40 ml of buffer A (50 mm Tris-HCl, pH 8.0, 300 mm NaCl, 1 mm tris(2-carboxyethyl)phosphine, 1 SIGMAFAST EDTA-free protease inhibitor mixture tablet (Sigma)) per liter of cell culture. The resulting cell suspensions were lysed by homogenization using an Emulsiflex-C3 (Avestin Inc.) at a pressure of 70–100 megapascals (three passes total). The insoluble cell lysates were then removed by centrifugation at 25,000 × g for 45 min, and the soluble supernatants were loaded onto 5-ml Ni2+-nitrilotriacetic acid columns pre-equilibrated with buffer A containing 5 mm imidazole. The majority of the contaminating E. coli proteins were removed from each column by a wash step using 10 column volumes of buffer A containing 20 mm imidazole followed by elution of the purified Alg44 constructs using three column volumes of buffer A containing 250 mm imidazole.
The selenomethionyl-incorporated Alg44(14–122) L69M and native Alg44(14–122) L69M/R95A proteins were then dialyzed against buffer B (20 mm Tris-HCl, pH 8.0, 150 mm NaCl, 1 mm tris(2-carboxyethyl)phosphine) for 16 h, followed by digestion with the SUMO protease (1000:1 (w/w)) for 1 h at room temperature. The resulting digestions were then passed over Ni2+-nitrilotriacetic acid columns pre-equilibrated in buffer A, and the flow-through containing the untagged protein was collected. Alg44(14–122) L69M and Alg44(14–122) L69M/R95A were then further purified over a HiLoad 16/60 Superdex 75 gel filtration column (GE Healthcare) pre-equilibrated in buffer B, and the eluates were stored at 4 °C until required. Alg44(1–122) and its Q16L, R17A, R21A, D44A, S46A, R87A, and R95A mutants were purified over a HiLoad 16/60 Superdex 75 gel filtration column (GE Healthcare) pre-equilibrated in buffer C (20 mm Tris-HCl, pH 8.0, 150 mm NaCl, and 5% (v/v) glycerol), and the eluates were stored at 4 °C until required.
Preparation of c-di-GMP
The enzymatic production of c-di-GMP was carried out as described by Whitney et al. (16). Briefly, 3.4 μm P. aeruginosa WspRR242A was incubated at 37 °C in 50 mm Tris-HCl, pH 7.5, 50 mm NaCl, 5 mm MgCl2, and 1 mm GTP for 16 h. The WspRR242A protein was then precipitated from the mixture by heating at 88 °C for 5 min and removed by syringe filtration (0.20 μm; Sarstedt). Triethylammonium bicarbonate was then added to the mixture to a final concentration of 5 mm prior to loading onto a 3-ml Resource RPC column (GE Healthcare). c-di-GMP was then eluted from the column using a linear gradient of 0–50% (v/v) ethanol. c-di-GMP eluted as a single peak, and its identity was confirmed by MALDI-TOF mass spectrometry (SPARC BioCentre, Hospital for Sick Children). Purified c-di-GMP was then lyophilized and stored at −20 °C until required. For subsequent experiments, the solution concentration of c-di-GMP was quantified using an extinction coefficient (ϵ260) of 26,000 m−1 cm−1.
Crystallization and Structure Determination
Selenomethionyl-incorporated Alg44(14–122) L69M and native Alg44(14–122) L69M/R95A were concentrated to 10 mg ml−1 by spin ultrafiltration (10 kDa molecular mass cut-off, Millipore) and screened against commercially available sparse matrix crystal screens (MCSG1–4, Microlytic) in the presence of 2.5 mm c-di-GMP (without any preincubation of the proteins and ligand). Crystal trials were set up in 48-well VDX plates using the hanging drop vapor diffusion technique. Protein and crystallization solutions were mixed in a 1:1 ratio with a final drop size of 3 μl suspended over 250 μl of crystallization solution and stored at 20 °C.
Crystals of selenomethionyl-incorporated Alg44(14–122) L69M appeared in condition 16 of the MCSG3 screen (0.1 m citric acid-NaOH, pH 3.5, 25% (w/v) PEG 3350) after 4 days. These crystals were optimized through systematic variation of the precipitant concentration and buffer pH, resulting in diffraction quality crystals that were grown in 0.1 m citric acid-NaOH, pH 2.9, 24% (w/v) PEG 3350. Crystals were cryoprotected in the crystallization solution supplemented with 20% (v/v) ethylene glycol prior to flash freezing in liquid nitrogen. X-ray diffraction data were collected on beamline X29A at the National Synchrotron Light Source at Brookhaven National Laboratory. Low resolution (180 images of 2° Δϕ oscillation) and high resolution (360 images of 1.0° Δϕ oscillation) data sets were collected on an ADSC Q315r CCD detector with a 260-mm crystal-to-detector distance and an exposure time of 0.4 s/image. The data were merged, integrated, and scaled using the HKL2000 software program. A total of three (of three) selenium sites were located using HKL2MAP, and density-modified phases were calculated using SOLVE/RESOLVE. The resulting density-modified Se-SAD map was of excellent quality and allowed for automated model building with Phenix AutoBuild (29). Subsequent model adjustments were made manually in COOT (30) between iterative rounds of refinement, which was carried out with PHENIX.REFINE (31). The progress of the refinement was monitored as a function of the reduction and convergence of Rwork and Rfree (Table 1).
TABLE 1.
X-ray data collection and refinement statistics
| Alg44(14–122) L69M + c-di-GMP | Alg44(14–122) L69M/R95A + c-di-GMP | |
|---|---|---|
| Data collection | ||
| Wavelength (Å) | 0.979 | 1.075 |
| Space group | P3121 | P6322 |
| Cell dimensions | ||
| a, b, c (Å) | 57.2, 57.2, 178.4 | 106.1, 106.1, 122.3 |
| α, β, γ (degrees) | 90.0, 90.0, 120.0 | 90.0, 90.0, 120.0 |
| Resolution (Å) | 50.0-1.80 (1.86-1.80)a | 50.00-1.70 (1.76-1.70) |
| Total no. of reflections | 758,769 | 1,745,650 |
| Total no. of unique reflections | 32,536 | 45,320 |
| Rmerge (%)b | 6.3 (68.5) | 14.7 (61.2) |
| I/σI | 56.7 (5.4) | 36.7 (7.3) |
| Completeness (%) | 100.0 (100.0) | 100.0 (100.0) |
| Redundancy | 23.3 (21.4) | 38.5 (34.9) |
| Refinement | ||
| Rwork/Rfree (%)c | 18.7/21.8 | 16.5/20.1 |
| No. of atoms | ||
| Protein | 2431 | 2604 |
| c-di-GMP | 276 | 138 |
| Water | 169 | 377 |
| Average B-factors (Å2) | ||
| Protein | 38.2 | 23.8 |
| c-di-GMP | 41.8 | 22.4 |
| Water | 42.4 | 34.6 |
| r.m.s. deviations | ||
| Bond lengths (Å) | 0.009 | 0.010 |
| Bond angles (degrees) | 1.375 | 1.374 |
| Ramachandran plot (%)d | ||
| Total favored | 96.5 | 97.7 |
| Total allowed | 99.4 | 100.0 |
| Coordinate error (Å)e | 0.20 | 0.15 |
a Values in parentheses correspond to the highest resolution shell.
b Rmerge = Σ Σ|I(k) − 〈I〉 /ΣI(k), where I(k) and 〈I〉 represent the diffraction intensity values of the individual measurements and the corresponding mean values. The summation is over all unique measurements.
c Rwork = Σ ‖Fobs| − k|Fc‖/|Fo|, where Fo and Fc are the observed and calculated structure factors, respectively. Rfree is the sum extended over a subset of reflections (5%) excluded from all stages of the refinement.
d As calculated using MOLPROBITY (51).
e Maximum likelihood-based coordinate error, as determined by PHENIX (31).
Crystals of Alg44(14–122) L69M/R95A appeared in condition 72 of the MCSG3 screen (0.1 m HEPES-NaOH, pH 7.5, 30% (v/v) PEG 400, 0.2 m NaCl) after 7 days. These crystals were directly flash-frozen from the hit condition, and x-ray diffraction data were collected on beamline X29A at the National Synchrotron Light Source. Data were processed using HKL2000, and the structure was determined using the PHENIX AutoMR wizard with the wild-type protein as a search model and refined as described above.
Limited Proteolysis
Alg44(1–122) at a concentration of 1 mg ml−1 was incubated with porcine trypsin (Sigma) at a ratio of 1000:1 (w/w) in the absence and presence of 1 mm c-di-GMP. Time points were taken at 0, 15, 30, 60, 120, 180, and 240 min by taking 5-μl aliquots of the reaction mixture directly added into Laemmli buffer and heating it to 95 °C for 5 min. Samples were run on SDS-PAGE using 16% polyacrylamide Tris-glycine gels and stained with Coomassie Brilliant Blue G-250. Identification of the trypsin cleavage site was done by Edman sequencing (SPARC BioCentre, The Hospital for Sick Children).
Isothermal Titration Calorimetry
Alg44(1–122), Q16L, R17A, R21A, D44A, S46A, R87A, and R95A variants and c-di-GMP were prepared in buffer C, and each solution was degassed before experimentation. ITC measurements were performed with a VP-ITC microcalorimeter (MicroCal Inc., Northampton, MA). Titrations were carried out with 500 μm c-di-GMP in the syringe and the following concentrations of the indicated Alg44(1–122) samples in the cuvette: 15 μm wild type, 40 μm Q16L, 30 μm R17A, 15 μm R21A, 15 μm D44A, 15 μm S46A, 15 μm R87A, and 40 μm R95A. Each titration experiment consisted of 25 10-μl injections with 240-s intervals between each injection. The heats of dilution for titrating c-di-GMP into buffer were subtracted from the sample data prior to analysis. The ITC data were analyzed using the Origin version 5.0 software (MicroCal Inc.) and fit using the appropriate binding model.
Strain Construction
Chromosomal point mutations were constructed using an unmarked, non-polar allelic replacement strategy (32). A fragment containing the last 150 base pairs at the 3′ end of alg8 and the first 450 base pairs from the 5′ end of alg44 was amplified from P. aeruginosa PAO1 genomic DNA, allowing for ∼200 base pairs on either side of the codons corresponding to Gln-16 and Arg-95 of Alg44. This fragment was inserted into the suicide vector pENTRPEX18Gm using Gateway destination cloning (Invitrogen), and the plasmid pENTRPEX18Gm::alg44 was verified by sequence analysis (Center for Applied Genomics, Hospital for Sick Children). The Q16L, R17A, R21A, D44A, S46A, R87A, and R95A point mutants were generated from pENTRPEX18Gm::alg44 using the QuikChange® Lightning site-directed mutagenesis kit (Agilent Technologies), and each plasmid was verified by sequence analysis. Allelic exchange plasmids were conjugated into P. aeruginosa PAO1 by biparental mating with E. coli SM10 (33). Single recombinant mutants were selected on LB agar containing 30 μg ml−1 gentamicin and 25 μg ml−1 Irgasan. Double recombinant mutants were selected on LB agar without NaCl containing 15% (w/v) sucrose and were confirmed by sequence analysis.
Stimulation of Alginate Production
The full-length gene corresponding to the alternate sigma factor algU (algT) was amplified from P. aeruginosa PAO1 genomic DNA, using primers that introduced a P. aeruginosa-compatible ribosome binding site (RBS), and ligated into the IPTG-inducible P. aeruginosa expression vector pPSV39 (34). The plasmid pPSV39::RBS-algU was verified by sequence analysis and was transformed into P. aeruginosa strains by electroporation. Alginate production was induced by plating transformants on LB agar containing 30 μg ml−1 gentamicin and 1 mm IPTG.
Alginate Purification
P. aeruginosa strains, containing pPSV39::RBS-algU, were streaked onto LB agar containing 30 μg ml−1 gentamicin and 1 mm IPTG and allowed to grow overnight. The next day, cells were scraped from the plate and resuspended in 20 ml of 0.9% NaCl, followed by centrifugation at 2000 × g for 30 min to separate cells from the dissolved alginate. The cell pellet was washed once with 5 ml of 0.9% NaCl, and the alginate-containing supernatants were combined. Cell pellets were dried and weighed for analysis. Alginate was precipitated from the supernatant by the addition of an equal volume of ice-cold isopropyl alcohol, followed by centrifugation (2000 × g for 30 min) to pellet the alginate. Alginate pellets were resuspended in 25 ml of 0.9% NaCl and precipitated again with an equal volume of ice-cold isopropyl alcohol. Alginate pellets were dried and resuspended in 1 ml of double-distilled H2O for subsequent analysis.
Quantification of Alginate
The concentration of purified alginate was determined by the carbazole method of Knutson and Jeanes (35). Briefly, 30 μl of purified alginate was mixed with 1 ml of ice-cold borate-sulfuric acid reagent (100 mm H3BO3 in concentrated H2SO4), followed by the addition of 30 μl of carbazole reagent (0.1% (w/v) in anhydrous ethanol). The solution was heated to 55 °C for 30 min and subsequently cooled on ice. Alginate concentration was measured spectrophotometrically at 530 nm, using alginic acid from brown seaweed as a standard.
Antibody Production and Purification
Alg44(1–122) was purified as described above. Purified Alg44(1–122) was used to generate antiserum from rabbits using a 70-day standard protocol (Cedarlane Laboratories). The α-Alg44 antiserum was further purified using a method adapted from Salamitou et al. (36). Briefly, 300 μg of purified Alg44(1–122) was loaded onto a 14% Tris-HCl polyacrylamide gel and transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was stained with Ponceau S, and the band corresponding to Alg44(1–122) was cut out and blocked using phosphate-buffered saline (PBS), pH 7, with 0.1% Tween 20 and 5% skim milk powder for 1 h. The membrane was then incubated with α-Alg44 antiserum overnight at 4 °C, followed by 2 h at room temperature. After washing with PBS, the α-Alg44 antibodies were eluted from the membrane by the addition of 700 μl of 0.2 m glycine, pH 2.2, for 15 min, followed by 300 μl of 1 m K2HPO4 to neutralize the solution. Antibodies were dialyzed into PBS for 24 h, mixed 1:1 with 100% glycerol, and used at a dilution of 1:3000.
Western Immunoblots
Cells were grown in LB to mid-log phase and harvested by centrifugation. After removal of the spent media, the cell pellet was resuspended in 50 μl of 2× Laemmli buffer and boiled for 10 min. 2.5 μl of sample was loaded onto a 14% polyacrylamide Tris-glycine gel and transferred to a PVDF membrane. The membrane was blocked in 5% skim milk in Tris-buffered saline with Tween 20 (TBST) for 30 min at room temperature. The membrane was subsequently probed with purified α-Alg44 antisera at a 1:3000 dilution or with commercial α-RNA polymerase antibody (Neoclone Biotechnologies) at 1:5000 in 1% skim milk in TBST for 1 h at room temperature. Blots were washed and probed with goat α-rabbit horseradish peroxidase (HRP)-conjugated secondary antibody (Bio-Rad) and developed using Pierce ECL Plus Western blotting substrate (Thermo Scientific).
RESULTS
Alg44PilZ Is Homodimeric and Binds a Dimer of c-di-GMP at Its N Terminus
Previously, PhoA fusion analysis showed that Alg44 contains an N-terminal PilZ domain, a central transmembrane domain, and a C-terminal periplasmic membrane fusion protein domain (37) (Fig. 1A). To examine the c-di-GMP binding properties of Alg44, studies of Alg44PilZ utilized constructs that omitted the transmembrane helix and the periplasmic domain. For structural analysis, the first 13 amino acids of the N terminus and the linker region connecting the PilZ domain to the inner membrane were omitted because bioinformatics analyses suggested that these protein segments contain a significant amount of disorder (data not shown). After generation of several constructs with varying N and C termini, the boundaries of Alg44 that were amenable to crystallization were identified as residues 14–122. Alg44(14–122) does not contain any methionine residues that can be utilized for phasing; therefore, a methionine was introduced at Leu-69 by site-directed mutagenesis. Multiple mutants were made and assessed, and the L69M mutant exhibited solubility properties that were most similar to the wild-type protein. In addition, a homology model of Alg44 predicted that this residue would be on the opposite face of the protein to the c-di-GMP binding site and therefore should not interfere with nucleotide binding. Selenomethionine-incorporated Alg44(14–122) L69M crystallized readily in the presence of c-di-GMP, and the 1.8 Å structure was determined by the single-wavelength anomalous dispersion (SAD) technique. Alg44(14–122) L69M in complex with c-di-GMP crystallized in the trigonal space group P3121 with three protomers in the asymmetric unit. The density-modified SAD maps were of excellent quality and allowed for automated model building for the majority of the protein except for the N-terminal c-di-GMP binding region of Alg44(14–122) L69M as well as c-di-GMP itself, which were built manually. The final model was refined to an Rwork and Rfree of 18.7 and 21.8%, respectively (Table 1).
FIGURE 1.
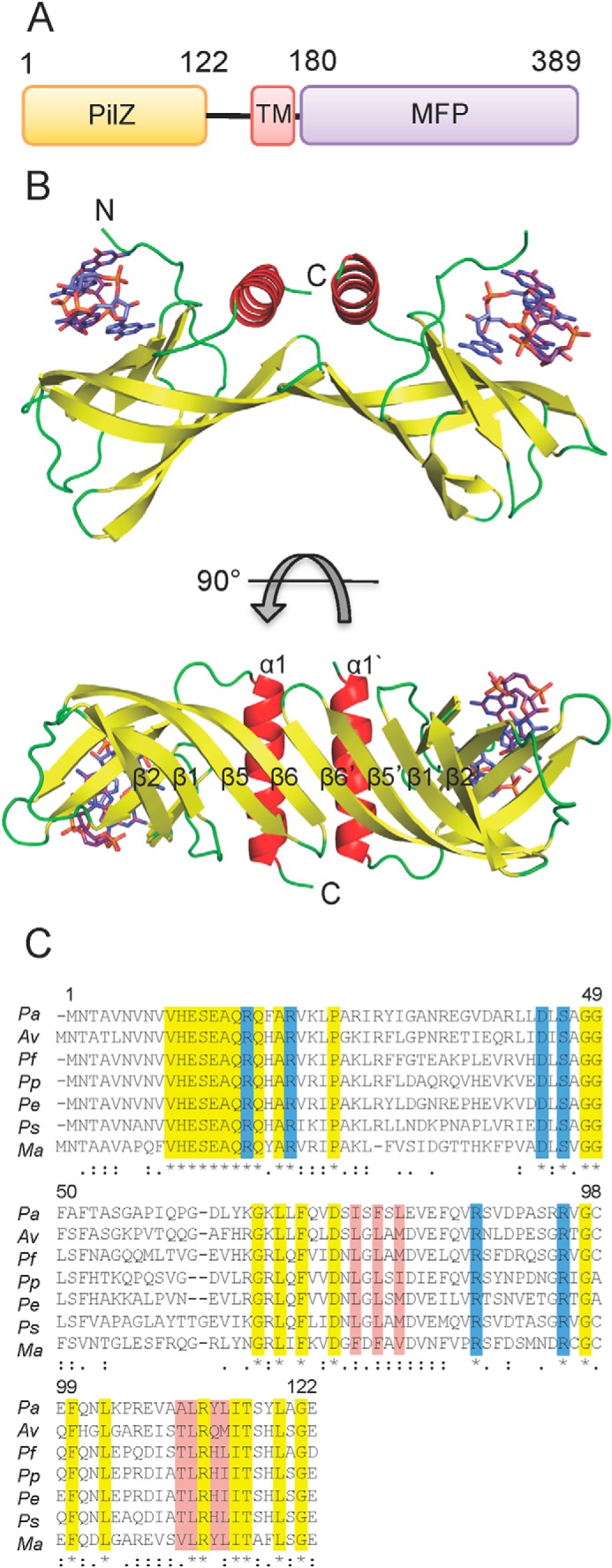
Overall structure and conserved residues of Alg44PilZ. A, domain organization of full-length Alg44. TM and MFP, predicted transmembrane domain and the membrane fusion protein domain, respectively. The approximate boundaries of each domain are indicated on the diagram. B, structure of L69M Alg44PilZ displayed as a schematic representation shown from two orthogonal views. The locations of the N and C termini for the monomer on the left are indicated with N and C, respectively. The self-intercalated c-di-GMP dimer is shown as a stick representation with carbon, nitrogen, oxygen, and phosphorous atoms colored purple, blue, red, and orange, respectively. C, multiple-sequence alignment of Alg44PilZ. Symbols below the alignment indicate invariant (*, highlighted yellow), highly conserved (:), and moderately conserved (.) amino acids. Residues that comprise the c-di-GMP binding site and the homodimerization interface are highlighted in blue and red, respectively. Pa, P. aeruginosa; Av, Azotobacter vinelandii; Pf, P. fluorescens; Pp, P. putida; Pe, Pseudomonas entomophila; Ps, Pseudomonas syringae; Ma, Marinobacter algicola. The amino acid numbering corresponds to the P. aeruginosa sequence.
Alg44(14–122) L69M adopts a split barrel-like fold composed of six anti-parallel β-strands followed by an α-helix that sits across one end of the barrel in an orientation that is roughly perpendicular to the barrel axis (Fig. 1B). The topology of Alg44(14–122) L69M is similar to that of other PilZ domain-containing proteins that have been solved in complex with c-di-GMP, which includes V. cholerae VC0042 (26), P. putida PP4397 (23), and P. aeruginosa PA4608 (21). A search of the Protein Data Bank using the DALI server (38) indicates that Alg44(14–122) L69M has the highest structural similarity to P. aeruginosa PA4608 (Protein Data Bank code 2L74, DALI Z-score = 9.3, r.m.s. deviation of 2.9 Å over 100 equivalent Cα positions) and P. putida PP4397 (Protein Data Bank code 2GJG, DALI Z-score = 7.8, r.m.s. deviation of 2.5 Å over 95 equivalent Cα positions). The most notable difference between Alg44(14–122) L69M and other PilZ domains of known structure is its unique mode of homodimerization. Although VCA0042 and PP4397 are PilZ domain-containing proteins that homodimerize, the interaction interface in these proteins is largely mediated by their YcgR-N domains. In Alg44(14–122) L69M, however, homodimerization is mediated by β-sheet augmentation of the PilZ domain between the β6 strands of each split barrel monomer, resulting in a β-sheet composed of the β2-β1-β5-β6-β6′-β5′-β1′-β2′ strands (Fig. 1B). In addition, side chains from the β6 strand (Ile-76, Phe-78, and Leu-80) and the α1 helix (Ala-110, Leu-111, Tyr-113, and Leu-114) contribute significant buried surface area at the dimerization interface, which is predominantly hydrophobic in nature. Analysis of this interface by the PDBe PISA Web server (39) estimates a buried interface of 687 Å2 and a ΔiG value of −9.7 kcal/mol, suggesting that homodimer formation is energetically favorable. Moreover, the residues that make up this interface exhibit a high degree of conservation among Alg44 orthologs (Fig. 1C). Molecular weight analysis using analytical size exclusion chromatography indicates that Alg44(14–122) in the solution state is a dimer both in the absence and presence of c-di-GMP, suggesting that it is highly stable and that ligand binding does not modulate its oligomeric state (Fig. 2).
FIGURE 2.
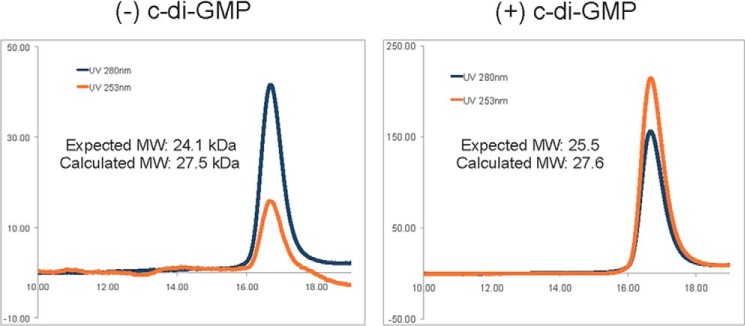
c-di-GMP binding does not modulate the oligomeric state of Alg44PilZ. Analytical size exclusion profiles of Alg44PilZ in the presence and absence of 1 mm c-di-GMP. Absorbance at 280 nm was used to monitor the elution of Alg44PilZ, whereas absorbance at 253 nm (λmax of c-di-GMP) was used as a qualitative indicator of c-di-GMP binding. Protein standards used to calibrate the column are indicated by inverted triangles; A, aldolase; C, conalbumin; O, ovalbumin; R, ribonuclease A. The molecular masses of aldolase, conalbumin, ovalbumin, and ribonuclease A are 158.0, 75.0, 43.0, and 13.7 kDa, respectively. The calculated dimeric molecular mass of Alg44PilZ in the absence and presence of c-di-GMP is 27.5 and 27.6 kDa, respectively, compared with their expected molecular masses of 24.1 and 25.5 kDa.
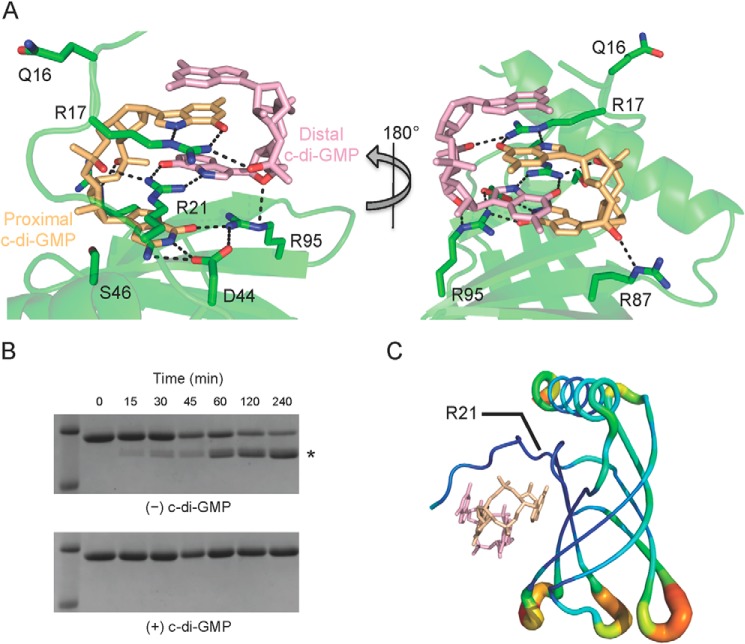
Examination of the c-di-GMP binding site shows that six conserved residues make polar contacts with the dimeric self-intercalated ligand. Arg-17, Arg-21, Asp-44, and Ser-46 are part of the canonical RXXXR and DXSXXG motifs that have been observed to bind c-di-GMP in other PilZ domain-containing proteins. The Nϵ and Nη1 atoms of Arg-17 make hydrogen bonding interactions with the guanine N7 and O6 atoms, respectively, of the proximal c-di-GMP molecule, and its Nη1 atom also interacts with one of the phosphate groups of the distal c-di-GMP molecule (Fig. 3A). Similarly, the Nη1 and Nη2 atoms of Arg-21 hydrogen bond with the guanine N7 and O6 atoms, respectively, of the distal c-di-GMP molecule, and its Nη2 atom also interacts with a phosphate moiety of the proximal c-di-GMP molecule. Asp-44 makes hydrogen bonding interactions between its Oδ1 atom and the guanine N1 and N2 atoms of the proximal c-di-GMP molecule. In addition, its Oδ2 atom interacts with the Nη1 atom of Arg-95. The hydroxyl group of Ser-46 appears to be too far away from c-di-GMP (3.6 Å) to be involved in ligand binding despite its strict conservation among PilZ domains. This observation is not unique to Alg44, because the corresponding serine residues in P. aeruginosa PA4608 and P. putida PP4397 are also not within hydrogen bonding distance of c-di-GMP (4.3 and 3.7 Å, respectively). The remaining two c-di-GMP-binding residues, Arg-87 and Arg-95, are unique to Alg44 at the sequence level, and among Alg44 orthologs they are completely conserved. The Nϵ atom of Arg-87 interacts with a phosphate group of the proximal c-di-GMP molecule, whereas the Nϵ atom of Arg-95 forms a hydrogen bond with the ethereal oxygen of a ribose group on the distal c-di-GMP molecule. In addition to its previously stated interaction with the Oδ1 atom of Asp-44, the Nη1 atom of Arg-95 also forms a hydrogen bond with the O6 atom of the proximal c-di-GMP molecule. Interestingly, the side chains of Arg-200 and Lys-164 of P. putida PP4397 appear to interact with c-di-GMP in the same manner as Arg-87 and Arg-95 of Alg44, despite originating from different locations in the primary sequence (Fig. 4). However, the contribution of these residues to c-di-GMP binding was not assessed in the analysis of the P. putida PP4397 binding site.
FIGURE 3.
The N terminus of Alg44PilZ binds dimeric c-di-GMP. A, close-up of the c-di-GMP binding site. Arg-17, Arg-21, Asp-44, and Ser-46 comprise the canonical RXXXR and DXSXXG motifs typically found in PilZ domains, whereas Arg-87 and Arg-95 also interact with c-di-GMP and are unique to Alg44. Gln-16 is the residue that immediately precedes the RXXXR motif (position X residue) that has been suggested to be important for c-di-GMP binding. Nitrogen and oxygen atoms are colored in blue and red, respectively. For clarity, only c-di-GMP atoms that interact with Alg44PilZ residues are colored. Protein carbon atoms and non-Alg44PilZ-interacting proximal and distal c-di-GMP atoms are colored in green, gold, and light purple, respectively. B, time course of trypsin proteolysis of Alg44PilZ in the absence (top) and presence (bottom) of c-di-GMP. The proteolytic fragment indicated by the asterisk starts at Arg-21 as determined by Edman degradation (SPARC BioCentre, Hospital for Sick Children). C, temperature factor putty representation of Alg44PilZ. Temperature factors are illustrated qualitatively by both the thickness of the backbone trace and its color with blue through to red representing a continuum of low to high temperature factors. The location of Arg-21 is indicated.
FIGURE 4.
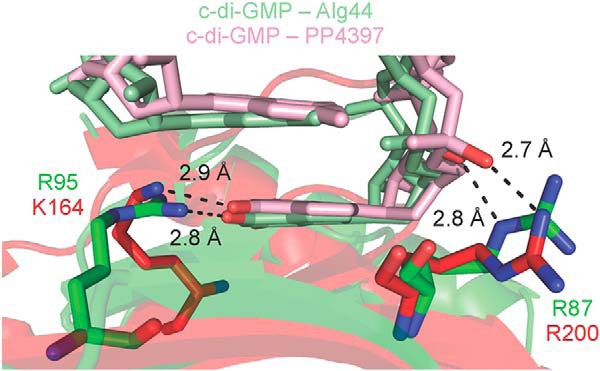
Arg-87 and Arg-95 of Alg44PilZ may be functionally equivalent to Arg-200 and Lys-164 of P. putida PP4397. Alg44PilZ and PP4397 are shown as schematic representations in green and red, respectively, with their c-di-GMP binding residues displayed as sticks. The c-di-GMP bound to Alg44PilZ is displayed as light green sticks with oxygen atoms that are interacting with Alg44PilZ residues colored red. The c-di-GMP bound to PP4397 is displayed as pink sticks with oxygen atoms that are interacting with Alg44PilZ residues colored red.
The amino acid residue that immediately precedes the RXXXR motif (referred to as “position X”) has been shown to be important in other characterized PilZ domains. Alg44 contains a glutamine at position X, consistent with the previous observation that a hydrophilic amino acid at this position facilitates the binding of dimeric c-di-GMP (23). However, Gln-16 does not appear to make contact with c-di-GMP (Fig. 3A).
A solution NMR study of the PilZ domain protein PA4608 from P. aeruginosa demonstrated that the N-terminal c-di-GMP binding region of this protein undergoes a disorder-to-order transition upon binding the dinucleotide. The result of this rearrangement is the creation of a highly negative surface on one side of the protein that the authors propose forms the molecular basis for downstream signaling (21). Although the holo-Alg44(14–122) L69M structure does not appear to have the same charge-clustering phenomenon, we sought to determine whether the N terminus of Alg44 undergoes a similar ordering on ligand binding. Because our crystallization attempts of apo-Alg44(14–122) were unsuccessful, we used limited proteolysis to probe the dynamics of the N-terminal region. To this end, Alg44(1–122) was incubated with trypsin in the absence and presence of c-di-GMP, and its proteolytic degradation pattern was analyzed by SDS-PAGE (Fig. 3B). In the absence of c-di-GMP, Alg44(1–122) rapidly degraded to a lower molecular weight species, whereas in the presence of c-di-GMP, the molecular weight remained the same as that of the undigested control. Edman sequencing of this degradation product indicated that apo-Alg44(1–122) was being proteolyzed at the N-terminal side of Arg-21, confirming that the N terminus of the protein is disordered in solution but upon binding c-di-GMP becomes ordered and resistant to trypsin degradation (Fig. 3C).
Mechanism of c-di-GMP Binding
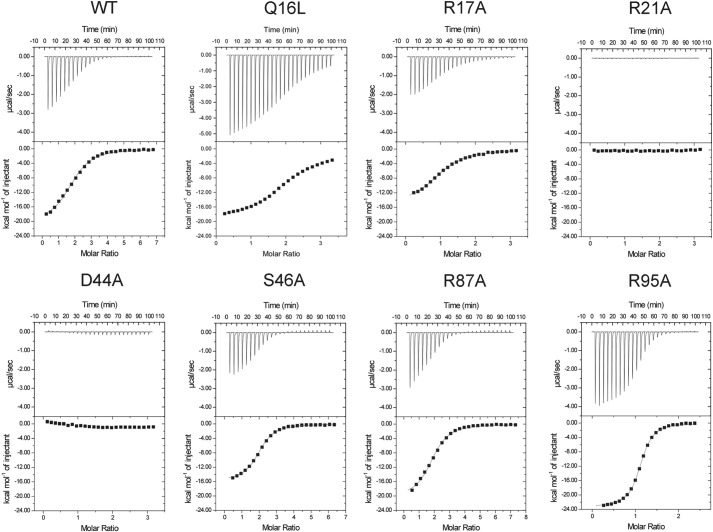
For calorimetric analysis of the c-di-GMP binding site, a construct encompassing residues 1–122 of Alg44 was generated (Alg44(1–122)). The inclusion of the first 13 amino acids of Alg44 did not change the solution properties of the protein compared with the crystallization construct (Alg44(14–122) L69M) as determined by size exclusion chromatography and circular dichroism spectroscopy (data not shown). To determine the importance of each of the previously mentioned residues in interactions with the dinucleotide, ITC analysis was performed on wild-type Alg44(1–122) as well as on R17A, R21A, D44A, S46A, R87A, and R95A site-directed mutants (Fig. 5 and Table 2). Because VCA0042 contains a leucine at position X and can only bind monomeric c-di-GMP, the corresponding Alg44 position X mutant, Q16L, was analyzed to probe binding stoichiometry. Although Gln-16 does not appear to make contact with c-di-GMP in the crystal structure of Alg44(14–122) (Fig. 3A), we speculated that it could still play a role in c-di-GMP interactions, given its proximity to the truncated N terminus (deletion of which was required for crystallization) as well as the limited proteolysis studies with Alg44(1–122) that suggest an N-terminal conformational change upon c-di-GMP binding.
FIGURE 5.
ITC titrations of c-di-GMP with wild-type Alg44PilZ and the indicated site-specific mutants. In each experiment, the top panel displays the heats of injection, whereas the bottom panel shows the normalized integration data as a function of the molar syringe and cell concentrations. Where binding was observed, the black line in the bottom panel of each experiment represents the fit of the integrated data to an independent sites binding model. The calculated dissociation constants (KD), binding stoichiometries, and thermodynamic parameters are listed in Table 2.
TABLE 2.
Thermodynamic parameters of c-di-GMP binding to wild-type Alg44PilZ and the indicated site-specific mutants
| Binding model | KD | ΔH | −TΔS | n | |
|---|---|---|---|---|---|
| μm | kcal/mol | kcal/mol | |||
| Wild type | Independent sites | 3.13 ± 0.25 | −20.14 ± 0.39 | 12.60 | 1.86 ± 0.03 |
| Q16L | Independent sites | 5.92 ± 0.29 | −21.27 ± 0.22 | 14.06 | 2.14 ± 0.01 |
| R17A | Independent sites | 5.56 ± 0.21 | −15.19 ± 0.19 | 8.02 | 1.03 ± 0.01 |
| R21A | NDa | ||||
| D44A | ND | ||||
| S46A | Independent sites | 1.52 ± 0.07 | −15.79 ± 0.14 | 7.84 | 1.97 ± 0.01 |
| R87A | Independent sites | 2.21 ± 0.19 | −20.29 ± 0.43 | 12.58 | 1.87 ± 0.03 |
| R95A | Independent sites | 0.73 ± 0.03 | −23.35 ± 0.10 | 14.96 | 1.09 ± 0.01 |
a ND, not determined.
Due to the dimeric nature of the protein, the binding of c-di-GMP to the wild type and mutant variants was fit to an independent sites binding model. For Alg44(1–122), this yielded a KD of 3.13 ± 0.25 μm and a ligand/protein stoichiometry of 1.86 ± 0.03, suggesting that, as found in the crystal structure, two molecules of c-di-GMP bind each monomer of Alg44(1–122) (Table 2). Analysis of the Q16L, S46A, and R87A mutants revealed KD values and stoichiometries that were similar to those of the wild-type protein, suggesting that these residues do not perturb the stoichiometry and thermodynamics of the c-di-GMP/Alg44(1–122) interaction. In contrast, the R21A and D44A mutants reduced c-di-GMP binding affinity to below the detection limit of the calorimeter, indicating that in the absence of either of these residues, c-di-GMP is unable to bind Alg44(1–122) with any appreciable affinity. The final pair of alanine mutants, R17A and R95A, were also fit to an independent sites binding model. However, unlike the WT, Q16L, S46A, and R87A samples, the calculated stoichiometry between c-di-GMP and the R17A and R95A mutants was 1.03 ± 0.01:1 and 1.09 ± 0.01:1, respectively, suggesting that they are only capable of binding a monomer of c-di-GMP (Table 2).
In order to confirm the stoichiometry results obtained by ITC, structural studies were carried out on the R95A mutant. However, Alg44(14–122) L69M/R95A in the presence of c-di-GMP did not crystallize in the conditions used for the L69M protein. We hypothesized that the failure to crystallize was probably due to the loss of crystal contacts as a result of the difference in ligand/protein stoichiometry, and as a consequence, Alg44(14–122) L69M/R95A was rescreened for new crystallization conditions using commercially available sparse matrix screens. A new crystal form was obtained, which diffracted to 1.7 Å, exhibited the space group symmetry of P6322, and contained three protomers in the asymmetric unit. Upon structure determination by molecular replacement using the wild-type protein as a search model, it was clear from the electron density that there was only a single c-di-GMP molecule present per Alg44(14–122) L69M/R95A monomer (Fig. 6A). The final model of Alg44(14–122) L69M/R95A in complex with c-di-GMP was refined to an Rwork of 16.5% and Rfree of 20.1%.
FIGURE 6.
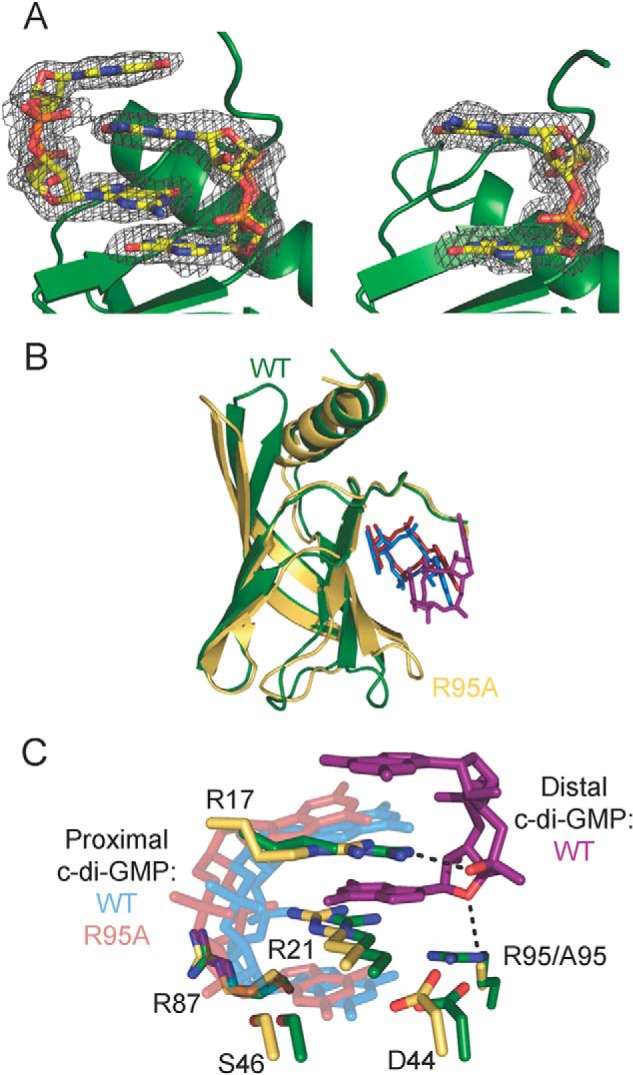
Comparison of Alg44PilZ L69M and L69M/R95A structures. A, (|Fo| − |Fc|) electron density map, with c-di-GMP molecules omitted from the electron density calculation, contoured at 3σ and shown as a gray mesh, of c-di-GMP bound to L69M (left) and L69M/R95A (right) Alg44(14–122). Each protein is shown as a schematic representation in green, whereas c-di-GMP is shown as sticks with carbon, nitrogen, oxygen and, phosphorous atoms colored yellow, blue, red, and orange, respectively. B, structural overlay of the L69M Alg44PilZ (green) and the L69M/R95A mutant (yellow) structures with bound c-di-GMP. C, structural overlay of the c-di-GMP binding residues from the L69M Alg44PilZ and the L69M/R95A mutant structures. The proximal and distal c-di-GMP molecules of the L69M Alg44PilZ structure are colored light blue and purple, respectively. The single bound c-di-GMP molecule of the Alg44PilZ L69M/R95A mutant structure is colored red. The black dashed lines indicate polar contacts between Arg-17 and Arg-95 and the distal c-di-GMP molecule of the wild-type structure.
The overall Alg44(14–122) L69M/R95A structure closely resembles that of the L69M mutant protein with an overall Cα r.m.s. deviation of 0.8 Å (Fig. 6B). Inspection of the c-di-GMP binding site reveals that the position of the c-di-GMP molecule in the L69M/R95A structure is in a position very similar to that of the proximal c-di-GMP molecule in the L69M structure except that its overall position is shifted by about ∼1 Å relative to the split barrel core of the PilZ domain (Fig. 6C). The result of this shift is that Ser-46 is now within hydrogen bonding distance (3.3 Å) of the guanine N2 atom of c-di-GMP, possibly accounting for both the increased affinity and binding enthalpy of c-di-GMP to this mutant. The bonding distances between the other residues involved in c-di-GMP binding differ no more than 0.1 Å between the L69M and L69M/R95A structure. Another notable change between the two structures is that the two guanine moieties are no longer co-planar in the R95A structure. This probably arises from the different crystal packing arrangements observed between the two structures. Although crystal packing is mediated by c-di-GMP in both crystal forms, the manner in which it does so is significantly different. In the L69M structure, hydrogen-bonding interactions between atoms from the guanine and phosphate groups of opposing c-di-GMP molecules facilitate crystal lattice formation. In contrast, in molecules A and B in the L69M/R95A structure, the opposing c-di-GMP molecules interact with one another through a π-π stacking interaction between guanine bases, which probably accounts for the deviation from co-planarity observed between the two guanine groups within each c-di-GMP molecule. The c-di-GMP moiety in molecule C of the L69M/R95A structure is not involved in crystal contacts, and its guanine groups are co-planar as per the L69M structure.
Alginate Production by P. aeruginosa Requires the Molecular Determinants for Dimeric c-di-GMP Binding
To determine whether the dimeric form of c-di-GMP is required for alginate production, the same point mutants examined by calorimetry were introduced onto the chromosome of P. aeruginosa PAO1 by allelic exchange. Because PAO1 does not secrete alginate under standard laboratory growth conditions, alginate production was stimulated by plasmid-born expression of the extracytoplasmic function sigma factor AlgT, which is a well characterized positive regulator of the alginate biosynthesis and export genes. In this system, induction of algT expression results in 97 mg of alginate per g of dry cellular mass as determined by the colorimetric carbazole assay for uronic acids (35). Importantly, PAO1 with empty vector lacking algT generated only 4 mg of uronic acids per g of dry cellular mass, demonstrating that the majority of uronic acids measured by this assay are due to alginate production stimulated by algT expression.
Next, we examined the amount of alginate produced by strains of P. aeruginosa bearing each of the aforementioned point mutations encoded in the native alg44 locus (Fig. 7A). Consistent with the calorimetric data that demonstrated that mutation of Gln-16, Ser-46, and Arg-87 did not alter the stoichiometry or thermodynamics of c-di-GMP binding, strains expressing the Q16L, S46A, and R87A mutant variants of Alg44 exhibited levels of alginate production comparable with the wild-type background. In addition, the R21A and D44A variants, which showed no detectable c-di-GMP binding in vitro, were found to be unable to secrete alginate in vivo. Last, when the c-di-GMP stoichiometry-determining residues Arg-17 and Arg-95 were mutated to alanine on the P. aeruginosa chromosome, the resulting strains were also unable to produce alginate. Analysis of Alg44 levels by Western blot demonstrates that the lack of alginate production is not due to a loss of protein expression, because comparable amounts of Alg44 are expressed in all strains (Fig. 7B). This demonstrates the necessity for binding of dimeric c-di-GMP to Alg44 to stimulate alginate biosynthesis.
FIGURE 7.
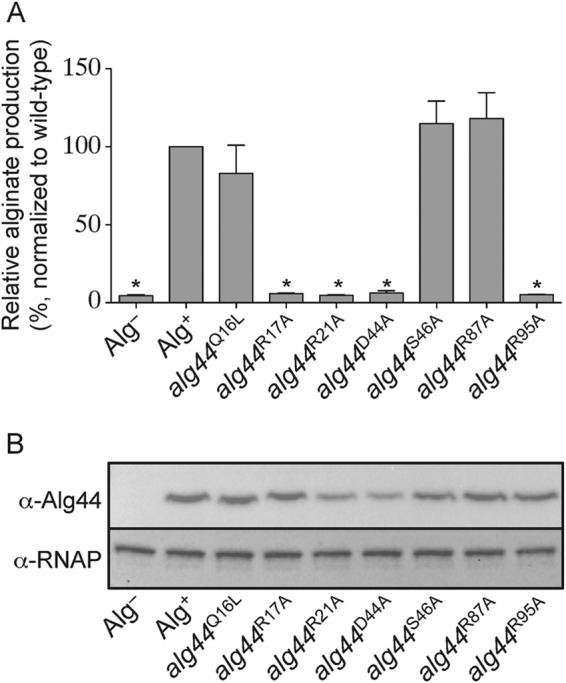
Amino acid residues necessary for dimeric c-di-GMP binding in vitro are required for alginate production in vivo. A, alginate production levels in P. aeruginosa PAO1 containing the pPSV39 vector control (Alg−) or pPSV39::algT (Alg+ and alg44 site-specific mutants) grown in the presence of 1 mm IPTG. Alginate levels in strains harboring the indicated alg44 site-specific mutant alleles were normalized to wild type (Alg+). Error bars, S.D. (n = 3). Asterisks, alginate production levels that are significantly different from wild type (p < 0.05). B, Western blot analysis of the above-mentioned strains probed using α-Alg44 antibody or α-RNA polymerase (RNAP). RNAP was used as a loading control.
DISCUSSION
In this work, we sought to determine the number of c-di-GMP molecules required for Alg44-dependent activation of alginate production in P. aeruginosa. Unexpectedly, the structure of Alg44PilZ L69M in complex with c-di-GMP shows that this protein adopts a unique homodimeric arrangement previously unseen in the PilZ family of proteins. This observation raises the possibility that unlike the well characterized cellulose synthase complex, which requires a single PilZ domain and a single glycosyltransferase domain for polysaccharide synthesis, alginate polymerization by the putative alginate synthase complex consisting of Alg8 and Alg44 may adopt a higher order structure containing two PilZ domains and, potentially, two glycosyltransferase domains. Reconstitution and characterization of a functional alginate synthase is the focus of ongoing research in our laboratory.
Each monomer of Alg44PilZ L69M binds to the dimeric self-intercalated form of c-di-GMP using a constellation of six highly conserved residues. Of these residues, Arg-21 and Asp-44 are essential for binding, whereas Arg-17 and Arg-95 select for the dimeric form of c-di-GMP. Unlike other characterized c-di-GMP receptors, mutation of position X residue Gln-16 to leucine did not alter c-di-GMP binding stoichiometry and had no affect on alginate production. Crystal structures of several PilZ domain-containing proteins have shown that for some proteins, such as in VCA0042 (26), a single bound molecule of c-di-GMP is present, whereas in others, including PP4397 and PA4608 (21, 23), a self-intercalated dimer is found. In addition to observations from crystal structures, the authors of the PP4397 study were also able to alter the binding stoichiometry between c-di-GMP and PP4397 from 2:1 to 1:1 by mutating the arginine residue at position X to a leucine residue (23). Unfortunately, the biological function of each of these proteins is unknown; thus, the significance of the oligomeric state of c-di-GMP in these structures could not be ascertained. In contrast, the PilZ domain of Alg44 is a known post-translational regulator of alginate biosynthesis and secretion in P. aeruginosa (10, 37). Therefore, the observation that Alg44PilZ requires binding of dimeric c-di-GMP to stimulate alginate production demonstrates that this oligomeric state contains the molecular determinants for alginate production in P. aeruginosa.
A study by Pultz et al. showed that c-di-GMP binding affinity determines the response threshold for c-di-GMP receptors found in Salmonella (40). This model provides a plausible explanation for the observation that many diguanylate cyclases appear to control only certain c-di-GMP-regulated phenotypes and not others (41). Bacteria are not known to contain any compartments that limit c-di-GMP diffusion, so it might be expected that an increase in intracellular c-di-GMP concentration would activate all c-di-GMP-responsive proteins in a given bacterium. Thus, differential ligand binding based on affinity may explain why some c-di-GMP-regulated phenotypes are observed, whereas others are not. The data presented herein add another layer of detail to this model and suggest that the oligomeric state of c-di-GMP when bound to a given receptor is probably related to its affinity for c-di-GMP. It may be that proteins only capable of binding a monomer of c-di-GMP become responsive at lower concentrations of c-di-GMP compared with those that bind the dimeric form of c-di-GMP. Consistent with this idea is the FimX protein, a c-di-GMP receptor involved in regulating type IV pili (T4P)-mediated twitching motility in P. aeruginosa (42). FimX binds monomeric c-di-GMP with a KD of 100 nm, resulting in functional T4P at cellular c-di-GMP concentrations as low as 300 nm, a concentration at which exopolysaccharide synthesis is not active (13, 43, 44). However, once cellular levels of c-di-GMP reach a concentration sufficient for biofilm production (estimated to be 5–10 μm (45)), there is enough c-di-GMP present to favor its binding to exopolysaccharide-activating receptors, including Alg44, P. aeruginosa PelD, and Gluconacetobacter xylinus BcsA, which all may require the binding of two molecules of c-di-GMP for activation (16, 46). Moreover, the mechanism of product inhibition for diguanylate cyclases involves allosteric inhibition by two molecules of c-di-GMP at a conserved RXXD motif (termed the “I-site”) that is spatially distant from their active sites (47, 48). Experimental determination of the inhibition constant (Ki) for this process is estimated to be around ∼1 μm (49), which is similar to the low micromolar binding constants observed for Alg44, PelD, and BcsA (16, 46).
NMR analysis of c-di-GMP oligomerization indicates that under standard buffering conditions, the monomer/dimer equilibrium constant is around 1 mm, suggesting that at the nanomolar to micromolar concentrations found in the cell, c-di-GMP will be predominantly monomeric (50). In order to reconcile the many crystal structures of proteins bound to dimeric c-di-GMP, Gentner et al. (50) proposed that the dimerization process might occur on the protein with the amino acids that comprise the binding site serving as a template for dimer binding. This statement is consistent with our experimental results in that Alg44PilZ was only able to bind a dimer of c-di-GMP when certain amino acid residues were present. In the absence of these dimer-inducing residues, only monomeric c-di-GMP bound to Alg44. Further structural and biophysical analysis of other c-di-GMP binding domains with measurable phenotypes will allow for assessment of the generalizability of this proposed model.
This work was supported, in whole or in part, by National Institutes of Health, NIAID, Grant AI-19146 (to D. E. O.). This work was also supported by Canadian Institutes of Health Research Grant MT13337 (to P. L. H.) and Veterans Administration Medical Research Grant IO1BX000477 (to D. E. O.). Beam line X29 at the National Synchrotron Light Source is supported by the United States Department of Energy and the National Institutes of Health National Center for Research Resources.
The atomic coordinates and structure factors (codes 4RT0 and 4RT1) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- c-di-GMP
- bis-(3′,5′)-cyclic dimeric guanosine monophosphate
- ITC
- isothermal titration calorimetry
- IPTG
- isopropyl 1-thio-β-d-galactopyranoside
- SAD
- single-wavelength anomalous dispersion
- r.m.s.
- root mean square
- SUMO
- small ubiquitin-like modifier.
REFERENCES
- 1. Tamayo R., Pratt J. T., Camilli A. (2007) Roles of cyclic diguanylate in the regulation of bacterial pathogenesis. Annu. Rev. Microbiol. 61, 131–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mills E., Pultz I. S., Kulasekara H. D., Miller S. I. (2011) The bacterial second messenger c-di-GMP: mechanisms of signalling. Cell Microbiol. 13, 1122–1129 [DOI] [PubMed] [Google Scholar]
- 3. Murray T. S., Egan M., Kazmierczak B. I. (2007) Pseudomonas aeruginosa chronic colonization in cystic fibrosis patients. Curr. Opin. Pediatr. 19, 83–88 [DOI] [PubMed] [Google Scholar]
- 4. Ramsey D. M., Wozniak D. J. (2005) Understanding the control of Pseudomonas aeruginosa alginate synthesis and the prospects for management of chronic infections in cystic fibrosis. Mol. Microbiol. 56, 309–322 [DOI] [PubMed] [Google Scholar]
- 5. Franklin M. J., Nivens D. E., Weadge J. T., Howell P. L. (2011) Biosynthesis of the Pseudomonas aeruginosa extracellular polysaccharides, alginate, Pel, and Psl. Front. Microbiol. 2, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wozniak D. J., Wyckoff T. J. O., Starkey M., Keyser R., Azadi P., O'Toole G. A., Parsek M. R. (2003) Alginate is not a significant component of the extracellular polysaccharide matrix of PA14 and PAO1 Pseudomonas aeruginosa biofilms. Proc. Natl. Acad. Sci. U.S.A. 100, 7907–7912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colvin K. M., Irie Y., Tart C. S., Urbano R., Whitney J. C., Ryder C., Howell P. L., Wozniak D. J., Parsek M. R. (2012) The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environ. Microbiol. 14, 1913–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hogardt M., Heesemann J. (2013) Microevolution of Pseudomonas aeruginosa to a chronic pathogen of the cystic fibrosis lung. Curr. Top. Microbiol. Immunol. 358, 91–118 [DOI] [PubMed] [Google Scholar]
- 9. Yang L., Hengzhuang W., Wu H., Damkiaer S., Jochumsen N., Song Z., Givskov M., Høiby N., Molin S. (2012) Polysaccharides serve as scaffold of biofilms formed by mucoid Pseudomonas aeruginosa. FEMS Immunol. Med. Microbiol. 65, 366–376 [DOI] [PubMed] [Google Scholar]
- 10. Merighi M., Lee V. T., Hyodo M., Hayakawa Y., Lory S. (2007) The second messenger bis-(3′-5′)-cyclic-GMP and its PilZ domain-containing receptor Alg44 are required for alginate biosynthesis in Pseudomonas aeruginosa. Mol. Microbiol. 65, 876–895 [DOI] [PubMed] [Google Scholar]
- 11. Hickman J. W., Harwood C. S. (2008) Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor. Mol. Microbiol. 69, 376–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krasteva P. V., Fong J. C. N., Shikuma N. J., Beyhan S., Navarro M. V. A. S., Yildiz F. H., Sondermann H. (2010) Vibrio cholerae VpsT regulates matrix production and motility by directly sensing cyclic di-GMP. Science 327, 866–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Navarro M. V. A. S., De N., Bae N., Wang Q., Sondermann H. (2009) Structural analysis of the GGDEF-EAL domain-containing c-di-GMP receptor FimX. Structure 17, 1104–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Navarro M. V. A. S., Newell P. D., Krasteva P. V., Chatterjee D., Madden D. R., O'Toole G. A., Sondermann H. (2011) Structural basis for c-di-GMP-mediated inside-out signaling controlling periplasmic proteolysis. PLoS Biol. 9, e1000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee V. T., Matewish J. M., Kessler J. L., Hyodo M., Hayakawa Y., Lory S. (2007) A cyclic-di-GMP receptor required for bacterial exopolysaccharide production. Mol. Microbiol. 65, 1474–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Whitney J. C., Colvin K. M., Marmont L. S., Robinson H., Parsek M. R., Howell P. L. (2012) Structure of the cytoplasmic region of PelD, a degenerate diguanylate cyclase receptor that regulates exopolysaccharide production in Pseudomonas aeruginosa. J. Biol. Chem. 287, 23582–23593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Steiner S., Lori C., Boehm A., Jenal U. (2013) Allosteric activation of exopolysaccharide synthesis through cyclic di-GMP-stimulated protein-protein interaction. EMBO J. 32, 354–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Amikam D., Galperin M. Y. (2006) PilZ domain is part of the bacterial c-di-GMP binding protein. Bioinformatics 22, 3–6 [DOI] [PubMed] [Google Scholar]
- 19. Whitney J. C., Howell P. L. (2013) Synthase-dependent exopolysaccharide secretion in Gram-negative bacteria. Trends Microbiol. 21, 63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ryjenkov D. A., Simm R., Römling U., Gomelsky M. (2006) The PilZ domain is a receptor for the second messenger c-di-GMP: the PilZ domain protein YcgR controls motility in enterobacteria. J. Biol. Chem. 281, 30310–30314 [DOI] [PubMed] [Google Scholar]
- 21. Habazettl J., Allan M. G., Jenal U., Grzesiek S. (2011) Solution structure of the PilZ domain protein PA4608 complex with cyclic di-GMP identifies charge clustering as molecular readout. J. Biol. Chem. 286, 14304–14314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pratt J. T., Tamayo R., Tischler A. D., Camilli A. (2007) PilZ domain proteins bind cyclic diguanylate and regulate diverse processes in Vibrio cholerae. J. Biol. Chem. 282, 12860–12870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ko J., Ryu K.-S., Kim H., Shin J.-S., Lee J.-O., Cheong C., Choi B.-S. (2010) Structure of PP4397 reveals the molecular basis for different c-di-GMP binding modes by Pilz domain proteins. J. Mol. Biol. 398, 97–110 [DOI] [PubMed] [Google Scholar]
- 24. Morgan J. L. W., Strumillo J., Zimmer J. (2013) Crystallographic snapshot of cellulose synthesis and membrane translocation. Nature 493, 181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morgan J. L. W., McNamara J. T., Zimmer J. (2014) Mechanism of activation of bacterial cellulose synthase by cyclic di-GMP. Nat. Struct. Mol. Biol. 21, 489–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benach J., Swaminathan S. S., Tamayo R., Handelman S. K., Folta-Stogniew E., Ramos J. E., Forouhar F., Neely H., Seetharaman J., Camilli A., Hunt J. F. (2007) The structural basis of cyclic diguanylate signal transduction by PilZ domains. EMBO J. 26, 5153–5166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Winsor G. L., Lam D. K. W., Fleming L., Lo R., Whiteside M. D., Yu N. Y., Hancock R. E. W., Brinkman F. S. L. (2011) Pseudomonas Genome Database: improved comparative analysis and population genomics capability for Pseudomonas genomes. Nucleic Acids Res. 39, D596–D600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee J. E., Cornell K. A., Riscoe M. K., Howell P. L. (2001) Structure of E. coli 5′-methylthioadenosine/S-adenosylhomocysteine nucleosidase reveals similarity to the purine nucleoside phosphorylases. Structure 9, 941–953 [DOI] [PubMed] [Google Scholar]
- 29. Terwilliger T. C., Grosse-Kunstleve R. W., Afonine P. V., Moriarty N. W., Zwart P. H., Hung L. W., Read R. J., Adams P. D. (2008) Iterative model building, structure refinement and density modification with the PHENIX AutoBuild wizard. Acta Crystallogr. D Biol. Crystallogr. 64, 61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Emsley P., Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 31. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., Richardson J. S., Terwilliger T. C., Zwart P. H. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Choi K.-H., Schweizer H. P. (2005) An improved method for rapid generation of unmarked Pseudomonas aeruginosa deletion mutants. BMC Microbiol. 5, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Lorenzo V., Timmis K. N. (1994) Analysis and construction of stable phenotypes in Gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 235, 386–405 [DOI] [PubMed] [Google Scholar]
- 34. Rietsch A., Vallet-Gely I., Dove S. L., Mekalanos J. J. (2005) ExsE, a secreted regulator of type III secretion genes in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 102, 8006–8011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Knutson C. A., Jeanes A. (1968) A new modification of the carbazole analysis: application to heteropolysaccharides. Anal. Biochem. 24, 470–481 [DOI] [PubMed] [Google Scholar]
- 36. Salamitou S., Lemaire M., Fujino T., Ohayon H., Gounon P., Béguin P., Aubert J. P. (1994) Subcellular localization of Clostridium thermocellum ORF3p, a protein carrying a receptor for the docking sequence borne by the catalytic components of the cellulosome. J. Bacteriol. 176, 2828–2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oglesby L. L., Jain S., Ohman D. E. (2008) Membrane topology and roles of Pseudomonas aeruginosa Alg8 and Alg44 in alginate polymerization. Microbiology 154, 1605–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Holm L., Rosenström P. (2010) Dali server: conservation mapping in 3D. Nucleic Acids Res. 38, W545–W549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Krissinel E., Henrick K. (2007) Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 [DOI] [PubMed] [Google Scholar]
- 40. Pultz I. S., Christen M., Kulasekara H. D., Kennard A., Kulasekara B., Miller S. I. (2012) The response threshold of Salmonella PilZ domain proteins is determined by their binding affinities for c-di-GMP. Mol. Microbiol. 86, 1424–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kulasakara H., Lee V., Brencic A., Liberati N., Urbach J., Miyata S., Lee D. G., Neely A. N., Hyodo M., Hayakawa Y., Ausubel F. M., Lory S. (2006) Analysis of Pseudomonas aeruginosa diguanylate cyclases and phosphodiesterases reveals a role for bis-(3′-5′)-cyclic-GMP in virulence. Proc. Natl. Acad. Sci. U.S.A. 103, 2839–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang B., Whitchurch C. B., Mattick J. S. (2003) FimX, a multidomain protein connecting environmental signals to twitching motility in Pseudomonas aeruginosa. J. Bacteriol. 185, 7068–7076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jain R., Behrens A.-J., Kaever V., Kazmierczak B. I. (2012) Type IV pilus assembly in Pseudomonas aeruginosa over a broad range of cyclic di-GMP concentrations. J. Bacteriol. 194, 4285–4294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chin K.-H., Kuo W.-T., Yu Y.-J., Liao Y.-T., Yang M.-T., Chou S.-H. (2012) Structural polymorphism of c-di-GMP bound to an EAL domain and in complex with a type II PilZ-domain protein. Acta Crystallogr. D Biol. Crystallogr. 68, 1380–1392 [DOI] [PubMed] [Google Scholar]
- 45. Weinhouse H., Sapir S., Amikam D., Shilo Y., Volman G., Ohana P., Benziman M. (1997) c-di-GMP-binding protein, a new factor regulating cellulose synthesis in Acetobacter xylinum. FEBS Lett. 416, 207–211 [DOI] [PubMed] [Google Scholar]
- 46. Fujiwara T., Komoda K., Sakurai N., Tajima K., Tanaka I., Yao M. (2013) The c-di-GMP recognition mechanism of the PilZ domain of bacterial cellulose synthase subunit A. Biochem. Biophys. Res. Commun. 431, 802–807 [DOI] [PubMed] [Google Scholar]
- 47. Chan C., Paul R., Samoray D., Amiot N. C., Giese B., Jenal U., Schirmer T. (2004) Structural basis of activity and allosteric control of diguanylate cyclase. Proc. Natl. Acad. Sci. U.S.A. 101, 17084–17089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. De N., Pirruccello M., Krasteva P. V., Bae N., Raghavan R. V., Sondermann H. (2008) Phosphorylation-independent regulation of the diguanylate cyclase WspR. PLoS Biol. 6, e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Christen B., Christen M., Paul R., Schmid F., Folcher M., Jenoe P., Meuwly M., Jenal U. (2006) Allosteric control of cyclic di-GMP signaling. J. Biol. Chem. 281, 32015–32024 [DOI] [PubMed] [Google Scholar]
- 50. Gentner M., Allan M. G., Zaehringer F., Schirmer T., Grzesiek S. (2012) Oligomer formation of the bacterial second messenger c-di-GMP: reaction rates and equilibrium constants indicate a monomeric state at physiological concentrations. J. Am. Chem. Soc. 134, 1019–1029 [DOI] [PubMed] [Google Scholar]
- 51. Chen V. B., Arendall W. B., 3rd, Headd J. J., Keedy D. A., Immormino R. M., Kapral G. J., Murray L. W., Richardson J. S., Richardson D. C. (2010) MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]