Background: β-Catenin is an important mediator of angiogenesis; however, its molecular regulation is poorly understood.
Results: Wnt promotes phosphorylation of c-Cbl on Tyr-731, enhancing its dimerization and nuclear translocation to degrade nuclear β-catenin.
Conclusion: Wnt-mediated c-Cbl phosphorylation regulates nuclear β-catenin.
Significance: Targeting c-Cbl E3 ligase activity could destabilize β-catenin and inhibit pathological angiogenesis.
Keywords: Angiogenesis, E3 Ubiquitin Ligase, Tumor Cell Biology, Wnt Signaling, Zebrafish, β-Catenin, Cbl, Tumorigenesis, Tumors
Abstract
Wnt signaling plays important roles in both the tumor-induced angiogenesis and tumorigenesis through the transcriptionally active nuclear β-catenin. Recently, c-Cbl was identified as a unique E3 ubiquitin ligase targeting the active nuclear β-catenin. However, little is known about the molecular mechanisms by which c-Cbl regulates ubiquitination and degradation of active β-catenin. Here, we demonstrate that Wnt activation promotes the phosphorylation of c-Cbl at tyrosine 731(Tyr-731), which increases c-Cbl dimerization and binding to β-catenin. Tyr-731 phosphorylation and dimerization mediate c-Cbl nuclear translocation and lead to the degradation of nuclearly active β-catenin in the Wnt-on phase. c-Cbl activation also inhibits expression of the pro-angiogenic Wnt targets, IL-8 and VEGF. Phospho-Tyr-731-inactive mutant c-Cbl (Y731F) enhances and phosphomimetic mutant c-Cbl (Y731E) suppresses angiogenesis in zebrafish. Taken together, we have identified a novel mechanism for the regulation of active nuclear β-catenin by c-Cbl and its critical role in angiogenesis. This mechanism can be further explored to modulate both the pathological angiogenesis and the tumorigenesis.
Introduction
Angiogenesis, the process of growth of new blood vessels, is imperative for several steps of tumorigenesis, including the progression to malignant phenotypes and metastasis (1). Both the knock-out animal models (2–4) and the tumor angiogenesis studies (5–7) implicate Wnt signaling as an important regulator of angiogenesis in endothelial cells (ECs).2 The current model supports β-catenin as the key mediator of Wnt signaling. Constitutive degradation of cytosolic β-catenin maintaining low Wnt activity characterizes Wnt-off phase. Conversely, stabilization and nuclear translocation of β-catenin with Wnt ligand are the hallmarks of Wnt-on phase. As a transcriptional co-activator, nuclear β-catenin induces several pro-tumorigenic and pro-angiogenic target genes (4, 8, 9). Thus, the regulation of nuclear β-catenin (also called active β-catenin) is critical for both tumorigenesis and angiogenesis. The ubiquitin-proteasomal degradation pathway constitutes the major mechanism for cytosolic β-catenin degradation in the Wnt-off phase (10, 11); however, little was known about the homeostasis of nuclear β-catenin.
Casitas B-lineage lymphoma (c-Cbl) is an E3 ligase expressed in ECs and was initially described as an “anti-angiogenic protein” with a poorly understood mechanism (12–15). Recently, c-Cbl was identified as a unique E3 ubiquitin ligase promoting degradation of active β-catenin and negatively regulating angiogenesis through Wnt signaling (16). Similar to β-catenin, Wnt activation also results in the nuclear translocation of c-Cbl ensuring its persistent interaction and degradation of active β-catenin (16). Although these biochemical events represent important determinants of nuclear β-catenin regulation, the molecular mechanisms of these Wnt-mediated events have remained elusive.
Among several post-translational modifications on c-Cbl, phosphorylation particularly at three tyrosine residues at the carboxyl terminus, Tyr-700, Tyr-731, and Tyr-774, regulates its interactions in response to upstream signaling events (17). We set out to examine the role of c-Cbl phosphorylation in β-catenin degradation and posited that Wnt activation mediates c-Cbl phosphorylation to regulate its nuclear translocation and down-regulation of nuclear β-catenin. Our data demonstrate that Wnt activation promotes phosphorylation of c-Cbl at Tyr-731, a critical event enhancing c-Cbl dimerization and binding to β-catenin. This mechanism regulates nuclear translocation of c-Cbl and leads to modulation of nuclear β-catenin and angiogenesis.
MATERIALS AND METHODS
Cell Culture
Primary human aortic endothelial cells and umbilical vein endothelial cells (HUVECs) (Promocell, Germany) pooled from three donors were grown in endothelial growth medium-2 (EGM-2) (Promocell). EGM-2 was prepared by supplementing endothelial basal medium (EBM-2) with fetal bovine serum (2%), hydrocortisone (1 μg/ml), fibroblast growth factor-1 (10 ng/ml), epidermal growth factor (5 ng/ml), insulin-like growth factor (20 ng/ml), ascorbic acid (1 μg/ml), and heparin (90 μg/ml). These primary human cells were tested for Mycoplasma, hepatitis B virus, hepatitis C virus, and HIV-1 using ELISA. The cells were characterized by CD31, von Willebrand factor, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate-Ac-LDL uptake, and smooth muscle actin using immunofluorescence and were used over eight passages.
β-Catenin Morpholino
Previously characterized MOs (18) that target zebrafish ctnnb1 and ctnnb2 were co-injected in equimolar ratios at the final concentration of 0.01 mm. b-cat1MO is 5]-ATCAAGTCAGACTGGGTAGCCATGA-3, and bcat-2MO is 5′-CCTTTAGCCTGAGCGACTTCCAAAC-3. The mismatched MOs served as controls.
Synthesis of Capped mRNA
The vectors were linearized with NotI restriction enzyme, treated with proteinase K (Sigma), and extracted with phenol. Linearized plasmid DNA (1 μg/μl) in RNase-free water was used for in vitro capped mRNA synthesis using the mMessage mMachine® SP6 kit (Ambion) according to manufacturer's instructions, as described previously (10).
Zebrafish Embryo Injection and Phenotype Evaluation
Fli-eGFP transgenic adult male and female zebrafish (Danio rerio) were housed in a fish facility at the Dana Farber Cancer Institute, Harvard Medical School, at a 14:19-h light/dark cycle at a temperature of (26.5 °C) and a pH of (7.0–7.4) in a controlled multitank recirculating water system (Aquatic Habitats, Apopka, FL). The Dana Farber Cancer Institute, Harvard Medical School, animal welfare committee approved the experimental protocol. The capped RNA (5–10 ng) with or without 0.01 mm β-catenin MO (an equimolar combination of validated MOs against zebrafish ctnnb1 and ctnnb2) or controlled MO was injected to one- or two-cell stage embryos (18, 19). The embryos were grown at 28 °C for 3 days. The images of fish under same light exposure setting were obtained from 10 randomly selected fish per group at every experiment and analyzed for the length of the tail vessels. The vessels were marked from the junction of the body and the tail going caudally using Image-Pro and averaged per group, as described previously (20). The tail vessel plexus was marked and measured for fluorescent intensity using ImageJ.
RT-PCR
Embryos harvested at 24 h post-fertilization were subjected to total RNA extraction using RNAshredder and RNeasy as recommended by the manufacturer (Qiagen). RNA (1 μg measured by Nanodrop, Thermo Fisher Scientific) was used for cDNA preparation. RT-PCR was conducted using TaqMan probes (ABI) with D. rerio vegfa and D. rerio actin.
Cross-linking Assay
ECs serum-starved for 12–16 h and exposed to Wnt3a ligand for 3 h were treated with the cross-linker disuccinimidyl suberate (DSS) (Thermo Fisher Scientific) at 1 mm for 30 min at room temperature (as recommended by manufacturer Thermo Fisher Scientific) followed by quenching using Tris-HCl (pH 7.5) for 15 min. The lysates were resolved on SDS-PAGE and probed using FLAG tag antibody and probed with FLAG antibody. Monomer is considered as loading control, and dimers were normalized to monomers using ImageJ. The antibodies, immunoblotting, immunoprecipitation, cellular fractionation, GST purification, and ubiquitination assays and in vitro tube formation assay are as described previously (16).
Statistical Analysis
In all figures, data are expressed as average ± S.E. Student's t test followed by Bonferroni's correction was conducted to determine the statistical differences between the groups. p < 0.05 was considered significant.
RESULTS
Wnt-mediated Phosphorylation of c-Cbl Tyr-731 Regulates Its Binding to β-Catenin
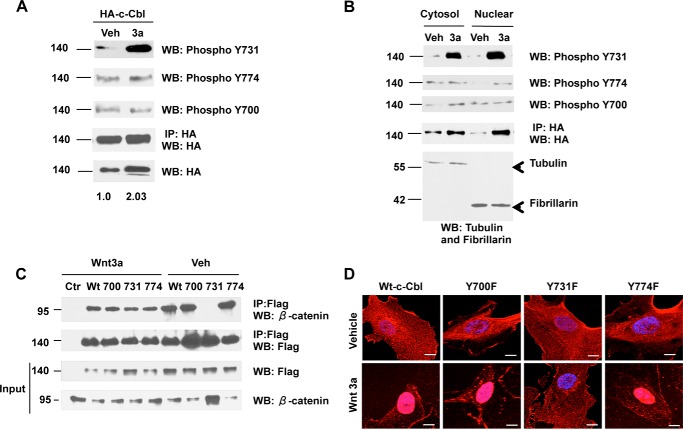
As phosphorylation at tyrosine 700, 731, and 774 regulates c-Cbl's binding to other interactors (17, 21, 22), we examined their role in β-catenin regulation. Wnt activation in ECs resulted in phosphorylation of c-Cbl tyrosine 731 (Fig. 1A), which was also detected in the nuclear fraction (Fig. 1B). Point mutants (Tyr to Phe as phospho-inactive) were employed to further examine its biological relevance. In the Wnt-off phase, c-Cbl Y731F did bind to β-catenin. However, unlike wild-type c-Cbl and Y700F and Y774F mutants, the interaction of Y731F and β-catenin reduced substantially with Wnt activation, despite an increase in β-catenin levels (Fig. 1C). Inactivation of Tyr-731 phosphorylation also abrogated c-Cbl's Wnt-mediated nuclear translocation (Fig. 1D and data not shown). These data underscore the importance of c-Cbl Tyr-731 phosphorylation in the Wnt-mediated c-Cbl nuclear translocation and β-catenin binding.
FIGURE 1.
Wnt mediates c-Cbl Tyr-731 phosphorylation, which in turn regulates β-catenin binding and nuclear translocation. A, Wnt-mediated c-Cbl Tyr-731 phosphorylation. ECs serum-starved for 18 h were stimulated with vehicle (Veh) or Wnt3a (50 ng/ml) for 3 h. Whole cell lysates were IPed (IP) using HA tag antibody and probed for phosphoantibody specific for Tyr-731. The membrane was stripped and reprobed for Tyr-700 and Tyr-774 and HA tag antibodies. Five percent of cell lysates were probed for HA normalized to actin. Representative immunoblot of three experiments is shown. WB, Western blot. B, c-Cbl Tyr-731 phosphorylation in the nuclear c-Cbl. Subcellular fractions from ECs expressing HA-c-Cbl that had been stimulated with Wnt as above were IPed with HA antibody and probed for c-Cbl phosphoantibodies Tyr-731 and Tyr-700 and reprobed for Tyr-774 and HA tag antibodies, respectively. Representative immunoblot of three experiments is shown. C, c-Cbl Y731F mutation compromises its binding to β-catenin in the Wnt-on phase. Whole cell lysates of ECs stably expressing FLAG c-Cbl wild-type (Wt) or point mutants Y700F, Y731F, or Y774F were stimulated with Wnt3a (100 ng/ml), IPed with FLAG, and immunoblotted using β-catenin. The blot was stripped and reprobed with FLAG antibody. Ten percent of lysates are shown as input loading control. Representative immunoblot of three experiments is shown. D, phospho-inactivating Y731F mutation compromises c-Cbl's ability to undergo Wnt-mediated nuclear translocation. Serum-starved ECs stably expressing FLAG-tagged constructs were stimulated with vehicle or Wnt3a, as described above, and were fixed for immunofluorescence using FLAG tag antibody and DAPI for nuclear staining. Laser confocal microscopy was performed, and representative cells from 100 randomly selected cells are shown. Scale bar, 100 pixel units.
c-Cbl Tyr-731 Phosphorylation Enhances β-Catenin Binding through Dimerization
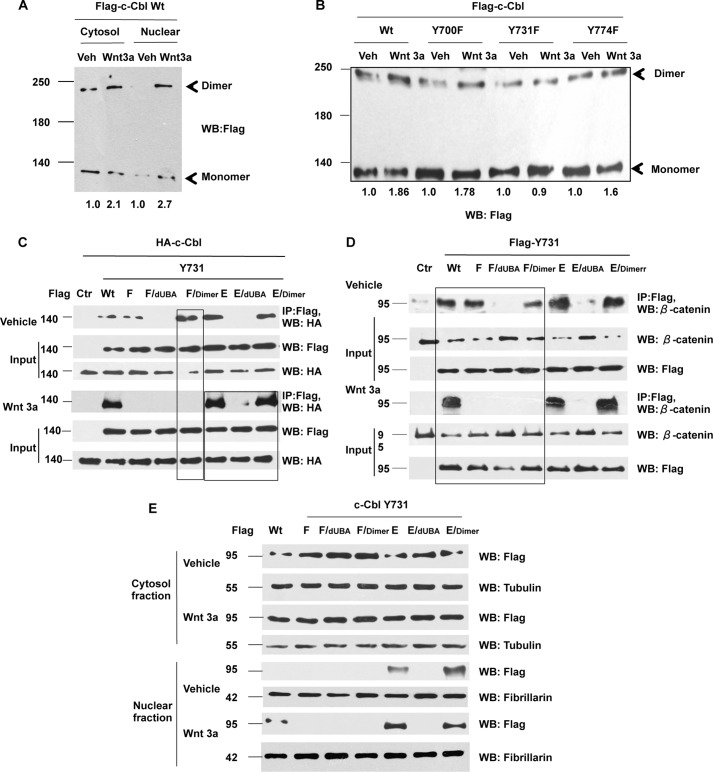
c-Cbl exists as monomers and dimers, whereas its dimerization is known to regulate interactions with other proteins (16, 17, 21). Because Tyr-731 phosphorylation enhances binding with β-catenin, we posited that Tyr-731 phosphorylation modulates binding with β-catenin through dimerization. This hypothesis was addressed using a set of constructs with combinations of point mutants (phospho-inactive, Y731F, and phospho-mimetic, Y731E) and ubiquitin-binding domain deletion (dUBA) or artificial dimerization motif (dimer), which is known to increase c-Cbl dimerization (16). Two orthogonal approaches, DSS, a cross-linking agent and a stabilizer of protein-protein interactions, and immunoprecipitation (IP) assays were used. The data showed that c-Cbl existed predominantly as monomers at baseline (expected 120 kDa) (Fig. 2, A and B) (23, 24). Wnt induction resulted in a 1.8–2-fold increase in the dimerized species, an event compromised by phospho-inactivating mutation at Tyr-731 (Fig. 2, A–C).
FIGURE 2.
c-Cbl Tyr-731 phosphorylation modulates β-catenin binding through dimerization. A, c-Cbl dimers are increased in the Wnt-on phase. HUVECs stably expressing FLAG-c-Cbl were serum-starved and stimulated with 50 ng/ml Wnt3a. Before harvest, the cells were treated with 1 mm DSS at room temperature for 30 min. The subcellular fractions were resolved in SDS-polyacrylamide gel probed with FLAG antibody. A representative of two independent experiments is shown. The dimers were normalized to monomers using ImageJ. B, c-Cbl wild-type but not Y731F shows Wnt-mediated dimerization. HUVECs transduced with wild-type or different c-Cbl point mutants were subjected to Wnt3a and DSS treatment as mentioned above before harvest. The whole cell lysates were resolved on SDS-polyacrylamide gel and probed. A representative of two independent experiments is shown. The dimers were normalized to monomers using ImageJ. C, Tyr-731 phosphorylation mediates Wnt-enhanced dimerization. Whole cell lysates from ECs stably co-expressing HA-c-Cbl and FLAG-c-Cbl constructs that were serum-starved and stimulated with Wnt3a 50 ng/ml for 3 h were IPed with FLAG antibody and immunoblotted using HA antibody. Five percent of lysates are used as inputs. Representative immunoblot of three experiments is shown. D, Y731F mutation compromises Wnt-enhanced c-Cbl-β-catenin binding through c-Cbl dimerization domain. Whole cell lysates from ECs stably expressing FLAG-tagged c-Cbl constructs that were stimulated with Wnt as above were IPed with FLAG antibody and immunoblotted using β-catenin antibody. Five percent of lysates are shown as input. Representative immunoblot of three experiments is shown. E, Wnt-mediated nuclear translocation is regulated by c-Cbl Tyr-731 phosphorylation. Wnt activation was performed as above in ECs stably expressing FLAG-c-Cbl wild type or mutants. Lysates were immunoblotted with FLAG antibody. Tubulin and fibrillarin serve as cytosolic and nuclear markers, respectively. Representative immunoblot of three experiments is shown. WB, Western blot; IP, immunoprecipitation; Veh, vehicle.
IP assays showed that the deletion of UBA in Y731F (F/dUBA) abrogated dimerization in both the phases of Wnt signaling, supporting the necessary role of UBA in c-Cbl dimerization (Fig. 2C). c-Cbl dimerization could be rescued by the artificial dimerization domain (F/dimer), only in the Wnt-off but not in the Wnt-on phase (Fig. 2C, 1st box). In contrast, the phosphomimicking Y731E mutant exhibited enhanced dimerization at baseline, even more than the wild-type c-Cbl in both phases of Wnt signaling (Fig. 2C, 2nd box). These data suggest that dimerization is necessary but not sufficient in the Wnt-on phase and that Tyr-731 phosphorylation enhances c-Cbl's dimerization in the Wnt-on phase.
As c-Cbl phosphorylation mediates its protein-protein interactions (17, 21, 22), we examined the effect of c-Cbl phosphorylation on c-Cbl-β-catenin binding using immunoprecipitation of endogenous β-catenin by different c-Cbl constructs (Fig. 2D). Although phospho-inactive Y731F c-Cbl bound β-catenin in the Wnt-off phase, its binding was substantially abrogated in the Wnt-on phase (Figs. 1C and 2D), which could not be rescued even with the artificial dimerization domain in Y731F (F/dimer) in the Wnt-on phase (box on Fig. 2D), underscoring a need for intact Tyr-731 in the Wnt-on phase for β-catenin binding. The phospho-mimetic c-Cbl Y731E showed binding to β-catenin more than wild-type c-Cbl. Taken together with the dimerization pattern, these data suggest that in the Wnt-on phase, c-Cbl Tyr-731 phosphorylation augments c-Cbl-β-catenin binding through its dimerization.
As binding to β-catenin regulates c-Cbl's nuclear translocation, we posited that the c-Cbl phosphorylation and dimerization regulates its Wnt-mediated nuclear translocation. Indeed, the nuclear translocation of c-Cbl mutants corresponded to their β-catenin binding pattern (Fig. 2E). Phospho-mimetic c-Cbl Y731E mutant alone or with functional dimerization and β-catenin binding abilities (E and E/dimer) localized to the nucleus even in the Wnt-off phase. The phospho-inactive mutants of c-Cbl Y731F showed no Wnt-mediated nuclear translocation, even when combined with the artificial dimerization motif (F/dimer). Collectively, these data indicate that in the Wnt-on phase, c-Cbl Tyr-731 phosphorylation is required to enhance c-Cbl dimerization, binding to β-catenin, and c-Cbl's nuclear translocation.
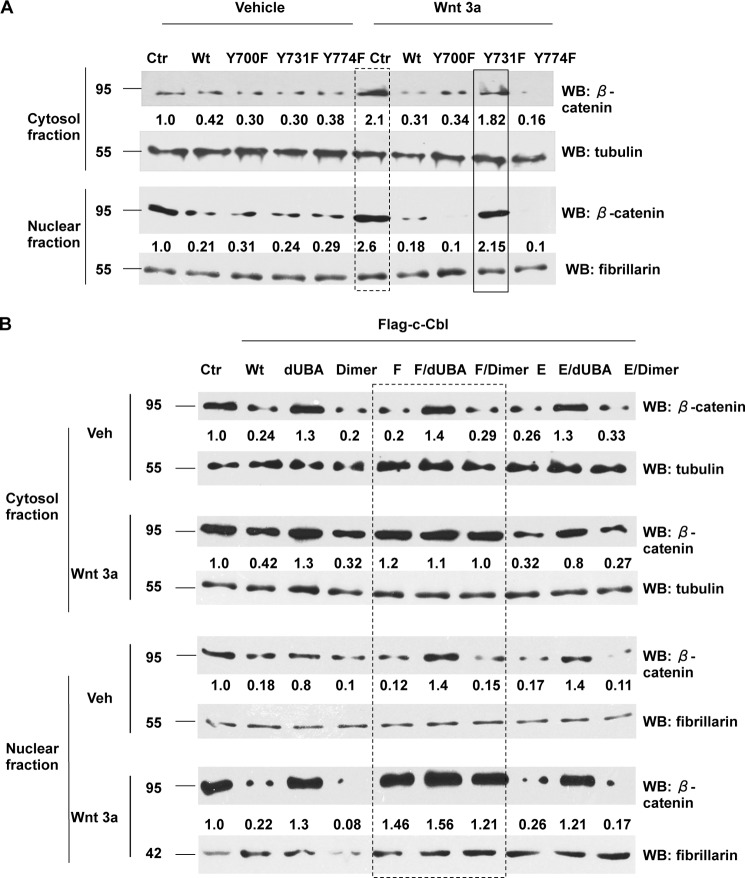
c-Cbl Tyr-731 Phosphorylation Regulates β-Catenin Ubiquitination and Degradation
Because binding of c-Cbl with β-catenin is required for β-catenin degradation (16) and Tyr-731 phosphorylation is critical for c-Cbl-β-catenin binding in the Wnt-on phase, we posited that c-Cbl Tyr-731 phosphorylation status regulates β-catenin degradation. To this effect, we examined endogenous β-catenin in both phases of Wnt signaling. The data showed that β-catenin regulation by different c-Cbl mutants is commensurate to their β-catenin binding pattern. In the Wnt-off phase, wild-type and all c-Cbl phosphomutants significantly down-regulated β-catenin. However, in the Wnt-on phase, Y731F lost this ability (Fig. 3A) and could not be rescued even by the artificial dimerization (F/dimer) (Fig. 3B and data not shown) in both the cytosol and nuclear fractions (box in Fig. 3B). However, Y731E and E/dimer suppressed β-catenin in both the phases of Wnt signaling.
FIGURE 3.
Tyr-731 phosphorylation regulates β-catenin down-regulation. A, phospho-inactive mutation of c-Cbl at Tyr-731 (Y731F) abrogates regulation of β-catenin in the Wnt-on phase. ECs stably expressing FLAG-tagged c-Cbl constructs were treated with Wnt3a and subjected to fractionation. The fractions were probed for β-catenin. Tubulin and fibrillarin served as markers of cytosolic and nuclear fractions and as loading controls, respectively. β-Catenin bands were normalized to loading controls and then compared with a control vehicle-treated sample. The box with dashed lines represents an increase in β-catenin with Wnt3a treatment, and the box with solid lines indicates a lack of reduction of β-catenin with Y731F mutation in the Wnt-on phase. Representative immunoblot from three experiments is shown. B, c-Cbl Tyr-731 phosphorylation regulates β-catenin through its dimerization in the Wnt-on phase. ECs stably expressing FLAG-c-Cbl constructs were Wnt-stimulated and subjected to fractionations, and the fractions were probed as above. Representative immunoblot from three experiments is shown. Ctr, control; WB, Western blot; Veh, vehicle.
c-Cbl-mediated β-catenin ubiquitination also followed its binding pattern (data not shown). In the Wnt-off phase, Y731F ubiquitinated β-catenin in an UBA-dependent manner. However, in the Wnt-on phase, even the artificial dimerization domain was not sufficient to rescue the ability of Y731F to ubiquitinate β-catenin. Collectively, these data underscore the critical role of Tyr-731 phosphorylation in down-regulation of β-catenin in the Wnt-on phase.
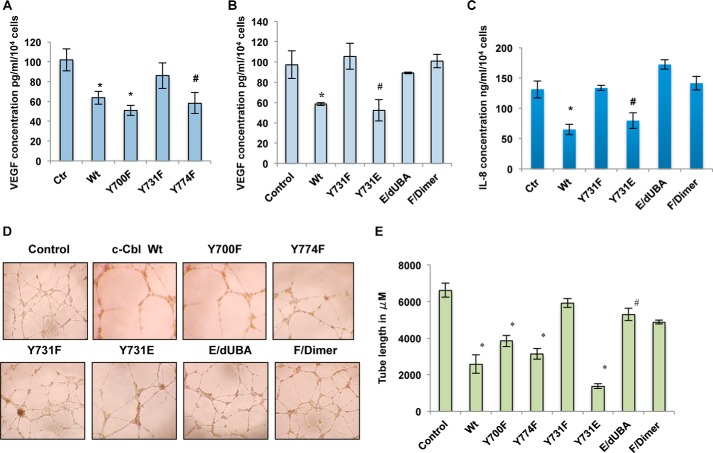
c-Cbl Tyr-731 Phosphorylation Regulates Pro-angiogenic Wnt Target Genes and Angiogenesis
Having shown that c-Cbl suppresses angiogenesis through Wnt signaling by degrading β-catenin and inhibiting pro-angiogenic Wnt target genes, IL-8 and VEGF (16), we next examined whether the c-Cbl phosphorylation status regulates the pro-angiogenic Wnt targets and angiogenesis. Of all phosphomutants, Y731F lost the inhibitory effect on VEGF, IL-8 levels, and in vitro tube formation (Fig. 4, A–E and data not shown). In contrast, Y731E inhibited VEGF, IL-8 levels, and in vitro tube formation more than the wild-type c-Cbl, an ability compromised by the deletion of UBA (E/dUBA). However, the addition of an artificial dimerization motif in Y731F (F/dimer) could not rescue the regulation of VEGF, IL-8, and in vitro angiogenesis (Fig. 4, B–D). These data indicate that although necessary, c-Cbl dimerization is not sufficient for its anti-angiogenic effect and that Tyr-731 phosphorylation regulates angiogenesis through its dimerization.
FIGURE 4.
c-Cbl Tyr-731 phosphorylation regulates pro-angiogenic Wnt target genes and angiogenesis. A, phospho-inactive c-Cbl Y731F mutant fails to suppress VEGF. The media of ECs collected as mentioned above were analyzed for VEGF levels. The mean of triplicates samples is shown. Student's t test was performed to determine statistical significance. In Wnt-treated samples, * indicates compared with control (Ctr), p = 0.05 for wild type (Wt) and 0.01 for Y700F and Y774F. Error bars, S.E. B, c-Cbl Tyr-731 regulates Wnt target gene VEGF depending on its dimerization status. Media harvested from ECs stably expressing FLAG-tagged c-Cbl constructs as above were analyzed for VEGF levels. The mean of triplicates samples is shown. Student's t test was performed to determine statistical significance. Compared with control, p = 0.001 for Wt c-Cbl, 0.026 for Y731E. Error bars, S.E. C, c-Cbl Tyr-731 regulates IL-8 depending on its dimerization status. Media harvested from ECs stably expressing control or various FLAG-c-Cbl constructs and serum-starved and pretreated with vehicle or Wnt3a (50 ng/ml) were analyzed for IL-8 levels. The mean of triplicates samples is shown. Student's t test was performed to determine statistical significance. Compared with control, p = 0.026 for c-Cbl WT and 0.003 for Y731E. Error bars, S.E. D, c-Cbl Tyr-731 phosphorylation status regulates angiogenesis. HUVECs stably expressing control or various FLAG-tagged c-Cbl constructs were serum-starved and treated with Wnt3a (50 ng/ml). Cells were then seeded in a 96-well plate coated with Matrigel and analyzed for tube formation within 24 h. Representative image of three independent experiments is shown. E, tube lengths were measured with ImageJ. Mean of six images shown. Mean of tube length from two separate experiments performed in triplicate is shown. Student's t test with Bonferroni's correction was performed to determine statistical significance. *, compared with control, p = 0.001 WT, Y700F, and Y774F, and p = 0.02 for Y731E. Compared with Y731E, p = 0.001 for E/dUBA. Compared with Y731F, p = 0.18 for F/dimer. Error bars, S.E.
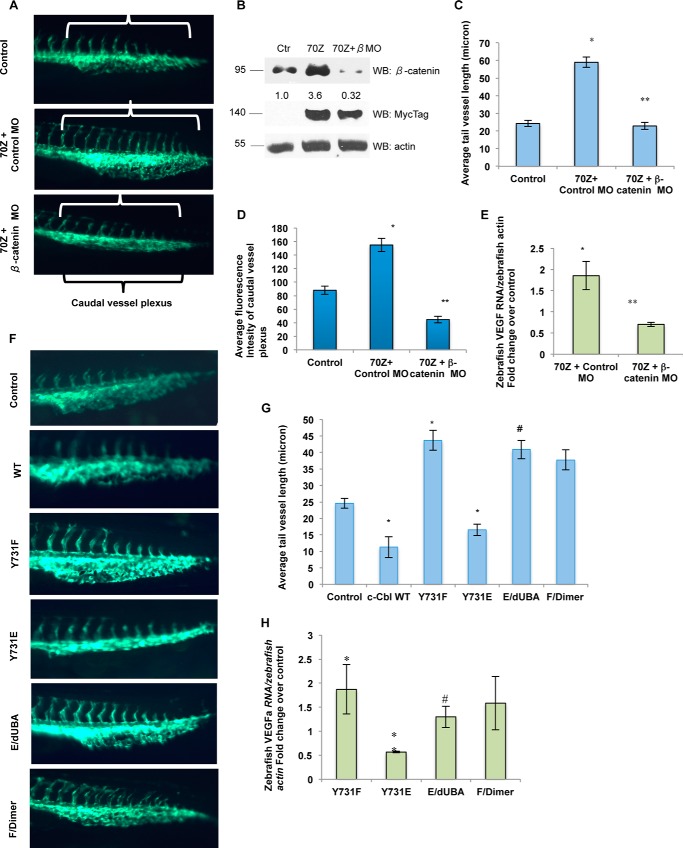
The Fli-eGFP transgenic zebrafish model is a well established tool to examine angiogenesis in vivo, where the ECs are genetically engineered to express enhanced GFP (20, 25). The number and the length of tail vessels, tail microvasculature, and caudal vessel plexus serve as the biological read-outs (20, 25). c-Cbl expression suppressed these angiogenic features (Table 1 and data not shown). 70Z is a naturally occurring E3 ligase-deficient mutant lacking the linker region from amino acids 366 to 382, which binds but fails to down-regulate β-catenin (16). In line with its dominant negative role, zebrafish injected with 70Z showed a significant increase in the length and early bifurcation of tail vessels and caudal vessel plexus and an increase in β-catenin (Fig. 5, A–D, and Table 1). These data provides an in vivo validation of the anti-angiogenic role of c-Cbl.
TABLE 1.
Number of injected Fli-eGFP zebrafish with c-Cbl, 70Z, and 70ZG306E
| Capped RNA | % phenotype in total no. of live zebrafish injected |
|---|---|
| LacZ, 5 ng | 0 |
| LacZ, 10 ng | 1 |
| c-Cbl, 5 nga | 53 (n = 65) |
| c-Cbl, 10 nga | 73 (n = 59) |
| 70Z, 5 ngb | 74 (n = 85) |
| 70Z, 10 ngb | 84 (n = 82) |
| 70ZG306E, 10 ngb | 78 (n = 92) |
a Reduced angiogenesis phenotype characterized by tail vessel length and fluorescent intensity of caudal venous plexus.
b Increased angiogenesis phenotype characterized by tail vessel length and fluorescent intensity of caudal venous plexus.
FIGURE 5.
c-Cbl regulates in vivo angiogenesis through Wnt signaling dependent on Tyr-731 phosphorylation status. A, β-catenin morpholino (MO) significantly abrogates 70Z-mediated angiogenesis. Fli-eGFP transgenic zebrafish two-cell stage embryos injected with 10 ng of mRNA with or without 0.01 mm combined zebrafish ctnnb1 and ctnnb2 MOs (β-catenin MO). LacZ or control MO-injected embryos served as controls. Representative image from randomly selected fish from total injected over three independent experiments is shown (Table 2). The white brackets mark increased tail vessels with bifurcation, and the black bracket marks the caudal vessel plexus. B, 70Z up-regulates endogenous β-catenin in zebrafish. The lysates from 30 de-yolked zebrafish were probed for β-catenin and Myc tag. Representative immunoblot of three experiments is shown. C, 70Z increases intersegmental vessel length in a β-catenin-dependent manner. Mean length of tail vessels of 10 randomly selected fish from total injected (Table 2) is shown. The images under same light exposure settings were obtained from 10 randomly selected fish per group from total live fish over three independent experiments and analyzed for the length of the tail vessels. The tail vessels were marked from the junction of the body and the tail going caudally using Image-Pro® and averaged per group. Error bars, S.E. Student's t test with Bonferroni correction was performed to determine statistical significance. *, compares LacZ control to 70Z, p = 0.024. **, compares 70Z + control MO with 70Z + β-catenin MO, p = 0.001. D, β-catenin MO significantly abrogates 70Z-induced increased caudal vessel plexus. The images of fish under same light exposure settings were obtained for 10 randomly selected fish per group from total live fish over three independent experiments and analyzed for the intensity of caudal vessel plexus using ImageJ. The mean intensity is shown. Student's t test was performed to determine statistical significance. Compared with the control, p = 0.018 for 70Z + control MO. Compared with 70Z + control MO p = 0.0031 with 70Z + β-catenin MO. Error bars, S.E. E, β-catenin MO significantly inhibits c-Cbl-70Z-mediated increase in VEGFa. Zebrafish injected with 5 ng of mRNA and 0.01 mm β-catenin MO as described above were harvested at 24 h post-fertilization for RT-PCR. VEGFa was normalized to zebrafish actin and compared with control animals. Mean of three experiments is shown. *, compares control to 70Z + control MO, p = 0.026; **, compares 70Z + control MO to 70Z + β-catenin MO, p = 0.019. Error bars, S.E. F, c-Cbl regulates angiogenesis dependent on its Tyr-731 phosphorylation status. Fli-eGFP transgenic zebrafish two-cell stage embryos injected as above using 10 ng of mRNA, and the images were obtained and analyzed as above. Representative image from randomly selected fish from total injected are shown (Table 3). G, c-Cbl phosphorylation and dimerization dictates its ability to increase tail vessel length. Tail vessel lengths of Fli-eGFP fish injected with different constructs were analyzed using ImageJ as described above. Mean length of tail vessels from 10 randomly selected fish from total live fish over three independent experiments is shown. Student's t test with Bonferroni correction was performed to determine statistical significance. *, compared with the control, p = 0.01 for c-Cbl WT; p = 0.031 for Y731F; p = 0.026 for Y731E. Compared with Y731E, p = 0.031 for E/UBA. Compared with Y731F, p = 0.103 for F/dimer. Error bars, S.E. H, c-Cbl Tyr-731 phosphorylation regulates VEGFa in zebrafish. Zebrafish injected with 10 ng of mRNAs were harvested at 24 h hpf for RT-PCR for zebrafish VEGFa normalized to zebrafish actin and compared with the controls. Mean of three experiments is shown. p = 0.041 for Y731F; p = 0.001 for Y731E. Compared with Y731E, p = 0.036 for E/UBA. Compared with Y731F p = 0.21 for F/dimer. Error bars, S.E. WB, Western blot. #, p = 0.04.
c-Cbl regulates angiogenesis through other downstream mediators such as receptor tyrosine kinases (12, 13). Specific contribution of Wnt signaling was examined by two orthogonal approaches, 70ZG306E, which up-regulates β-catenin but not some of the receptor tyrosine kinases (16, 26), and β-catenin MO (18, 19). In line with the specific effect on β-catenin, c-Cbl 70ZG306E enhanced angiogenesis (Table 1 and data not shown). β-Catenin MO significantly abrogated 70Z-induced β-catenin levels, an increase in tail vessel length and caudal vessel plexus (Fig. 5, A–D, and Table 2). The effect on zebrafish VEGFa, a target of Wnt, followed the pattern of β-catenin regulation. c-Cbl inhibited and 70Z up-regulated vegfa in a β-catenin-dependent manner (Fig. 5E). All these data indicate that c-Cbl regulates angiogenesis through β-catenin.
TABLE 2.
Number of injected Fli-eGFP zebrafish with 70Z and β-catenin MO
| Capped RNA | % phenotype in total no. of live zebrafish |
|---|---|
| LacZ + control MO | 2.3 (n = 84) |
| 70Z + control MO | 79 (n = 82) |
| 70Z + β-catenin MO | 67 (n = 102) |
We next examined the role of c-Cbl phosphorylation on zebrafish angiogenesis. Phospho-inactive c-Cbl Y731F, which increased β-catenin, also enhanced it, and the phosphomimicking c-Cbl Y731E substantially reduced angiogenesis and zebrafish vegfa mRNA (Fig. 5, F and G, and Table 3). The ability of Y731E to inhibit angiogenesis was significantly compromised by dUBA (E/dUBA). But the artificial dimerization motif in Y731F (F/dimer) failed to rescue this effect, confirming that UBA is not sufficient for c-Cbl's anti-angiogenic effect and that Tyr-731 regulates angiogenesis through dimerization.
TABLE 3.
Number of injected Fli-eGFP zebrafish with c-Cbl constructs
| Capped RNA | % phenotype in total no. of live zebrafish injected |
|---|---|
| LacZ | 2 (n = 56) |
| c-Cbl Y731F | 78 (n = 89) |
| c-Cbl Y731E | 82 (n = 91) |
| Y731E/dUBA | 71 (n = 83) |
| Y731F/dimer | 73 (n = 106) |
DISCUSSION
c-Cbl binding to β-catenin is a critical determinant of its regulation of transcriptionally active nuclear β-catenin. This study implicates Wnt-mediated Tyr-731 phosphorylation of c-Cbl as an important post-translational modification regulating its binding to active β-catenin in the Wnt-on phase. In doing so, we uncovered an intriguing interplay of c-Cbl Tyr-731 phosphorylation and dimerization under different states of Wnt activity.
Dependence of β-catenin regulation on c-Cbl dimerization changed with Wnt status. In the Wnt-off phase, the abrogation of dimerization compromised the binding of both Y731F and Y731E to β-catenin (Fig. 2, B and C) underscoring the dimerization as a necessary requirement for β-catenin binding (16). Also, c-Cbl dimerization was sufficient for its binding to β-catenin, as the artificial dimerization motif rescued the binding of both Y731F and Y731E (F/dimer and E/dimer) to β-catenin (Fig. 2C). In contrast, despite the intact dimerization domain, Y731F lost binding to β-catenin in the Wnt-on phase. The phosphomimicking Y731E mutant showed enhanced β-catenin binding even at baseline. These data implicate c-Cbl dimerization and Tyr-731 phosphorylation status as predominant determinants of c-Cbl-β-catenin binding in the Wnt-off and the Wnt-on phase, respectively.
However, in the Wnt-on phase, c-Cbl dimerization is also important. In the Wnt-on phase, deletion of UBA in Y731E (E/dUBA) substantially abrogated its binding to β-catenin strongly suggesting that Tyr-731 phosphorylation regulates c-Cbl-β-catenin binding through its dimerization. Overall, the data support the notion that in the Wnt-off phase, c-Cbl dimerization is independent of c-Cbl's phosphorylation status and is necessary and sufficient for the c-Cbl-β-catenin interaction. However, in the Wnt-on phase, Wnt-mediated Tyr-731 phosphorylation through its dimerization enhances c-Cbl-β-catenin binding (Fig. 6).
FIGURE 6.
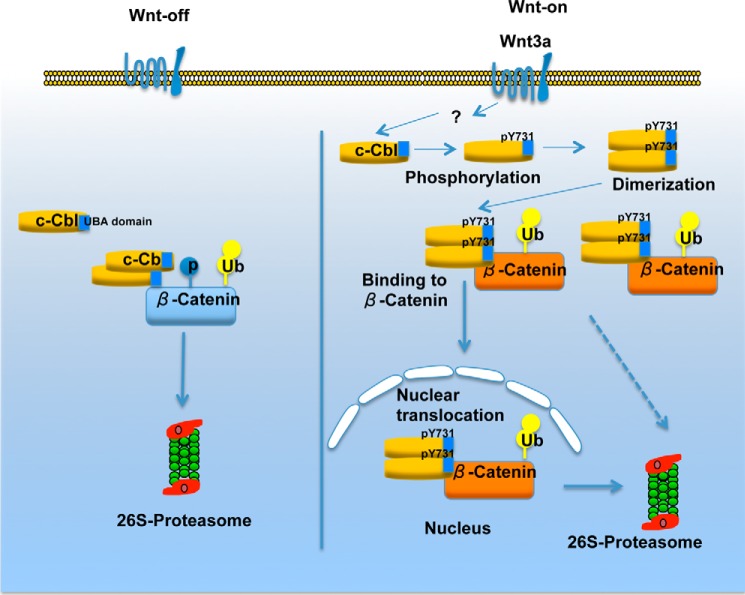
Suggested model of c-Cbl regulating β-catenin. In the Wnt-off phase, c-Cbl exists in cytosol predominantly as monomers, but dimers are also observed. The dimerized species of c-Cbl binds and degrades β-catenin. Wnt-on phase is characterized by hypophosphorylated β-catenin, a species that interacts with c-Cbl, but not other E3 ligases such as β-TrCP or Jade-1. β-Catenin translocates into the nucleus to activate transcription of Wnt target genes (active β-catenin). In parallel, Wnt activation phosphorylates Tyr-731 on c-Cbl to increase its dimerization through the UBA domain facilitating its nuclear translocation. The dimerization also enhances binding and degradation of active β-catenin. Thus, in the Wnt-on phase, c-Cbl chases and suppresses a rapidly expanding pool of active β-catenin.
These observations beg an interesting question. How does c-Cbl Tyr-731 phosphorylation enhance dimerization, β-catenin binding, and c-Cbl nuclear translocation with Wnt activation? There can be different explanations. It is possible that Wnt-mediated Tyr-731 phosphorylation results in a conformational change to enhance c-Cbl dimerization. In turn, dimerization of c-Cbl bestows stoichiometric and/or kinetic advantages for binding to a rapidly expanding pool of active β-catenin molecules during the Wnt-on phase. It is also possible that similar to β-catenin, at baseline c-Cbl can be a part of a multiprotein complex tethered to a yet unknown partner precluding its translocation into the nucleus. Tyr-731 phosphorylation could alter c-Cbl's binding with this putative inhibitory partner, relinquishing it to translocate into the nucleus along with β-catenin. This may also explain why basal c-Cbl-β-catenin binding is insufficient for c-Cbl nuclear translocation during the Wnt-on phase. Although the above models warrant further investigation, the current data uncover Wnt-mediated c-Cbl Tyr-731 phosphorylation regulating its dimerization as a novel mechanism of β-catenin regulation.
The phosphorylation and dimerization of c-Cbl are intertwined events. Bartkiewicz et al. (23) showed that in the presence of EGF, the deletion of UBA of c-Cbl reduced its dimerization, tyrosine phosphorylation, and binding to EGF receptors (22, 23). The authors ascribed it to a reduced number of c-Cbl molecules integrated into EGF receptor complex due to a lack of dimerization, suggesting phosphorylation as a function of dimerization (23). Although their data indicate that c-Cbl dimers are targets of phosphorylation (23), it was not clear whether phosphorylation in turn influenced dimerization. The present data show phosphorylation of c-Cbl Tyr-731 as an upstream event in Wnt signaling. However, as an extension of their model, the data also show that Wnt-mediated c-Cbl Tyr-731 phosphorylation enhances c-Cbl dimerization (Fig. 2, A–C). Thus, c-Cbl dimerization and phosphorylation remain closely interdependent events.
Phosphorylation in canonical Wnt signaling employs GSK-3β and CK1, serine and threonine kinases (9). Because Wnt3a mediates tyrosine phosphorylation of c-Cbl (Fig. 1, A and B), this indicates a possibility of cross-talk. Wnt stimulation increases VEGF secretion (Fig. 4, A and B), which can potentially initiate VEGF signaling. Engagement of VEGF ligand to the VEGFR-2 receptor activates VEGFR-2 signaling, which is known to phosphorylate tyrosine on c-Cbl (27), generating a negative feedback loop. Therefore, Tyr-731 phosphorylation may be a result from the participation of kinases other than those employed in canonical Wnt signaling and underscores nonlinearity in signaling events activated by Wnt ligands.
The above data along with our previous work and that of others (10, 11, 16) support the following model. c-Cbl exists in cytosol as both the monomers and the dimers in the Wnt-off phase. The dimerized species of c-Cbl mediates β-catenin binding and degradation. Wnt-on phase is characterized by hypophosphorylated β-catenin, a species that is degraded by c-Cbl, but not the other E3 ligases such as β-TrCP or Jade-1. β-Catenin molecules translocates into the nucleus to activate transcription of Wnt target genes (active β-catenin). In parallel, Wnt activation phosphorylates Tyr-731 on c-Cbl to increase its dimerization through the UBA domain, binding with β-catenin and c-Cbl nuclear translocation. c-Cbl, thus “chases” and degrades the active β-catenin in the nucleus (Fig. 6).
This study describes Wnt-mediated c-Cbl Tyr-731 phosphorylation as a critical determinant of its binding, and regulation of nuclearly active β-catenin and can be further explored as a biomarker in angiogenesis and cancer. c-Cbl modulating Wnt signaling is consistent with its tumor suppressor activity. The germ line c-Cbl mutations have developmental and functional consequences (28). Hyperactive Wnt signaling has been implicated in several of those conditions (29, 30), which now can be linked through the present data. Overall, the c-Cbl-Wnt-β-catenin relationship has implications both in angiogenesis and tumorigenesis. c-Cbl represents a novel therapeutic target in both of these biological processes.
Acknowledgments
We thank Brent Weinstein (National Institutes of Health) for providing Fli-eGFP transgenic zebrafish. We thank Lili Yu, Veronica Akle, K. Kopotiyenk, and Irina Zhadnova (Boston University School of Medicine) for help with initial zebrafish experiments. We thank the core facilities of Boston University School of Medicine and Dr. Michael Kirber for immunofluorescence imaging at the core facility of the Department of Medicine, Boston University School of Medicine.
This work was supported, in whole or in part, by National Institutes of Health Grant RO1 CA175382 from NCI (to V. C. C.), Grant R01 GM49039 (to E. R. E.), Grants CA191970 and R01EY017955 (to N. R.), Grant T32 HL007501 from NHLBI (to I. H.), and Grant R00CA134743 (to H. F.). This work was also supported by American Cancer Society Grant IRG-72-001-36 (to H. F.) and the Sharon Anderson American Society of Nephrology Fellowship Award (to M. S.).
- EC
- endothelial cell
- c-Cbl
- Casitas B-lineage lymphoma
- HUVEC
- human umbilical vein endothelial cell
- IP
- immunoprecipitation
- DSS
- disuccinimidyl suberate
- MO
- morpholino
- UBA
- ubiquitin-binding domain
- dUBA
- ubiquitin-binding domain deletion.
REFERENCES
- 1. Weis S. M., Cheresh D. A. (2011) Tumor angiogenesis: molecular pathways and therapeutic targets. Nat. Med. 17, 1359–1370 [DOI] [PubMed] [Google Scholar]
- 2. Reis M., Liebner S. (2013) Wnt signaling in the vasculature. Exp. Cell Res. 319, 1317–1323 [DOI] [PubMed] [Google Scholar]
- 3. Parmalee N. L., Kitajewski J. (2008) Wnt signaling in angiogenesis. Curr. Drug Targets 9, 558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nusse R. (2005) Wnt signaling in disease and in development. Cell Res. 15, 28–32 [DOI] [PubMed] [Google Scholar]
- 5. Planutis K., Planutiene M., Holcombe R. F. (2014) A novel signaling pathway regulates colon cancer angiogenesis through Norrin. Sci. Rep. 4, 5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramachandran I., Thavathiru E., Ramalingam S., Natarajan G., Mills W. K., Benbrook D. M., Zuna R., Lightfoot S., Reis A., Anant S., Queimado L. (2012) Wnt inhibitory factor 1 induces apoptosis and inhibits cervical cancer growth, invasion and angiogenesis in vivo. Oncogene 31, 2725–2737 [DOI] [PubMed] [Google Scholar]
- 7. Hu J., Dong A., Fernandez-Ruiz V., Shan J., Kawa M., Martínez-Ansó E., Prieto J., Qian C. (2009) Blockade of Wnt signaling inhibits angiogenesis and tumor growth in hepatocellular carcinoma. Cancer Res. 69, 6951–6959 [DOI] [PubMed] [Google Scholar]
- 8. Dejana E. (2010) The role of wnt signaling in physiological and pathological angiogenesis. Circ. Res. 107, 943–952 [DOI] [PubMed] [Google Scholar]
- 9. MacDonald B. T., Tamai K., He X. (2009) Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chitalia V. C., Foy R. L., Bachschmid M. M., Zeng L., Panchenko M. V., Zhou M. I., Bharti A., Seldin D. C., Lecker S. H., Dominguez I., Cohen H. T. (2008) Jade-1 inhibits Wnt signalling by ubiquitylating β-catenin and mediates Wnt pathway inhibition by pVHL. Nat. Cell Biol. 10, 1208–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Latres E., Chiaur D. S., Pagano M. (1999) The human F box protein β-Trcp associates with the Cul1/Skp1 complex and regulates the stability of β-catenin. Oncogene 18, 849–854 [DOI] [PubMed] [Google Scholar]
- 12. Singh A. J., Meyer R. D., Navruzbekov G., Shelke R., Duan L., Band H., Leeman S. E., Rahimi N. (2007) A critical role for the E3-ligase activity of c-Cbl in VEGFR-2-mediated PLCγ1 activation and angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 104, 5413–5418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meyer R. D., Husain D., Rahimi N. (2011) c-Cbl inhibits angiogenesis and tumor growth by suppressing activation of PLCγ1. Oncogene 30, 2198–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Husain D., Meyer R. D., Mehta M., Pfeifer W. M., Chou E., Navruzbekov G., Ahmed E., Rahimi N. (2010) Role of c-Cbl-dependent regulation of phospholipase Cγ1 activation in experimental choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 51, 6803–6809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meyer R. D., Srinivasan S., Singh A. J., Mahoney J. E., Gharahassanlou K. R., Rahimi N. (2011) PEST motif serine and tyrosine phosphorylation controls vascular endothelial growth factor receptor 2 stability and down-regulation. Mol. Cell. Biol. 31, 2010–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chitalia V., Shivanna S., Martorell J., Meyer R., Edelman E., Rahimi N. (2013) c-Cbl, a ubiquitin E3 ligase that targets active β-catenin: a novel layer of Wnt signaling regulation. J. Biol. Chem. 288, 23505–23517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Swaminathan G., Tsygankov A. Y. (2006) The Cbl family proteins: ring leaders in regulation of cell signaling. J. Cell. Physiol. 209, 21–43 [DOI] [PubMed] [Google Scholar]
- 18. Bellipanni G., Varga M., Maegawa S., Imai Y., Kelly C., Myers A. P., Chu F., Talbot W. S., Weinberg E. S. (2006) Essential and opposing roles of zebrafish β-catenins in the formation of dorsal axial structures and neurectoderm. Development 133, 1299–1309 [DOI] [PubMed] [Google Scholar]
- 19. Zhang M., Zhang J., Lin S. C., Meng A. (2012) β-Catenin 1 and β-catenin 2 play similar and distinct roles in left-right asymmetric development of zebrafish embryos. Development 139, 2009–2019 [DOI] [PubMed] [Google Scholar]
- 20. Hartsough E. J., Meyer R. D., Chitalia V., Jiang Y., Marquez V. E., Zhdanova I. V., Weinberg J., Costello C. E., Rahimi N. (2013) Lysine methylation promotes VEGFR-2 activation and angiogenesis. Sci. Signal. 6, ra104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmidt M. H., Dikic I. (2005) The Cbl interactome and its functions. Nat. Rev. Mol. Cell Biol. 6, 907–918 [DOI] [PubMed] [Google Scholar]
- 22. Liu J., Kimura A., Baumann C. A., Saltiel A. R. (2002) APS facilitates c-Cbl tyrosine phosphorylation and GLUT4 translocation in response to insulin in 3T3-L1 adipocytes. Mol. Cell. Biol. 22, 3599–3609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bartkiewicz M., Houghton A., Baron R. (1999) Leucine zipper-mediated homodimerization of the adaptor protein c-Cbl. A role in c-Cbl's tyrosine phosphorylation and its association with epidermal growth factor receptor. J. Biol. Chem. 274, 30887–30895 [DOI] [PubMed] [Google Scholar]
- 24. Yokouchi M., Kondo T., Houghton A., Bartkiewicz M., Horne W. C., Zhang H., Yoshimura A., Baron R. (1999) Ligand-induced ubiquitination of the epidermal growth factor receptor involves the interaction of the c-Cbl RING finger and UbcH7. J. Biol. Chem. 274, 31707–31712 [DOI] [PubMed] [Google Scholar]
- 25. Nicoli S., Presta M. (2007) The zebrafish/tumor xenograft angiogenesis assay. Nat. Protoc. 2, 2918–2923 [DOI] [PubMed] [Google Scholar]
- 26. Lupher M. L., Jr., Reedquist K. A., Miyake S., Langdon W. Y., Band H. (1996) A novel phosphotyrosine-binding domain in the N-terminal transforming region of Cbl interacts directly and selectively with ZAP-70 in T cells. J. Biol. Chem. 271, 24063–24068 [DOI] [PubMed] [Google Scholar]
- 27. Rahimi N. (2009) A role for protein ubiquitination in VEGFR-2 signalling and angiogenesis. Biochem. Soc. Trans. 37, 1189–1192 [DOI] [PubMed] [Google Scholar]
- 28. Niemeyer C. M., Kang M. W., Shin D. H., Furlan I., Erlacher M., Bunin N. J., Bunda S., Finklestein J. Z., Sakamoto K. M., Gorr T. A., Mehta P., Schmid I., Kropshofer G., Corbacioglu S., Lang P. J., et al. (2010) Germline CBL mutations cause developmental abnormalities and predispose to juvenile myelomonocytic leukemia. Nat. Genet. 42, 794–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harisis G. N., Chen N., Farmer P. J., Bodemer D., Li R., Sourial M., Southwell B. R., Balic A., Hutson J. M. (2013) Wnt signalling in testicular descent: a candidate mechanism for cryptorchidism in Robinow syndrome. J. Pediatr. Surg. 48, 1573–1577 [DOI] [PubMed] [Google Scholar]
- 30. Luscan A., Shackleford G., Masliah-Planchon J., Laurendeau I., Ortonne N., Varin J., Lallemand F., Leroy K., Dumaine V., Hivelin M., Borderie D., De Raedt T., Valeyrie-Allanore L., Larousserie F., Terris B., et al. (2014) The activation of the WNT signaling pathway is a hallmark in neurofibromatosis type 1 tumorigenesis. Clin. Cancer Res. 20, 358–371 [DOI] [PubMed] [Google Scholar]