Abstract
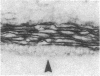
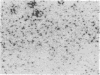
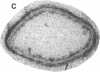
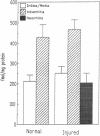
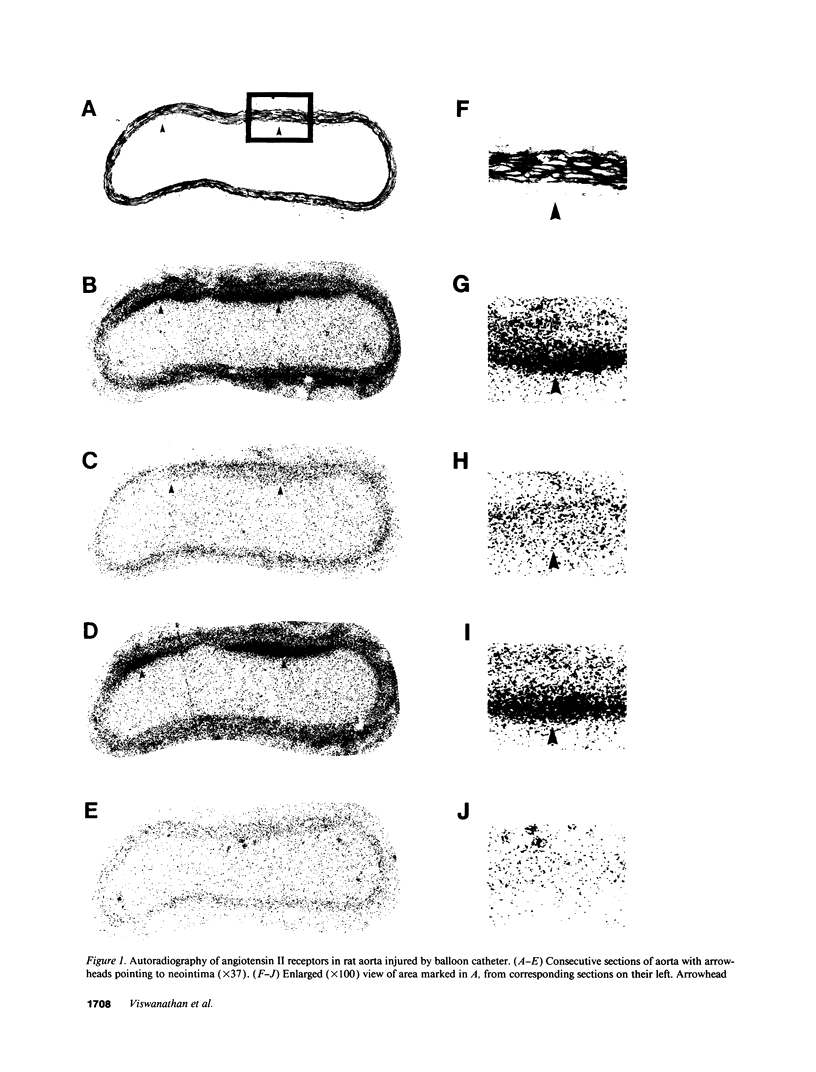
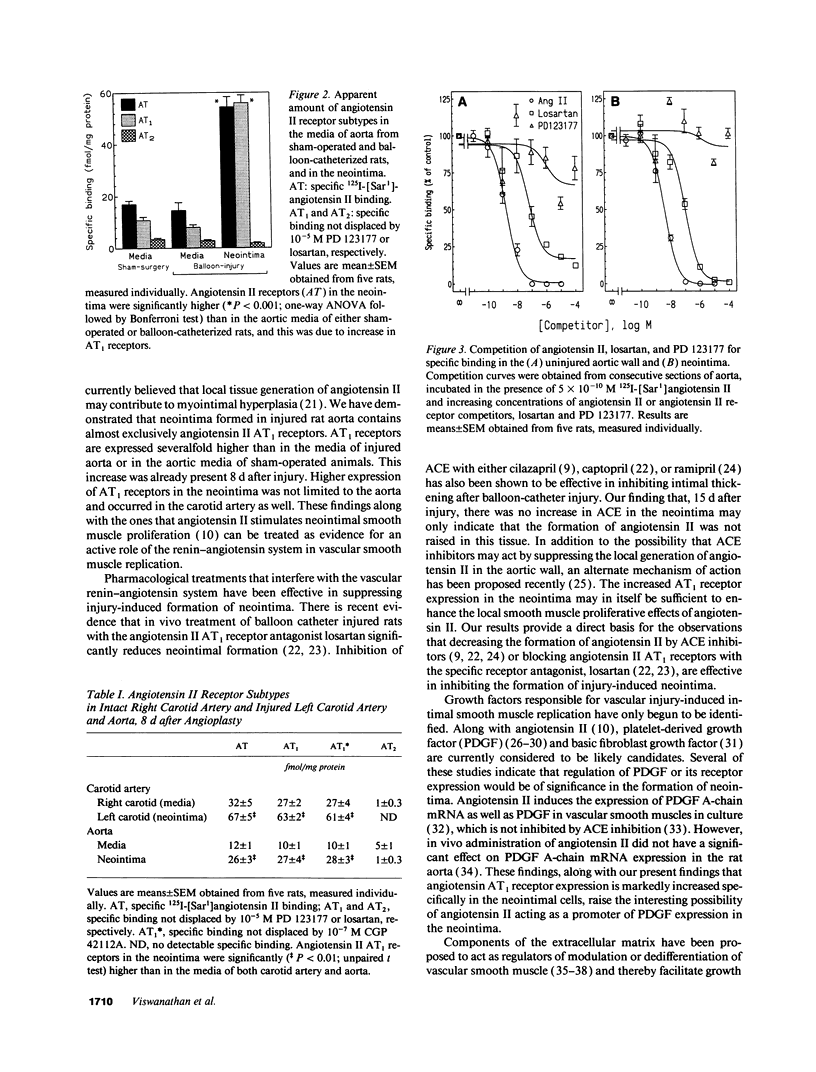
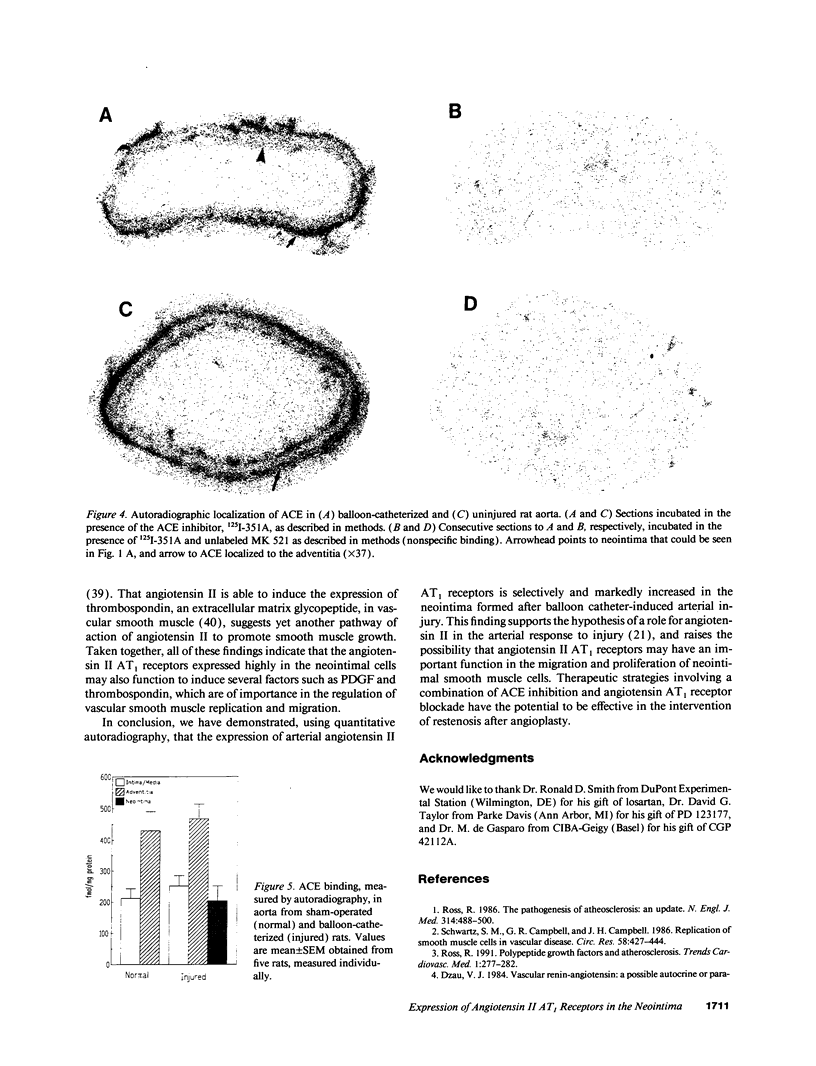
Angiotensin II is a vasoactive peptide and may act as a growth factor in vascular smooth muscle cells. Experimental injury of the rat aorta causes rapid migration of medial smooth muscle cells and their proliferation resulting in the formation of neointima. We have examined, using quantitative autoradiography, the expression of angiotensin II receptor subtypes AT1 and AT2, and angiotensin-converting enzyme, in the neointima formed in the rat thoracic aorta 15 d after balloon-catheter injury. In contrast to the normal aortic wall, which contained both AT1 and AT2 receptors (80% and 20%, respectively), neointimal cells expressed almost exclusively angiotensin II AT1 receptors. The apparent number of these receptors was fourfold higher in the neointima compared to that in the normal aortic wall. The affinities of the neointimal receptors to angiotensin II or to the AT1 receptor antagonist, losartan, were not different from those in the normal aortic wall. Angiotensin-converting enzyme binding in the neointima was not different from that in the media of the uninjured aorta. Our data suggest that angiotensin II AT1 receptors may have a significant role in injury-induced vascular smooth muscle proliferation and migration.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett T. B., Benditt E. P. Platelet-derived growth factor gene expression in human atherosclerotic plaques and normal artery wall. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2810–2814. doi: 10.1073/pnas.85.8.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumpus F. M., Catt K. J., Chiu A. T., DeGasparo M., Goodfriend T., Husain A., Peach M. J., Taylor D. G., Jr, Timmermans P. B. Nomenclature for angiotensin receptors. A report of the Nomenclature Committee of the Council for High Blood Pressure Research. Hypertension. 1991 May;17(5):720–721. doi: 10.1161/01.hyp.17.5.720. [DOI] [PubMed] [Google Scholar]
- Campbell D. J. Circulating and tissue angiotensin systems. J Clin Invest. 1987 Jan;79(1):1–6. doi: 10.1172/JCI112768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capron L., Heudes D., Chajara A., Bruneval P. Effect of ramipril, an inhibitor of angiotensin converting enzyme, on the response of rat thoracic aorta to injury with a balloon catheter. J Cardiovasc Pharmacol. 1991 Aug;18(2):207–211. doi: 10.1097/00005344-199108000-00005. [DOI] [PubMed] [Google Scholar]
- Chang R. S., Lotti V. J. Angiotensin receptor subtypes in rat, rabbit and monkey tissues: relative distribution and species dependency. Life Sci. 1991;49(20):1485–1490. doi: 10.1016/0024-3205(91)90048-g. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Correa F. M., Plunkett L. M., Saavedra J. M. Quantitative distribution of angiotensin-converting enzyme (kininase II) in discrete areas of the rat brain by autoradiography with computerized microdensitometry. Brain Res. 1986 Jun 11;375(2):259–266. doi: 10.1016/0006-8993(86)90746-8. [DOI] [PubMed] [Google Scholar]
- Daemen M. J., Lombardi D. M., Bosman F. T., Schwartz S. M. Angiotensin II induces smooth muscle cell proliferation in the normal and injured rat arterial wall. Circ Res. 1991 Feb;68(2):450–456. doi: 10.1161/01.res.68.2.450. [DOI] [PubMed] [Google Scholar]
- Dudley D. T., Panek R. L., Major T. C., Lu G. H., Bruns R. F., Klinkefus B. A., Hodges J. C., Weishaar R. E. Subclasses of angiotensin II binding sites and their functional significance. Mol Pharmacol. 1990 Sep;38(3):370–377. [PubMed] [Google Scholar]
- Dzau V. J. Significance of the vascular renin-angiotensin pathway. Hypertension. 1986 Jul;8(7):553–559. doi: 10.1161/01.hyp.8.7.553. [DOI] [PubMed] [Google Scholar]
- Dzau V. J. Vascular renin-angiotensin: a possible autocrine or paracrine system in control of vascular function. J Cardiovasc Pharmacol. 1984;6 (Suppl 2):S377–S382. [PubMed] [Google Scholar]
- Fager G., Hansson G. K., Gown A. M., Larson D. M., Skalli O., Bondjers G. Human arterial smooth muscle cells in culture: inverse relationship between proliferation and expression of contractile proteins. In Vitro Cell Dev Biol. 1989 Jun;25(6):511–520. doi: 10.1007/BF02623563. [DOI] [PubMed] [Google Scholar]
- Farhy R. D., Ho K. L., Carretero O. A., Scicli A. G. Kinins mediate the antiproliferative effect of ramipril in rat carotid artery. Biochem Biophys Res Commun. 1992 Jan 15;182(1):283–288. doi: 10.1016/s0006-291x(05)80142-1. [DOI] [PubMed] [Google Scholar]
- Ferns G. A., Raines E. W., Sprugel K. H., Motani A. S., Reidy M. A., Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991 Sep 6;253(5024):1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Kocher O., Bloom W. S., Vandekerckhove J., Weber K. Actin expression in smooth muscle cells of rat aortic intimal thickening, human atheromatous plaque, and cultured rat aortic media. J Clin Invest. 1984 Jan;73(1):148–152. doi: 10.1172/JCI111185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden M. A., Au Y. P., Kirkman T. R., Wilcox J. N., Raines E. W., Ross R., Clowes A. W. Platelet-derived growth factor activity and mRNA expression in healing vascular grafts in baboons. Association in vivo of platelet-derived growth factor mRNA and protein with cellular proliferation. J Clin Invest. 1991 Feb;87(2):406–414. doi: 10.1172/JCI115011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haudenschild C. C., Schwartz S. M. Endothelial regeneration. II. Restitution of endothelial continuity. Lab Invest. 1979 Nov;41(5):407–418. [PubMed] [Google Scholar]
- Kauffman R. F., Bean J. S., Zimmerman K. M., Brown R. F., Steinberg M. I. Losartan, a nonpeptide angiotensin II (Ang II) receptor antagonist, inhibits neointima formation following balloon injury to rat carotid arteries. Life Sci. 1991;49(25):PL223–PL228. doi: 10.1016/0024-3205(91)90298-p. [DOI] [PubMed] [Google Scholar]
- Kocher O., Skalli O., Cerutti D., Gabbiani F., Gabbiani G. Cytoskeletal features of rat aortic cells during development. An electron microscopic, immunohistochemical, and biochemical study. Circ Res. 1985 Jun;56(6):829–838. doi: 10.1161/01.res.56.6.829. [DOI] [PubMed] [Google Scholar]
- Libby P., Warner S. J., Salomon R. N., Birinyi L. K. Production of platelet-derived growth factor-like mitogen by smooth-muscle cells from human atheroma. N Engl J Med. 1988 Jun 9;318(23):1493–1498. doi: 10.1056/NEJM198806093182303. [DOI] [PubMed] [Google Scholar]
- Lindner V., Lappi D. A., Baird A., Majack R. A., Reidy M. A. Role of basic fibroblast growth factor in vascular lesion formation. Circ Res. 1991 Jan;68(1):106–113. doi: 10.1161/01.res.68.1.106. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Daemen M. J., Schwartz S. M. Alpha 1-adrenergic stimulation of platelet-derived growth factor A-chain gene expression in rat aorta. J Biol Chem. 1990 Jan 15;265(2):1082–1088. [PubMed] [Google Scholar]
- Majesky M. W., Reidy M. A., Bowen-Pope D. F., Hart C. E., Wilcox J. N., Schwartz S. M. PDGF ligand and receptor gene expression during repair of arterial injury. J Cell Biol. 1990 Nov;111(5 Pt 1):2149–2158. doi: 10.1083/jcb.111.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftilan A. J., Pratt R. E., Dzau V. J. Induction of platelet-derived growth factor A-chain and c-myc gene expressions by angiotensin II in cultured rat vascular smooth muscle cells. J Clin Invest. 1989 Apr;83(4):1419–1424. doi: 10.1172/JCI114032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarali A. J., Gutkind J. S., Saavedra J. M. Calibration of 125I-polymer standards with 125I-brain paste standards for use in quantitative receptor autoradiography. J Neurosci Methods. 1989 Dec;30(3):247–253. doi: 10.1016/0165-0270(89)90135-0. [DOI] [PubMed] [Google Scholar]
- Powell J. S., Clozel J. P., Müller R. K., Kuhn H., Hefti F., Hosang M., Baumgartner H. R. Inhibitors of angiotensin-converting enzyme prevent myointimal proliferation after vascular injury. Science. 1989 Jul 14;245(4914):186–188. doi: 10.1126/science.2526370. [DOI] [PubMed] [Google Scholar]
- Powell J. S., Müller R. K., Rouge M., Kuhn H., Hefti F., Baumgartner H. R. The proliferative response to vascular injury is suppressed by angiotensin-converting enzyme inhibition. J Cardiovasc Pharmacol. 1990;16 (Suppl 4):S42–S49. doi: 10.1097/00005344-199016004-00010. [DOI] [PubMed] [Google Scholar]
- Reidy M. A. A reassessment of endothelial injury and arterial lesion formation. Lab Invest. 1985 Nov;53(5):513–520. [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Schelling P., Fischer H., Ganten D. Angiotensin and cell growth: a link to cardiovascular hypertrophy? J Hypertens. 1991 Jan;9(1):3–15. [PubMed] [Google Scholar]
- Schwartz S. M., Campbell G. R., Campbell J. H. Replication of smooth muscle cells in vascular disease. Circ Res. 1986 Apr;58(4):427–444. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- Scott-Burden T., Bühler F. R. Regulation of smooth muscle proliferative phenotype by heparinoid--matrix interactions. Trends Pharmacol Sci. 1988 Mar;9(3):94–98. doi: 10.1016/0165-6147(88)90175-7. [DOI] [PubMed] [Google Scholar]
- Scott-Burden T., Resink T. J., Hahn A. W., Bühler F. R. Induction of thrombospondin expression in vascular smooth muscle cells by angiotensin II. J Cardiovasc Pharmacol. 1990;16 (Suppl 7):S17–S20. [PubMed] [Google Scholar]
- Viswanathan M., Tsutsumi K., Correa F. M., Saavedra J. M. Changes in expression of angiotensin receptor subtypes in the rat aorta during development. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1361–1367. doi: 10.1016/0006-291x(91)91723-p. [DOI] [PubMed] [Google Scholar]
- Wilcox J. N., Smith K. M., Williams L. T., Schwartz S. M., Gordon D. Platelet-derived growth factor mRNA detection in human atherosclerotic plaques by in situ hybridization. J Clin Invest. 1988 Sep;82(3):1134–1143. doi: 10.1172/JCI113671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. C., Hart S. D., Zaspel A. M., Chiu A. T., Ardecky R. J., Smith R. D., Timmermans P. B. Functional studies of nonpeptide angiotensin II receptor subtype-specific ligands: DuP 753 (AII-1) and PD123177 (AII-2). J Pharmacol Exp Ther. 1990 Nov;255(2):584–592. [PubMed] [Google Scholar]
- Wong P. C., Reilly T. M., Timmermans P. B. Effect of a monoclonal antibody to angiotensin II on hemodynamic responses to noradrenergic stimulation in pithed rats. Hypertension. 1989 Nov;14(5):488–497. doi: 10.1161/01.hyp.14.5.488. [DOI] [PubMed] [Google Scholar]