Background: Basophils play a great role in the induction of Th2 cell responses in different disease contexts.
Results: Basophils highly expressed OX40 ligand (OX40L) after activation. Adoptive transfer of activated basophils triggered a robust Th2 response and airway inflammation.
Conclusion: Basophils primed Th2 responses via OX40-OX40L interaction in asthma.
Significance: OX40L on basophils may be a novel therapeutic target in asthma.
Keywords: asthma, immunology, inflammation, lung, T helper cells, Basophils, OX40-OX40L, Th2 response
Abstract
Asthma is characterized by increased airway submucosal infiltration of T helper (Th) cells and myeloid cells that co-conspire to sustain a chronic inflammation. While recent studies have demonstrated that the myeloid basophils promote Th2 cells in response to various types of allergens, the underlying mechanisms are poorly understood. Here, we found for the first time that in a mouse model of allergic asthma basophils highly expressed OX40 ligand (OX40L) after activation. Interestingly, blockade of OX40-OX40L interaction suppressed basophils-primed Th2 cell differentiation in vitro and ameliorated ovalbumin (OVA)-induced allergic eosinophilic inflammation mediated by Th2 activation. In accordance, the adoptive transfer of basophils derived from mediastinal lymph nodes (MLN) of OVA-immunized mice triggered a robust Th2 response and eosinophilic inflammation in wild-type mice but largely muted in OX40−/− mice and mice receiving OX40L-blocked basophils. Taken together, our results reveal a critical role of OX40L presented by the activated basophils to initiate Th2 responses in an allergic asthma model, implicating OX40-OX40L signaling as a potential therapeutic target in the treatment of allergic airway inflammation.
Introduction
Asthma is a chronic disease that has seen its prevalence rise worldwide due to deteriorating environmental conditions. This has cost a heavy burden on patients and societies alike. Despite the uses of inhaled corticosteroids and long-acting beta agonists that focus on effective symptomatic relief, there is little advancement in disease modifying therapies that target the underlying immune mechanisms. Pathophysiologically, it is increasingly recognized that asthma is characterized by the increased airway submucosal infiltration of not only the previously identified culprits, CD4+ T helper (Th)3 cells and eosinophils but also the mast cells and basophils, which together causes mucus hypersecretion, intermittent airway hyperresponsiveness and airway remodeling (1, 2). Accumulative studies suggest that an aberrant Th2 immune response, traditionally defined by the overproduction of IL-4, IL-5, and IL-13, plays a central role in the pathogenesis of asthma (3, 4). Therefore, clarification of the mechanism underlying the initiation of Th2 responses is crucial to anti-asthmatic therapies based on key molecules targeting.
Basophils, which are cells derived from basophil/mast cell precursor cells in the bone marrow, comprise less than 1% of the blood leukocytes and share some characteristics with mast cells phenotypically and functionally, such that they both can act as important effector cells in allergic airway inflammation (5, 6). Numerous studies have demonstrated that basophils not only play crucial roles in the effector phase, but also critically participate in the initiation of Th2-centered immune responses (7–11). Using an ovalbumin (OVA)-induced allergic airway inflammation mouse model, we previously showed that basophils acted as a primary inducer of the Th2 immunity, evident by increased OVA-specific immunoglobulin E (OVA-sIgE) and IL-4 levels both in serum and bronchial alveolar lavage fluid (BALF), and increased proportion of Th2 cells in the spleen after transfusion of lung basophils derived from OVA-challenged mice into wild-type (WT) mice (12). We also found that pulmonary basophils were capable of antigen uptake, expressed an array of molecular markers such as CD40, CD80/86, and MHC II and released high levels of IL-4 following OVA sensitization and challenge. Taken together, these observations support the notion that basophils possess the activity to initiate Th2 cell responses (7–9).
However, the underlying mechanisms entitling basophils as the key initiator in the Th2 immune responses remain to be elucidated. The antigen recognition by TCR, ligation of co-stimulatory molecules and corresponding cytokines such as IL-4 for Th2 cells are crucial for the induction of naïve T cells to effector Th2 cells. Recent studies suggest that OX40 (CD134)-OX40 ligand (OX40L, CD252) interaction contributes greatly to the differentiation of some Th subsets, such as Th9 cells, Th2 cells, and Tregs (13–15). OX40, a member of tumor necrosis factor receptor (TNFR) superfamily, is mainly expressed on activated T cells, including CD4+ T cells and CD8+ T cells (16, 17). OX40L, which belongs to the TNF superfamily, is likely to be induced on activated professional antigen-presenting cells (APCs), such as B cells, macrophages, and mature conventional dendritic cells (DCs) (18–20). In addition, non-APCs such as NK cells, smooth muscle cells, and monocytes can also express OX40L in inflammatory diseases (21–23). Notably, OX40-OX40L interaction is bi-directional and thus plays a potential role in regulation of not only T cell function but also APC activation and maturation. OX40 signaling promotes T cell proliferation and survival, while OX40L signaling favors maturation and cytokine production of APCs (16, 24). Furthermore, OX40-OX40L interaction is crucial for optimal T cell activation through the NF-κB pathway, especially in CD4+ T cells (25). Interestingly, in the context of atopic dermatitis in mice, OX40-OX40L signaling pathway has a critical role in Th2 priming (14). Nevertheless, a key issue remains unaddressed regarding whether OX40-OX40L interaction is also involved in the Th2 initiation when being primed by basophils in asthma.
Data from animal and human studies have confirmed a critical role of OX40-OX40L interaction in allergic airway inflammation. Studies have showed an increased expression of OX40 and OX40L on the immune cells in the airway submucosa of patients with mild asthma, and their expression was related to the level of IL-4 and the number of eosinophils in the lung (26). In a murine model, OX40−/− mice sensitized and challenged with OVA showed significant alleviation of inflammation, characterized by reduced levels of serum IgE and inflammatory cytokines, as well as a decreased number of eosinophils in lungs compared with that in WT mice (27, 28). In this study, we aim to further determine if basophils play a primary role in inducing naïve T cells to differentiate into Th2 cells and promoting Th2 responses and eosinophilia via OX40-OX40L interaction in a murine model of OVA-induced allergic eosinophilic airway inflammation.
EXPERIMENTAL PROCEDURES
Mice
OX40−/− mice (C57BL/6 genetic background) and DO11.10 mice (BALB/c genetic background) were purchased from the Jackson Laboratory (Bar Harbor, ME) and Model Animal Research Center of Nanjing University, respectively. WT C57BL/6 mice and BALB/c mice (Shanghai Laboratory Animal Co., Ltd., China) were used. CD40−/− mice (BALB/c genetic background) were provided by Professor David Hinrichs at the Portland VA Medical Center. 6–8-week-old mice were used for all experiments and maintained in the specified-pathogen-free (SPF) facilities in the Research Center for Experimental Medicine of Ruijin Hospital affiliated with Shanghai Jiao Tong University School of Medicine. All animal procedures were approved by Ruijin Hospital Animal Ethics Committee.
Cell Culture, Isolation, and Identification of Bone Marrow-derived Basophils (BM-Bas)
To obtain BM-Bas, bone marrow cells were cultured at a density of 2 × 106 cells per 1 ml for 10 days in the presence of 15 ng/ml recombinant mouse IL-3 (rIL-3, R&D Systems) in RPMI 1640 medium supplemented with 10% (v/v) fetal bovine serum (FBS), 100 units/ml penicillin, and streptomycin, 55 μmol/liter β-mercaptoethanol (β-ME), 2 mmol/liter of l-glutamine, and 0.1 mmol/liter non-essential amino acids (RPMI 1640 complete medium) (9, 29). On days 4 and 7, half of the medium was replaced by fresh medium. On day 10, the cells were collected, washed twice, stained with PE/Cy7-conjugated anti-mouse FcϵRIα (clone MAR-1), FITC-conjugated anti-mouse CD49b (pan-NK cells, clone DX5), Alexa Fluor® 700-conjugated anti-mouse CD11c (clone N418) and APC-conjugated anti-mouse CD117 (c-Kit, clone 2B8) (Biolegend) and then sorted by fluorescence-activated cell sorter (FACS) in accordance with negative staining for CD117 and CD11c and positive staining for FcϵRIα and CD49b as well as small FSC and SSC within the lymphocyte gate. Sorted FSClow SSC low CD117− CD11c− CD49b+ FcϵRI+ BM-Bas cells were stained with propidium iodide (PI) and assessed cell viability by FACS. To characterize the sorted BM-Bas, cytospins were made. A part of them were stained with Wright-Giemsa and the others were fixed with 4% (v/v) paraformaldehyde, then permeated with 0.1% (v/v) Triton 100 and blocked with 10% (w/v) bovine serum albumin, stained with purified anti-mMCP-8 antibody (Ab) (clone TUG8, Biolegend) and FITC-conjugated rabbit polyclonal secondary Ab to rat, and counterstained with DAPI (4,6-diamidino-2-phenylindole) (30, 31).
Th2 Cell Differentiation in Vitro
Naïve CD4+ T cells (CD3+ CD4+ CD62L+ CD44low) from spleens were isolated via a negative selection principle by MagCellect (R&D Systems) following the manufacturer's instructions. For the initiation of Th2 cells, BM-Bas (2.5 × 105 per well) and naïve CD4+ T cells (5 × 105 per well) were seeded in 96-well plates in RPMI 1640 complete medium in the presence of rIL-2 (20 U/ml, R&D Systems), rIL-3 (30 ng/ml), DNP-OVA (100 μg/ml, Biosearch Technologies, Novato) and anti-DNP IgE (10 μg/ml, Sigma-Aldrich) for 5 days (7, 12). For some experiments, anti-mouse CD252 (20 μg/ml, OX40L blocking Ab, αOX40L, eBioscience) was added to the cultures. On day 3, fresh RPMI 1640 complete medium with rIL-2 (20 units/ml) was added. On day 5, the cells were re-stimulated using PMA (50 ng/ml, Sigma-Aldrich) and ionomycin (1 μg/ml, Sigma-Aldrich) for 6 h. At the last 2 h, Brefeldin A solution (1:1000, eBioscience) was treated. The cells were collected, stained and subjected to FACScan Flow Analyzer to analyze Th1/Th2 subsets. The levels of IL-4, IL-5, and IL-13 in the supernatant were determined by ELISA.
Western Blot Analysis
BM-Bas were prepared and washed twice with phosphate-buffered saline (PBS), and solubilized at ice-cold RIPA buffer (Beyotime) supplemented with phenylmethanesulfonyl fluoride (PMSF). The cell lysates were boiled in 1×SDS loading buffer, diluted with 1×SDS loading buffer to 2 μg/μl, electrophoresed on a sodium dodecyl sulfate-polyacrylamide gel and blotted. Membranes were blocked with 5% skim milk for 1 h, incubated with OX40L Ab (M-20, Santa Cruz Biotechnology) and β-actin Ab (Cell Signal Technology) overnight, then incubated with the corresponding horseradish peroxidase-conjugated second Ab for 1 h. To get chromogenic detection data, the blots were exposed to gel imaging system (Tanon 5500) after adding Thermo ECL substrate solution (32).
Flow Cytometry
For surface marker staining, cells were washed twice with PBS and further incubated for 30 min at 4 °C with specified FITC-, PE-, Alexa Fluor® 700-, and APC-conjugated Abs. For analysis of intracellular markers, cells were stained with PE-Cyanine7-conjugated anti-mouse CD4 (clone GK1.5, eBioscience), fixed with IC fixation buffer, permeabilized using permeabilization buffer and stained with specified Abs according to the manufacturer's instructions. The samples were subjected to FACScan Flow Analyzer. The BALF cells were stained with PE-conjugated CD49d (clone 9C10, Biolegend) and then subjected to BD Accuri C6 Flow Cytometer (33).
ELISA
Cell-culture supernatants, BALF, serum and mediastinal lymph nodes (MLN) lysate supernatants were prepared as described previously (12). The levels of IL-4, IL-5, and IL-13 were measured with sandwich ELISA kits (eBioscience) according to the manufacturer's instructions. For the measurement of serum OVA-sIgE, standard mouse OVA-specific IgE and anti-mouse IgE (Serotec, U.K.) were used as described previously (12).
Adoptive Transfer of Basophils
Female C57BL/6 mice were sensitized intraperitoneal with OVA (100 μg/mouse) complexed with aluminum hydroxide (Al(OH)3) on days 0 and 14 and subsequently challenged i.n. with OVA in PBS (100 μg/mouse) under isoflurane inhalation anesthesia on day 14. The mice here were referred to as immunized mice (34). On day 16 (48 h after challenge), the MLN cells were collected for isolation of basophils. A part of basophils were treated with anti-mouse CD252 (20 μg/ml) or an isotype Ab overnight before adoptive transfer in vitro.
2.5 × 105 purified MLN basophils from immunized mice were transferred twice into recipient mice (WT C57BL/6 mice or OX40−/− mice) at 14-day intervals (day 0 and 14) according to the protocols as described previously (7, 12). Recipient mice were challenged i.n. with OVA in PBS (100 μg/mouse) on days 14, 25, 26, and 27 under isoflurane inhalation anesthesia. Control mice were sensitized and challenged with PBS. Mice were sacrificed on day 19 and day 28. Blood was collected for serum preparation and BALF was performed with 3 × 0.4 ml ice-cold PBS. The BALF was centrifuged at 453 g for 5 min, and the cells were collected and stained with Ab for CD49d for detection of eosinophils by flow cytometry (33). The supernatant was kept for cytokine detection by ELISA. The lower right lobe of the lung was collected and fixed in 4% (v/v) paraformaldehyde for hematoxylin and eosin (H&E) and periodic acid Schiff (PAS) staining. MLN cells were seeded at a density of 2 × 106/ml and re-stimulated for 5 days in the presence of OVA (0.5 mg/ml). On day 5, the cells were collected, stained, and subjected to FACScan Flow Analyzer to analyze Th1/Th2 subsets.
Statistical Analysis
Flow cytometric analysis data were analyzed using Flow Jo software (Tree Star) and BD Accuri C6 software. Data are described by mean and S.D. Statistical analyses were performed by Student's t test or ANOVA analysis using GraphPad Prism 6.
RESULTS
The Number of Basophils in MLN and OX40L Expression on These Cells in the Early Phase of OVA-Immunized Mice
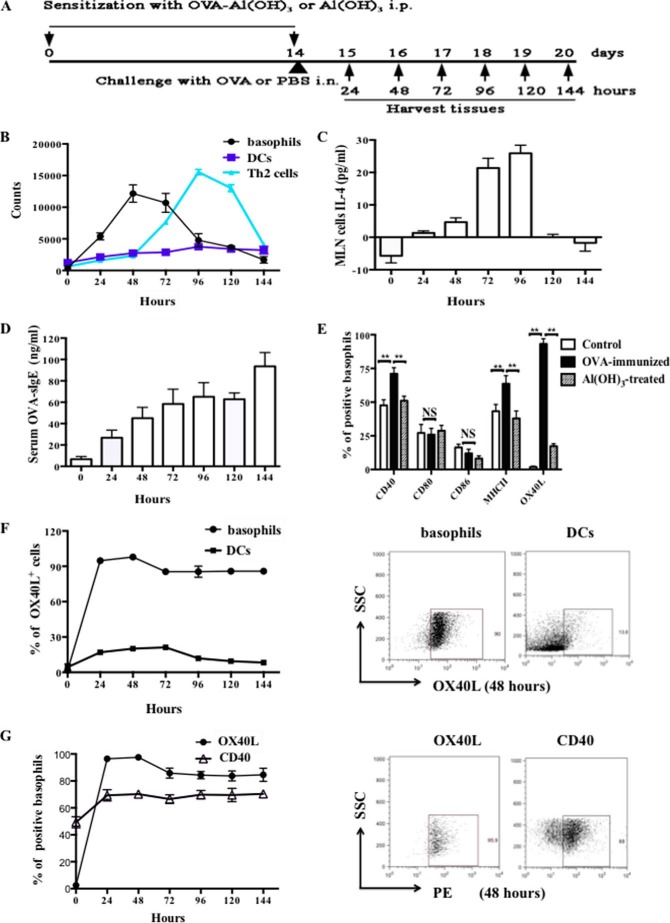
To confirm the role of basophils in the early phase of allergic airway inflammation, we examined the recruitment of basophils in MLN in OVA-immunized mice. WT C57BL/6 mice were sensitized by intraperitoneal injection of OVA in Al(OH)3 on days 0 and 14, followed by OVA challenge i.n. on day 14 (OVA-immunized group). Mice were sensitized by Al(OH)3 and challenged with PBS as Al(OH)3-treated group and untreated as control group, respectively. MLN were harvested at 24∼144 h after the challenge (Fig. 1A). Interestingly, we observed that basophils were recruited to the MLN within 24 h and the number increased robustly by 48 h after OVA challenge. Furthermore, the results showed that CD4+ IL-4+ T cells (Th2 cells) increased at 48 h, peaked at 96 h and sustained to 120 h after OVA challenge, whereas the number of MHC II+ CD11c+ DCs did not alter (Fig. 1B). These results demonstrated that the recruitment of basophils but not DCs to the MLN was earlier than that of Th2 cells. Consistent with these data, the level of IL-4 in MLN cells increased at 48 h and peaked at 72∼96 h after OVA challenge, thus confirming that IL-4 has key roles in modulating Th2 responses by basophils in OVA-immunized mice (Fig. 1C). Compared with our previous findings, serum OVA-sIgE level in early phase was a slight elevation, peaked to 93.54 ± 12.91 ng/ml at 144 h after OVA challenge on day 14 (Fig. 1D), which was significantly lower than that in asthmatic mice (2∼4 μg/ml).
FIGURE 1.
Basophils were recruited to MLN in the early phase of OVA-induced allergic inflammation and expressed high levels of OX40L. A, protocol of OVA-immunized mice and Al(OH)3-treated mice. Wild type (WT) C57BL/6 mice were sensitized by 100 μg of OVA complexed with Al(OH)3 or Al(OH)3 intraperitoneal on day 0 and 14 and challenged by 100 μg of OVA (OVA-immunized group) or PBS (Al(OH)3-treated group) i.n. on day 14, and untreated mice as control group. MLN were harvested at 24∼144 h after the challenge; B, kinetics of basophils, DCs, and Th2 cells in MLN from one mouse per time point. Dot at zero hour represents data from Al(OH)3-treated mice. The basophils were gated on FSClow SSClow CD117− CD11c− CD49b+ FcϵRI+ cells, and the DCs were gated on MHC II+ CD11c+ cells. Th2 cells were gated on CD4+ IL-4+ cells; C, MLN cells IL-4 levels in the OVA-immunized mice by ELISA. The bar graph at zero hour represents data from Al(OH)3-treated mice; D, serum OVA-specific IgE (OVA-sIgE) levels in the OVA-immunized mice by ELISA. The bar graph at zero hour represents data from Al(OH)3-treated mice; E, expression of co-stimulatory molecules on MLN basophils; F, left is the percentage of OX40L+ MLN basophils and OX40L+ MLN DCs. Dot at zero hour represents data from untreated mice. The right is the percentage of the cells from OVA-immunized mice at 48 h; G, left is the levels of OX40L and CD40 expression on MLN basophils from OVA-immunized mice. Dot at zero hour represents data from untreated mice. The right is the expression levels of OX40L and CD40 at 48 h; Results are presented as mean ± S.D. and are representative of three independent experiments. **, p < 0.01; NS, not significant.
It has been shown that mature APCs, including DCs, could express OX40L in the presence of factors leading to cell maturation, such as thymic stromal lymphopoietin (TSLP) (35). In this regard, we previously found that lung basophils from OVA-induced asthmatic mice expressed higher levels of co-stimulatory molecules such as CD40 and MHC II, compared with control mice (12). To identify the functional involvement of co-stimulatory molecules in the induction of Th2 immune responses by basophils, we examined the expression of CD40, CD80, CD86, MHC II, OX40L, or OX40 on MLN basophils at 48 h after OVA challenge. Interestingly, we found that among the array of co-stimulatory molecules, the level of OX40L expressed by basophils increased most remarkably after OVA stimulation, the percentage increased from 1.8 ± 0.3% in control group to 94.2 ± 2.1% in OVA-immunized mice group. However, OX40 level was not detectable (Fig. 1E). Furthermore, we analyzed the OX40L expression pattern on MLN basophils by flow cytometric analysis. As shown in Fig. 1F, virtually all basophils expressed high levels of OX40L at 48 h after OVA challenge and this tendency maintained for 144 h, whereas MHC II+ CD11c+ DCs expressed much lower OX40L. In addition, the level of CD40 expression on OVA-immunized basophils slightly increased compared with both Al(OH)3-treated basophils and control basophils, and maintained between 24 to 144 h. This kind of expression change of CD40 was much lower than that of OX40L (Fig. 1G). Collectively, these results indicated that OX40L on basophils might be the major player in early phase of asthma.
OX40L Expression on BM-Bas after Activation
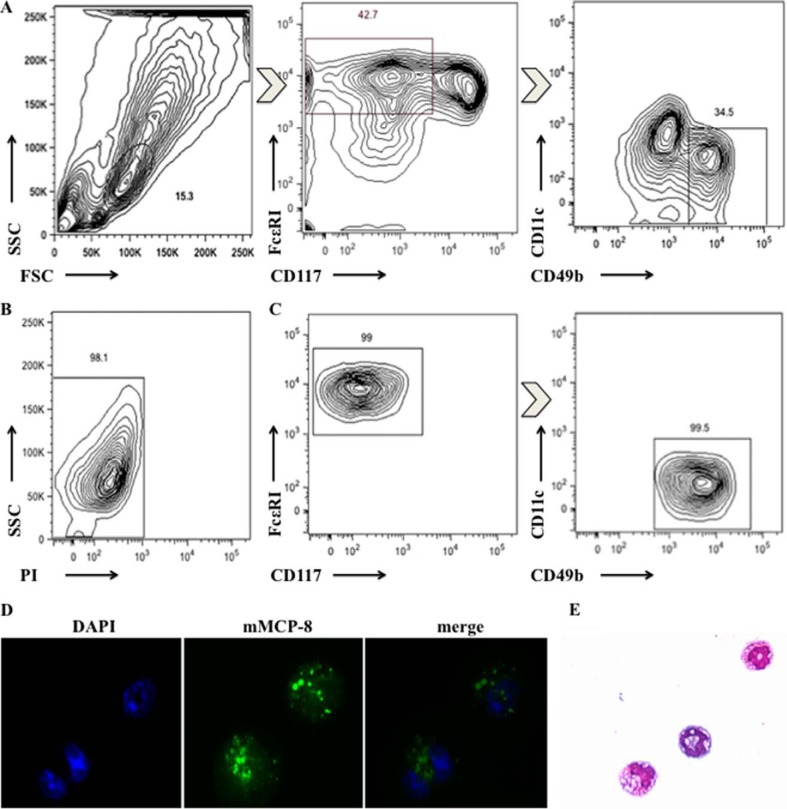
We further examined whether basophils derived from bone marrow in vitro expressed OX40L by flow cytometry. We cultured bone marrow cells for 10 days with rIL-3 (15 ng/ml) and enriched basophils by FACS according to FSClow SSClow CD117− CD11c− CD49b+ FcϵRI+ (Fig. 2A). The survival rate of BM-Bas was at least 98%, and the purity was greater than 98.5% (Fig. 2, B and C). In addition, to confirm that the highly enriched cells were indeed basophils, cytospins were stained with mMCP-8, which has been reported as a specific marker for basophils, and Wright-Giemsa staining, respectively (31, 36). Immunofluorescence analysis indicated that the population was ∼100% positive for mMCP-8 (Fig. 2D). Wright-Giemsa staining showed that these cells appeared morphologically small, were similar to lymphocytes in size, with lobed nucleus or ring-shaped nucleus (Fig. 2E). Collectively, these results provided evidence that the sorted FSClow SSClow CD117− CD11c− CD49b+ FcϵRI+ cells were highly pure and viable basophils.
FIGURE 2.
Characterization of sorted BM-Bas. A, gating strategy for sorting BM-Bas by fluorescence-activated cell sorter (FACS). FSClow SSClow CD117− CD11c− CD49b+ FcϵRI+ cells were gated; B, viability analysis on the sorted BM-Bas by FACS. Live cells (PI− cells) were gated; C, purity analysis on the sorted BM-Bas by FACS; D, immunofluorescence analysis of mMCP-8 expression on sorted BM-Bas (green fluorescence, ×1000); E, morphology of sorted BM-Bas (Wright-Giemsa staining, ×400). Data are representative of three independent experiments.
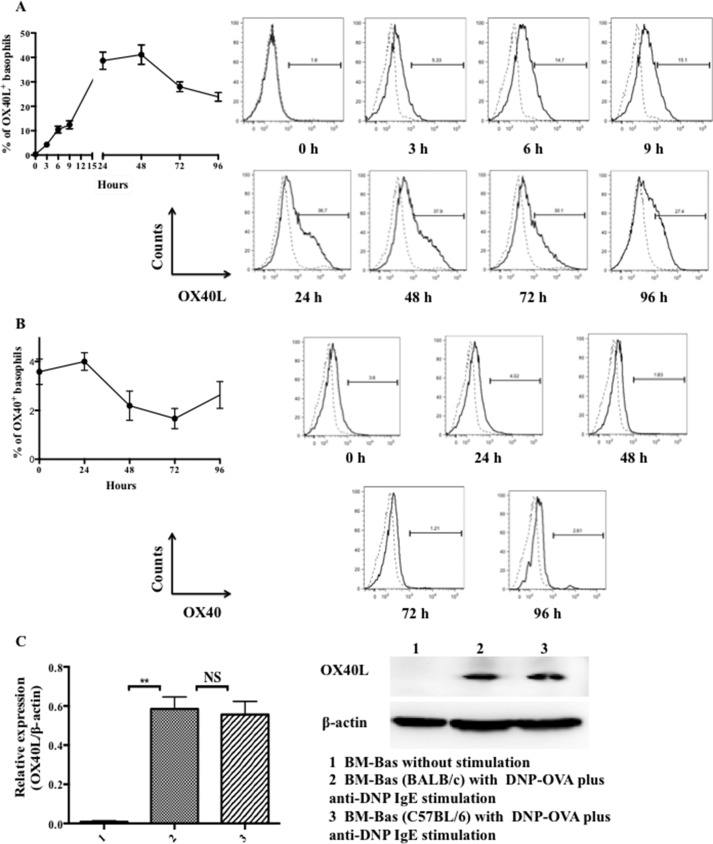
Purified BM-Bas were stimulated for 0∼96 h in the presence of DNP-OVA (100 μg/ml) plus anti-DNP IgE (10 μg/ml). As shown in Fig. 3, A and B stimulation with DNP-OVA plus anti-DNP IgE induced basophil expression of OX40L, but not OX40, in vitro. More importantly, we found for the first time that the OX40L expression was detected at as early as 3 h after activation by DNP-OVA plus anti-DNP IgE and peaked at 24∼48 h to a mean percentage of 41.1 ± 4.0% (Fig. 3A). In contrast, OX40 expression on basophils was not detected (with or without DNP-OVA plus anti-DNP IgE stimulation) (Fig. 3B). We further examined whether the genetic background of mice affected the kinetics of OX40L expression. The results showed that basophils from BALB/c and C57BL/6 mice exhibited similar OX40L expression pattern (data not shown). Furthermore, the OX40L level in basophils was determined by Western blot analysis in different states and different gene backgrounds. As shown in Fig. 3C, BM-Bas stimulated with DNP-OVA plus anti-DNP IgE for 48 h substantially expressed OX40L, but not in non-stimulated BM-Bas. The protein was about 28∼30 kDa, which was consistent with previous reports (37). Taken together, these data indicated that BM-Bas express OX40L in an inducible manner.
FIGURE 3.
BM-Bas express OX40L but not OX40 after activation. 5 × 105 sorted BM-Bas (from C57BL/6 mice) were cultured in vitro in the presence of DNP-OVA (100 μg/ml) plus anti-DNP IgE (10 μg/ml) for 0∼96 h. Expression of OX40L and OX40 were determined by flow cytometric analysis. Solid line and dashed line represent staining with OX40L (A) or OX40 (B) and isotype-control IgG, respectively; C, OX40L protein levels on BM-Bas with or without DNP-OVA plus anti-DNP IgE stimulation by Western blot analysis. Histograms show densitometry analysis of OX40L protein after normalization with β-actin. Results are presented as mean ± S.D. and are representative of three independent experiments. **, p < 0.01; NS, not significant.
Blocking OX40-OX40L Interaction with Anti-OX40L Ab Remarkably Attenuates Allergic Airway Inflammation
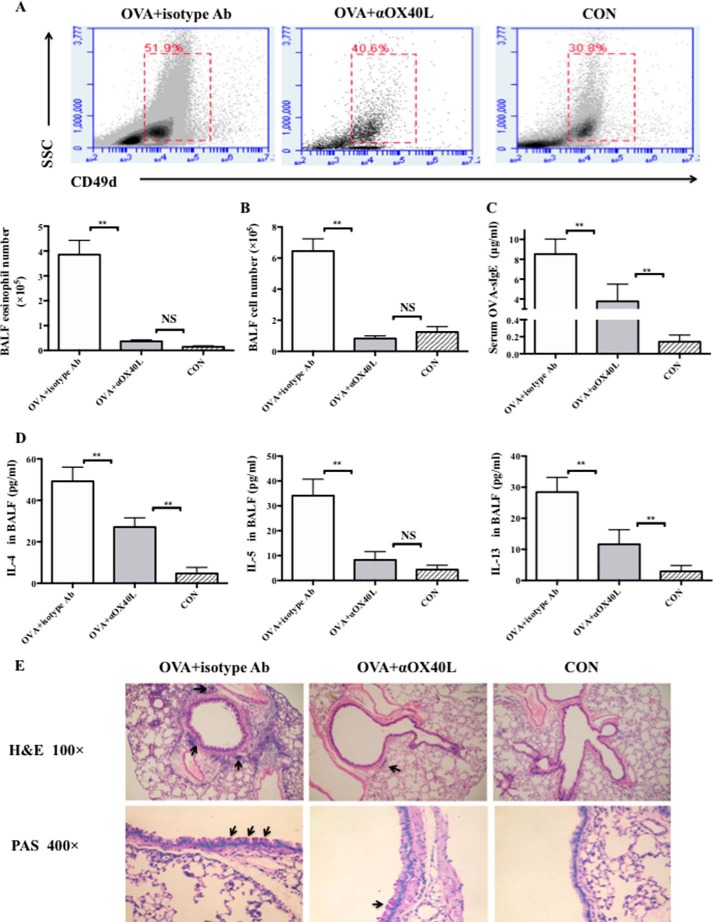
To test the role of OX40-OX40L interaction in the early phase of allergic airway inflammation, asthmatic WT C57BL/6 mice were sensitized by 100 μg of OVA complexed with Al(OH)3 intraperitoneal on days 0 and 14 and challenged by 100 μg OVA i.n. on days 14, 25, 26, and 27. In the early sensitization period on days 0, 4, 8, and 12, half of the mice were treated by 100 μg of OX40L blocking Ab (anti-mouse CD252, αOX40L) via tail vein injection to block OX40-OX40L interaction (OVA+αOX40L group). The others were given control isotype Ab (OVA+isotype Ab group). The control mice (CON group) were sensitized by Al(OH)3 on days 0 and 14 and challenged by PBS i.n. on days 14, 25, 26, and 27. Mice were sacrificed 24 h after the last challenge. Compared with OVA+isotype Ab group mice, inflammation in mice injected with αOX40L Ab was remarkably less severe, as characterized by a significant reduction in eosinophil and total cell counts in BALF (Fig. 4A and B). Since Th2-type cytokines, such as IL-4, IL-5, and IL-13, play crucial roles in the pathology of allergic asthma, we examined their levels and thus found that the levels of IL-4, IL-5, and IL-13 in BALF and OVA-sIgE in serum were decreased in OVA+αOX40L group compared with those in OVA+isotype Ab group (Fig. 4, C and D). Meanwhile, the H&E and PAS staining showed that OX40L blockade markedly reduced eosinophil infiltration in lungs and suppressed mucus formation as well (Fig. 4E).
FIGURE 4.
OX40-OX40L interaction played an important role in the early phase of OVA-induced allergic airway inflammation. WT C57BL/6 mice were used. Asthma mice were sensitized by 100 μg of OVA complexed with Al(OH)3 intraperitoneal on days 0 and 14 and challenged by 100 μg of OVA i.n. on days 14, 25, 26, and 27. Half of mice were treated with 100 μg of αOX40L via tail vein to block OX40-OX40L interaction (OVA+αOX40L group, n≥3), and the others were given control isotype Ab (OVA+isotype Ab group, n≥3) on days 0, 4, 8, and 12, respectively. The control mice (CON group, n≥3) were sensitized by Al(OH)3 on days 0 and 14 and challenged by PBS i.n. on days 14, 25, 26, and 27. Mice were sacrificed 24 h after the last challenge. A, flow cytometric analysis of eosinophils in BALF. CD49d+ SSCint eosinophils were gated; B, total cell numbers of BALF assessed by flow cytometric analysis; C, level of OVA-sIgE in serum was determined by ELISA; D, levels of IL-4, IL-5, and IL-13 of BALF were determined by ELISA; E, H&E and PAS staining of lung tissues from mice. Results are presented as mean ± S.D. and are representative of three independent experiments. **, p < 0.01; NS, not significant.
The Effects of OX40-OX40L Interaction on Th2 Cell Differentiation Primed by Basophils in Vitro
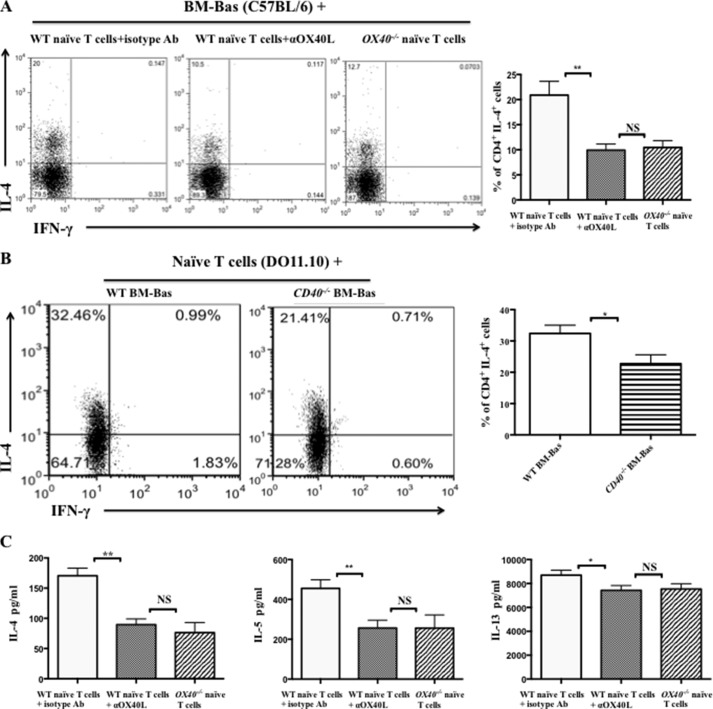
The mechanisms by which basophils induce naïve T cells to differentiate into Th2 cells were poorly understood. The data obtained above indicated that OX40L highly expressed on the basophils might bind OX40 to induce Th2 cell differentiation. To further examine this possibility, BM-Bas from B6 WT mice and B6 WT mice or OX40−/− mice splenic naïve T cells were co-cultured in the absence or presence of αOX40L with the stimulation of DNP-OVA plus anti-DNP IgE. The results showed that the percentage of CD4+ IL-4+ Th2 cells was significantly decreased in both OX40L blockade and OX40−/− naïve T cell cultures, from 20.9 ± 2.7% to 9.9 ± 1.2% and 10.4 ± 1.3% (Fig. 5A), respectively. In the present study, we also found that the expression of CD40 on basophils from OVA-immunized mice slightly increased (Fig. 1, E and G). Thus, we further assessed whether the CD40-CD40L interaction was involved in the Th2 cell differentiation primed by basophils. Naïve T cells from DO11.10 mice and BM-Bas from WT BALB/c mice or CD40−/− mice were co-cultured. The results showed that the percentage of CD4+ IL-4+ Th2 cells in CD40−/− basophil cultures was decreased, but less than that in OX40-OX40L blockade cultures (Fig. 5B). Furthermore, ELISA assay revealed that in the supernatant, the levels of IL-4 and IL-5 were significantly reduced, but IL-13 level was slightly decreased after blocking OX40-OX40L interaction (Fig. 5C).
FIGURE 5.
Blocking OX40-OX40L interaction reduced Th2 cell differentiation induced by basophils in vitro. A, flow cytometric analysis of Th1/Th2 subsets in co-culture of splenic naïve T cells (5 × 105/well) from B6 WT mice or OX40−/− mice (with C57BL/6 genetic background) and BM-Bas (2.5 × 105/well) from B6 WT mice following 5 days of treatment with DNP-OVA (100 μg/ml) plus anti-DNP IgE (10 μg/ml), rIL-2 (20 units/ml), and rIL-3 (30 ng/ml) in the absence or presence of αOX40L (20 μg/ml). Cells were gated on CD4+ T cells. The percentage of Th2 cell (CD4+ IL-4+ cells) in the co-culture cells (right); B, flow cytometric analysis of Th1/Th2 subsets in co-culture of splenic naïve T cells (5 × 105/well) from DO11.10 mice and BM-Bas (2.5 × 105/well) from WT BALB/c mice or CD40−/− mice (with BALB/c genetic background) following 5 days of treatment with DNP-OVA (100 μg/ml) plus anti-DNP IgE (10 μg/ml), rIL-2 (20 units/ml), and rIL-3 (30 ng/ml). Cells were gated on CD4+ T cells. The percentage of Th2 cell (CD4+ IL-4+ cells) in the co-culture cells (right); C, ELISA analysis of IL-4, IL-5 and IL13 levels in the supernatants of co-cultured cells stimulated with PMA (50 ng/ml) plus ionomycin (1 μg/ml) for 6 h and Brefeldin A (1:1000) for the last 2 h. Results are presented as mean ± S.D. and are representative of three independent experiments. *, p < 0.05; **, p < 0.01; NS, not significant.
OX40-OX40L Interaction Is Involved in the Initiation of Th2 Immune Responses Primed by Basophils in Allergic Airway Inflammation
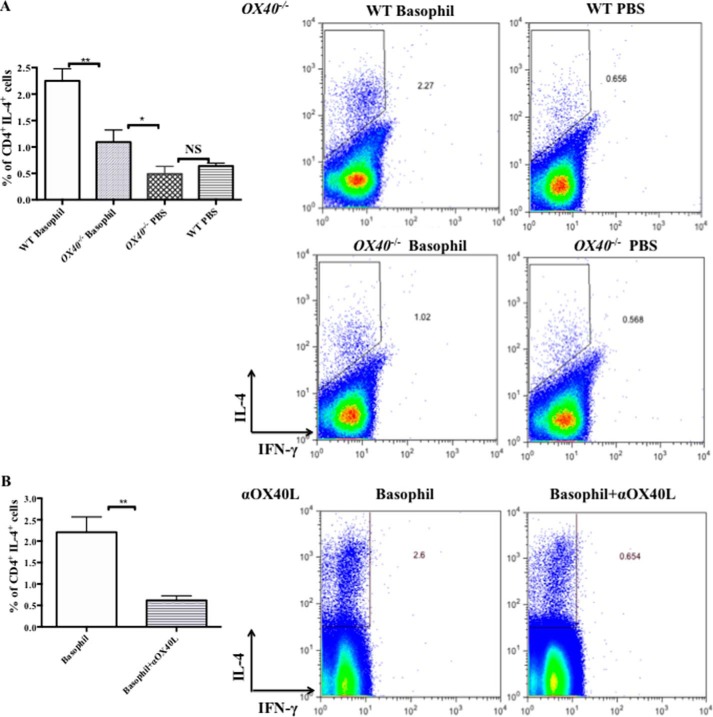
Recently, others and we have shown that basophils could act as a primary inducer of the Th2 immunity in a mouse model of OVA-induced allergic airway inflammation. Our results above suggested that basophils might initiate Th2 responses via OX40-OX40L interaction. Thus, we adoptive transferred 2.5 × 105 MLN basophils from immunized B6 mice into B6 WT mice and OX40−/− mice on days 0 and 14, and the control mice were given an administration of PBS (7, 12, 38). The method of MLN basophils purification was as same as that of BM-Bas (Fig. 2A). To assesse Th2 responses in recipients, MLN were harvested at 5 days after the second transfer and cultured for 5 days in vitro in the presence of OVA (0.5 mg/ml). As shown in Fig. 6A, the percentage of CD4+ IL-4+ Th2 cells in WT mice was as high as 2.5 fold of that in OX40−/− mice. In addition, we treated the sorted MLN basophils with αOX40L (Basophil+αOX40L group) or an isotype Ab (Basophil group) overnight before adoptive transfer into WT mice to prove the role of OX40L on basophil in Th2 responses. We found that the percentage of CD4+ IL-4+ Th2 cells in Basophil+αOX40L group was significantly decreased compared with that of isotype Ab treated basophil recipients, confirming that Th2 responses were inhibited when OX40-OX40L interaction was blocked (Fig. 6B).
FIGURE 6.
Blockade of OX40-OX40L interaction reduced Th2 responses initiated by adoptive transfer of MLN basophils. MLN FSClow SSClow CD117− CD11c− CD49b+ FcϵRI+ basophils (2.5 × 105 cells in 200 μl PBS) from OVA-immunized mice at 2 days after challenge were transferred into B6 WT mice (WT Basophil group) or OX40−/− mice (OX40−/− Basophil group) by intraperitoneal injection at days 0 and 14. On day 14, the mice were challenged by 100 μg of OVA i.n. Control mice (WT PBS group and OX40−/− PBS group) were treated with PBS intraperitoneal on days 0 and 14, and challenged by PBS on days 14. Each group had at least 3 mice. Mice were sacrificed on day 19. A part of basophils were treated with anti-mouse CD252 (20 μg/ml, OX40L blocking Ab, Basophil+αOX40L group) or an isotype Ab (Basophil group) overnight before adoptive transfer in vitro. MLN cells from recipients were cultured at a density of 2 × 106/ml in the presence of OVA (0.5 mg/ml) for 5 days. Cells were stained with Abs for CD4, IL-4, and IFN-γ and analyzed by FACS with CD4+ gating. A, flow cytometric analysis of CD4+ IL-4+ T cells from OX40−/− recipients and WT recipients; B, flow cytometric analysis of CD4+ IL-4+ T cells from WT mice receiving OX40L-blocked basophils or isotype Ab-treated basophils. Results are presented as mean ± S.D. and are representative of three independent experiments. *, p < 0.05; **, p < 0.01; NS, not significant.
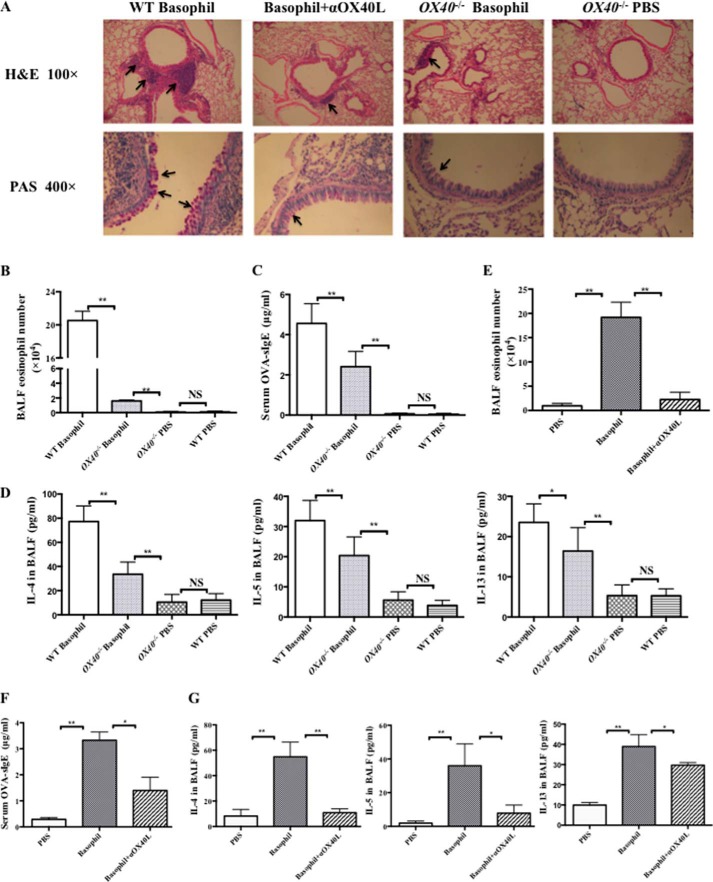
To test whether the suppression of Th2 responses mediated by OX40L influenced allergic airway inflammation, we assessed lung inflammation at day 28 in the transfer experiment. Compared with WT mice, both the OX40−/− recipients and mice receiving OX40L-blocked basophils displayed an alleviation of airway inflammation, which was reflected by decreased eosinophil infiltration in lungs (Fig. 7A) and in BALF (Fig. 7, B and E), as well as suppressed mucus formation (Fig. 7A) and Th2-type cytokines production, including IL-4, IL-5, and IL-13 in BALF (Fig. 7, D and G), and decreased levels of OVA-sIgE in serum (Fig. 7, C and F). Collectively, these results confirmed that basophil-associated OX40L was tightly involved in priming Th2 immune responses, thus leading to asthmatic inflammation.
FIGURE 7.
Blockade of OX40-OX40L interaction reduced allergic airway inflammation initiated by adoptive transfer of MLN basophils. MLN FSClow SSClow CD117− CD11c− CD49b+ FcϵRI+ basophils (2.5 × 105 cells in 200 μl of PBS) from OVA-immunized mice at 2 days after challenge were transferred into B6 WT mice (WT Basophil group) or OX40−/− mice (OX40−/− Basophil group) by intraperitoneal injection at days 0 and 14. On days 14, 25, 26, and 27, the mice were challenged by 100 μg of OVA i.n. Control mice (WT PBS group and OX40−/− PBS group) were treated with PBS intraperitoneal on days 0 and 14, and challenged by PBS on days 14, 25, 26, and 27. Each group had at least 3 mice. Mice were sacrificed on day 28. A part of basophils were treated with anti-mouse CD252 (20 μg/ml, OX40L blocking Ab, Basophil+αOX40L group) or an isotype Ab (Basophil group) overnight before adoptive transfer in vitro. A, H&E staining and PAS staining of lung tissues from different groups sacrificed 24 h after the last challenge; B, flow cytometric analysis of eosinophils in BALF gated on CD49d+ SSCint in OX40−/− recipients and WT recipients; C, level of OVA-sIgE from OX40−/− recipients and WT recipients was determined by ELISA; D, levels of IL-4, IL-5, and IL-13 of BALF from OX40−/− recipients and WT recipients were determined by ELISA; E, flow cytometric analysis of eosinophils in BALF gated on CD49d+ SSCint from WT mice receiving OX40L-blocked basophils or isotype Ab-treated basophils; F, level of OVA-sIgE from WT mice receiving OX40L-blocked basophils or isotype Ab-treated basophils was determined by ELISA; G, levels of IL-4, IL-5, and IL-13 of BALF from WT mice receiving OX40L-blocked basophils or isotype Ab-treated basophils were determined by ELISA. Results are presented as mean ± S.D. and are representative of three independent experiments. *, p < 0.05; **, p < 0.01; NS, not significant.
DISCUSSION
Recently, multiple studies have demonstrated the role of basophils in priming Th2 cell responses in different disease contexts. Some reports found that basophils were the only APCs to induce Th2 cells via IL-4 production and presentation of peptide-MHC class II complexes, and basophils were necessary and sufficient for the induction of Th2 cells in vivo and in vitro (7, 9, 29). Other studies proposed that DCs were the key APCs (39, 40), and in some contexts Th2 responses require DCs-basophils cooperation (38, 41). We have reported that basophils could induce Th2 cell responses in OVA-induced allergic airway inflammation (12), but the responsible mechanism is not understood. In addition to the secreted cytokines in the microenvironment, co-stimulatory molecules are essential for Th cell differentiation. Many studies show that signals from engagement of TNF family receptors and ligands contribute greatly to different aspects of T cell responses (42, 43). Furthermore, OX40 plays the most important role in the responses of CD4+ T cells, including cell differentiation and survival (44, 45). Here, we found that the expression and change of OX40L, the unique ligand for OX40 in vivo on MLN basophils, was much greater than other co-stimulatory molecules, thus we hypothesized that OX40L might mediate Th2 cell responses initiated by basophils. Our study further showed that blockade of OX40-OX40L interaction inhibited Th2 cell differentiation primed by basophils in vitro and ameliorated Th2 responses in a mouse model of OVA-induced airway inflammation. Furthermore, adoptive transfer of basophils from MLN of OVA-immunized mice to WT mice produced a robust Th2 response and subsequently allergic eosinophilic airway inflammation, whereas the Th2 response was significantly inhibited in OX40−/− mice receiving basophils and WT mice receiving OX40L-blocked basophils. Taken together, our results reveal a critical role of basophil-associated OX40L to the initiation of Th2 responses primed by basophils in the early phase of allergic asthma inflammation.
In OVA-immunized mice, there was a rapid recruitment of basophils after OVA challenge, which reached the peak point before the increase of Th2 cells in MLN when the number of DCs was not altered yet. Thus, these data strongly suggest that basophils play a pivotal role in the early phase of airway inflammation. However, this was not consistent with a previous report by Hammad et al., who observed that basophils were absent from MLN after house dust mite extract (HDM) administration (39). This discrepancy may be explained by the use of different experimental protocols, and especially antigens, which have dominant effects in determining basophil dependence. In this study, instead of the HDM i.n. protocol, we selected a widely-used OVA-induced allergic airway inflammation model. The mechanism and outcome of this model and the associated Th2-dominant phenotype has been well characterized, namely the mice are sensitized systemically by intraperitoneal OVA coupled with Al(OH)3 and then challenged OVA locally at the airway, leading to a Th2 response and eosinophilic asthma. We also observed that administration of the DNP-OVA/anti-DNP IgE complex was important for basophil activation in the Th2 differentiation experiment.
Notably, basophils from MLN in the OVA-immunized mice expressed high levels of OX40L and the peak of its expression was consistent with that of basophil number in MLN, indicating that the interaction between basophils presented OX40L and Th2 OX40 is involved in the Th2 responses primed by basophils. Based on the results of basophils from MLN, we investigated the expression of OX40L on BM-Bas in vitro and found that OX40L was expressed in an inducible manner. In a previous study, OX40L was up-regulated on B cells activated by CD40L-CD8 fusion protein plus αIgD dextran, bound to OX40 and promoted B cell proliferation, differentiation and immunoglobulin isotype switch (37). DCs, in the presence of TSLP, also expressed high levels of OX40L to activate T cells and promote T cell differentiation (14). Overall, the expression pattern of OX40L on basophils was similar to that on DCs and B cells, which was induced in the condition of maturation or inflammation context, indicating that basophils OX40L binds to OX40 to promote cell differentiation, maturation and inflammation. More importantly, the data that in vivo blockade of OX40-OX40L interaction in the early phase remarkably attenuated lung inflammation strongly indicated that OX40-OX40L interaction indeed played a critical role in the early phase of allergic airway inflammation in the present study, which in part was consistent with previous study (27).
Studies have demonstrated that CD4+ T cell differentiation might be regulated by cytokines and various co-stimulatory molecules. In addition, the contribution of signals from TNF/TNFR, including CD40/CD40L and OX40/OX40L, to Th2 cells differentiation has been reported (42, 43). Others and our previous study showed that basophils can induce Th2 cells differentiation through expressing co-stimulatory molecules and secreting early IL-4 (7, 9, 12). Additionally, IL-3- and TSLP-elicited basophils exhibit distinct phenotypic and functional characteristics depending on the cytokine milieu in which they promote Th2 cell differentiation and affect Th2 cytokines IL-4, IL-5, and IL-13 secretion (46). In the present study, the expression of CD40 on basophils increased in the OVA-immunized mice, which was consistent with our previous report (12). Thus, we assessed the roles of CD40-CD40L and OX40-OX40L interactions in the induction of Th2 cells differentiation primed by basophils in vitro, respectively, and found that both OX40-OX40L and CD40-CD40L pathways were indeed involved in Th2 cells differentiation. However, OX40-OX40L interactions played a preferential role in Th2 cell development in this mouse model. Furthermore, blockade of OX40-OX40L pathway led to a significant decrease in IL-4 and IL-5, but a slight reduction in IL-13 production in IL-3-treated cultures.
Finally, using adoptively transfer of MLN basophils derived from OVA-immunized mice into WT mice and OX40−/− mice, instead of the traditional OVA/alum sensitization method, we further found that both the WT and OX40−/− recipient mice developed OVA-specific Th2 responses in MLN, but the Th2 response observed in the latter was much subdued as indicated by reduced CD4+ IL-4+ Th2 cell percentages in the MLN. As expected, we found that lung inflammation was remarkably attenuated in the OX40−/− recipient mice compared with WT recipient mice. Importantly, decreased Th2 responses and ameliorated asthmatic inflammation were also observed in mice receiving OX40L-blocked basophils. These data confirmed that OX40-OX40L interaction is heavily involved in the initiation of a Th2 response primed by basophils, eventually leading to IgE-dependent allergic airway inflammation.
Our study has shown for the first time that OX40-OX40L interaction is involved in the Th2 responses driven by basophils in vitro and in IgE-dependent allergic airway inflammation in vivo. In the adoptive transfer model, the transferred basophils with OVA in the cytoplasm are the only resource of antigen-presenting cells that expressed high level of OX40L. Therefore, the roles of DCs and other APCs could be excluded, since there was no OVA uptake by them in the airway. Thus OX40L+ basophils are indispensable for initiating Th2 responses at the onset of IgE-dependent allergic airway inflammation. This result is not consistent with that reported by Leyva-Castillo et al., in which they observed a DC-T-Baso-T cascade (14). They found that TSLP-targeted DCs activated naïve T cells to produce IL-3 via OX40-OX40L interaction, which recruited basophils to directly or indirectly mediate the optimal CD4+ T cell expansion and Th2 priming in skin-draining lymph nodes (14). One explanation for the discrepancy is that the model of Th2 response priming may vary from a kind of disease context to another. According to the present study, we consider that basophils themselves can express high level of OX40L in the context of allergic airway inflammation, thus providing a direct pathway for T cell priming via the OX40-OX40L axis. There are some limitations in our study. The model we used is IgE-dependent allergic airway inflammation induced by OVA, which can mimic most of the manifestations in asthmatic patients, but still remains a mechanistic animal model. Further studies in asthmatic patients are needed to confirm the translatability of our findings.
Acknowledgments
We thank the staffs of Research Center for Experimental Medicine of Ruijin Hospital affiliated with Shanghai Jiao Tong University School of Medicine and flow cytometry core facility of Shanghai Jiao tong University School of Medicine, and Dr. Gary Brewer from University of Medicine and Dentistry of New Jersey for critical review of the article.
This work was supported by grants from the National Natural Science Foundation of China (81470217, 81270084, and 81070022) and the Shanghai Municipal Science and Technology Commission Foundation (13XD1402800).
- Th
- T helper
- BM-Bas
- bone marrow-derived basophils
- OVA-sIgE
- ovalbumin-specific immunoglobulin E
- MLN
- mediastinal lymph nodes
- αOX40L
- OX40L blocking antibody
- DC
- dendritic cell
- Ab
- antibody
- SPF
- specified-pathogen-free.
REFERENCES
- 1. Barnes P. J. (2008) Immunology of asthma and chronic obstructive pulmonary disease. Nature Reviews. Immunology 8, 183–192 [DOI] [PubMed] [Google Scholar]
- 2. Holgate S. T. (2012) Innate and adaptive immune responses in asthma. Nature Medicine 18, 673–683 [DOI] [PubMed] [Google Scholar]
- 3. Lloyd C. M., Hessel E. M. (2010) Functions of T cells in asthma: more than just T(H)2 cells. Nature Reviews. Immunology 10, 838–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hamid Q., Tulic M. (2009) Immunobiology of asthma. Annu. Rev. Physiol. 71, 489–507 [DOI] [PubMed] [Google Scholar]
- 5. Wedemeyer J., Tsai M., Galli S. J. (2000) Roles of mast cells and basophils in innate and acquired immunity. Curr. Opin. Immunol. 12, 624–631 [DOI] [PubMed] [Google Scholar]
- 6. Siracusa M. C., Kim B. S., Spergel J. M., Artis D. (2013) Basophils and allergic inflammation. J. Allergy Clinical Immunology 132, 789–801; quiz 788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yoshimoto T., Yasuda K., Tanaka H., Nakahira M., Imai Y., Fujimori Y., Nakanishi K. (2009) Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nature Immunology 10, 706–712 [DOI] [PubMed] [Google Scholar]
- 8. Wynn T. A. (2009) Basophils trump dendritic cells as APCs for T(H)2 responses. Nature Immunology 10, 679–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sokol C. L., Chu N. Q., Yu S., Nish S. A., Laufer T. M., Medzhitov R. (2009) Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nature Immunology 10, 713–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Beek A. A., Knol E. F., de Vos P., Smelt M. J., Savelkoul H. F., van Neerven R. J. (2013) Recent developments in basophil research: do basophils initiate and perpetuate type 2 T-helper cell responses? Int. Arch. Allergy Immunol. 160, 7–17 [DOI] [PubMed] [Google Scholar]
- 11. Karasuyama H., Mukai K., Obata K., Tsujimura Y., Wada T. (2011) Nonredundant roles of basophils in immunity. Annu. Rev. Immunol. 29, 45–69 [DOI] [PubMed] [Google Scholar]
- 12. Zhong W., Su W., Zhang Y., Liu Q., Wu J., Di C., Zhang Z., Xia Z. (2014) Basophils as a primary inducer of the T helper type 2 immunity in ovalbumin-induced allergic airway inflammation. Immunology 142, 202–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xiao X., Balasubramanian S., Liu W., Chu X., Wang H., Taparowsky E. J., Fu Y. X., Choi Y., Walsh M. C., Li X. C. (2012) OX40 signaling favors the induction of T(H)9 cells and airway inflammation. Nature Immunology 13, 981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leyva-Castillo J. M., Hener P., Michea P., Karasuyama H., Chan S., Soumelis V., Li M. (2013) Skin thymic stromal lymphopoietin initiates Th2 responses through an orchestrated immune cascade. Nature Communications 4, 2847. [DOI] [PubMed] [Google Scholar]
- 15. Gopisetty A., Bhattacharya P., Haddad C., Bruno J. C., Jr., Vasu C., Miele L., Prabhakar B. S. (2013) OX40L/Jagged1 cosignaling by GM-CSF-induced bone marrow-derived dendritic cells is required for the expansion of functional regulatory T cells. J. Immunol. 190, 5516–5525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Croft M. (2010) Control of immunity by the TNFR-related molecule OX40 (CD134). Annu. Rev. Immunol. 28, 57–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salek-Ardakani S., Moutaftsi M., Crotty S., Sette A., Croft M. (2008) OX40 drives protective vaccinia virus-specific CD8 T cells. J. Immunol. 181, 7969–7976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murata K., Ishii N., Takano H., Miura S., Ndhlovu L. C., Nose M., Noda T., Sugamura K. (2000) Impairment of antigen-presenting cell function in mice lacking expression of OX40 ligand. J. Exp. Med. 191, 365–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tanaka H., Demeure C. E., Rubio M., Delespesse G., Sarfati M. (2000) Human monocyte-derived dendritic cells induce naive T cell differentiation into T helper cell type 2 (Th2) or Th1/Th2 effectors. Role of stimulator/responder ratio. J. Exp. Med. 192, 405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weinberg A. D., Wegmann K. W., Funatake C., Whitham R. H. (1999) Blocking OX-40/OX-40 ligand interaction in vitro and in vivo leads to decreased T cell function and amelioration of experimental allergic encephalomyelitis. J. Immunol. 162, 1818–1826 [PubMed] [Google Scholar]
- 21. Zingoni A., Sornasse T., Cocks B. G., Tanaka Y., Santoni A., Lanier L. L. (2004) Cross-talk between activated human NK cells and CD4+ T cells via OX40-OX40 ligand interactions. J. Immunol. 173, 3716–3724 [DOI] [PubMed] [Google Scholar]
- 22. Burgess J. K., Carlin S., Pack R. A., Arndt G. M., Au W. W., Johnson P. R., Black J. L., Hunt N. H. (2004) Detection and characterization of OX40 ligand expression in human airway smooth muscle cells: a possible role in asthma? J. Allergy Clin. Immunol. 113, 683–689 [DOI] [PubMed] [Google Scholar]
- 23. Zhang J. Y., Wu X. L., Yang B., Wang Y., Feng G. H., Jiang T. J., Zeng Q. L., Xu X. S., Li Y. Y., Jin L., Lv S., Zhang Z., Fu J., Wang F. S. (2013) Upregulation of OX40 ligand on monocytes contributes to early virological control in patients with chronic hepatitis C. Eur. J. Immunol. 43, 1953–1962 [DOI] [PubMed] [Google Scholar]
- 24. Morimoto S., Kanno Y., Tanaka Y., Tokano Y., Hashimoto H., Jacquot S., Morimoto C., Schlossman S. F., Yagita H., Okumura K., Kobata T. (2000) CD134L engagement enhances human B cell Ig production: CD154/CD40, CD70/CD27, and CD134/CD134L interactions coordinately regulate T cell-dependent B cell responses. J. Immunol. 164, 4097–4104 [DOI] [PubMed] [Google Scholar]
- 25. Kawamata S., Hori T., Imura A., Takaori-Kondo A., Uchiyama T. (1998) Activation of OX40 signal transduction pathways leads to tumor necrosis factor receptor-associated factor (TRAF) 2- and TRAF5-mediated NF-κB activation. J. Biol. Chem. 273, 5808–5814 [DOI] [PubMed] [Google Scholar]
- 26. Siddiqui S., Mistry V., Doe C., Stinson S., Foster M., Brightling C. (2010) Airway wall expression of OX40/OX40L and interleukin-4 in asthma. Chest 137, 797–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jember A. G., Zuberi R., Liu F. T., Croft M. (2001) Development of allergic inflammation in a murine model of asthma is dependent on the costimulatory receptor OX40. J. Exp. Med. 193, 387–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoshino A., Tanaka Y., Akiba H., Asakura Y., Mita Y., Sakurai T., Takaoka A., Nakaike S., Ishii N., Sugamura K., Yagita H., Okumura K. (2003) Critical role for OX40 ligand in the development of pathogenic Th2 cells in a murine model of asthma. Eur. J. Immunol. 33, 861–869 [DOI] [PubMed] [Google Scholar]
- 29. Perrigoue J. G., Saenz S. A., Siracusa M. C., Allenspach E. J., Taylor B. C., Giacomin P. R., Nair M. G., Du Y., Zaph C., van Rooijen N., Comeau M. R., Pearce E. J., Laufer T. M., Artis D. (2009) MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nature Immunology 10, 697–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Metcalf D., Ng A. P., Baldwin T. M., Di Rago L., Mifsud S. (2013) Concordant mast cell and basophil production by individual hematopoietic blast colony-forming cells. Proc. Natl. Acad. Sci. U. S. A. 110, 9031–9035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ugajin T., Kojima T., Mukai K., Obata K., Kawano Y., Minegishi Y., Eishi Y., Yokozeki H., Karasuyama H. (2009) Basophils preferentially express mouse Mast Cell Protease 11 among the mast cell tryptase family in contrast to mast cells. J. Leukocyte Biology 86, 1417–1425 [DOI] [PubMed] [Google Scholar]
- 32. Zhang Y., Zhang L., Wu J., Di C., Xia Z. (2013) Heme oxygenase-1 exerts a protective role in ovalbumin-induced neutrophilic airway inflammation by inhibiting Th17 cell-mediated immune response. J. Biol. Chem. 288, 34612–34626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim M. S., Cho K. A., Cho Y. J., Woo S. Y. (2013) Effects of interleukin-9 blockade on chronic airway inflammation in murine asthma models. Allergy, Asthma Immunology Research 5, 197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Henderson W. R., Jr., Lewis D. B., Albert R. K., Zhang Y., Lamm W. J., Chiang G. K., Jones F., Eriksen P., Tien Y. T., Jonas M., Chi E. Y. (1996) The importance of leukotrienes in airway inflammation in a mouse model of asthma. J. Exp. Med. 184, 1483–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ito T., Wang Y. H., Duramad O., Hori T., Delespesse G. J., Watanabe N., Qin F. X., Yao Z., Cao W., Liu Y. J. (2005) TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 202, 1213–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lützelschwab C., Huang M. R., Kullberg M. C., Aveskogh M., Hellman L. (1998) Characterization of mouse mast cell protease-8, the first member of a novel subfamily of mouse mast cell serine proteases, distinct from both the classical chymases and tryptases. Eur. J. Immunol. 28, 1022–1033 [DOI] [PubMed] [Google Scholar]
- 37. Stüber E., Neurath M., Calderhead D., Fell H. P., Strober W. (1995) Cross-linking of OX40 ligand, a member of the TNF/NGF cytokine family, induces proliferation and differentiation in murine splenic B cells. Immunity 2, 507–521 [DOI] [PubMed] [Google Scholar]
- 38. Wakahara K., Van V. Q., Baba N., Bégin P., Rubio M., Delespesse G., Sarfati M. (2013) Basophils are recruited to inflamed lungs and exacerbate memory Th2 responses in mice and humans. Allergy 68, 180–189 [DOI] [PubMed] [Google Scholar]
- 39. Hammad H., Plantinga M., Deswarte K., Pouliot P., Willart M. A., Kool M., Muskens F., Lambrecht B. N. (2010) Inflammatory dendritic cells–not basophils–are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J. Exp. Med. 207, 2097–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ohnmacht C., Schwartz C., Panzer M., Schiedewitz I., Naumann R., Voehringer D. (2010) Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity 33, 364–374 [DOI] [PubMed] [Google Scholar]
- 41. Tang H., Cao W., Kasturi S. P., Ravindran R., Nakaya H. I., Kundu K., Murthy N., Kepler T. B., Malissen B., Pulendran B. (2010) The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nature Immunology 11, 608–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Croft M., Benedict C. A., Ware C. F. (2013) Clinical targeting of the TNF and TNFR superfamilies. Nature Reviews. Drug Discovery 12, 147–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Croft M., Duan W., Choi H., Eun S. Y., Madireddi S., Mehta A. (2012) TNF superfamily in inflammatory disease: translating basic insights. Trends Immunol. 33, 144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rogers P. R., Song J., Gramaglia I., Killeen N., Croft M. (2001) OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity 15, 445–455 [DOI] [PubMed] [Google Scholar]
- 45. Gramaglia I., Weinberg A. D., Lemon M., Croft M. (1998) Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J. Immunol. 161, 6510–6517 [PubMed] [Google Scholar]
- 46. Siracusa M. C., Saenz S. A., Hill D. A., Kim B. S., Headley M. B., Doering T. A., Wherry E. J., Jessup H. K., Siegel L. A., Kambayashi T., Dudek E. C., Kubo M., Cianferoni A., Spergel J. M., Ziegler S. F., Comeau M. R., Artis D. (2011) TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature 477, 229–233 [DOI] [PMC free article] [PubMed] [Google Scholar]