Background: Autocatalytic processing is required for Psd1p function. Molecular requirements for Psd1p autocatalysis are largely undefined.
Results: Psd1p autocatalysis occurs in yeast mutants lacking its substrate or mitochondrial-specific lipids. Furthermore, Psd1p re-directed to the endoplasmic reticulum undergoes autocatalysis and is functional in vivo.
Conclusion: Psd1p autocatalysis does not require its substrate or mitochondrial-specific lipids, proteins, or co-factors.
Significance: Once membrane-embedded, Psd1p is autocatalytically self-sufficient.
Keywords: membrane, membrane biogenesis, mitochondria, phosphatidylethanolamine, phospholipid, yeast, autocatalysis
Abstract
Phosphatidylethanolamine (PE) is a major cellular phospholipid that can be made by four separate pathways, one of which resides in the mitochondrion. The mitochondrial enzyme that generates PE is phosphatidylserine decarboxylase 1 (Psd1p). The pool of PE produced by Psd1p, which cannot be compensated for by the other cellular PE metabolic pathways, is important for numerous mitochondrial functions, including oxidative phosphorylation and mitochondrial dynamics and morphology, and is essential for murine development. To become catalytically active, Psd1p undergoes an autocatalytic processing step involving a conserved LGST motif that separates the enzyme into α and β subunits that remain non-covalently attached and are anchored to the inner membrane by virtue of the membrane-embedded β subunit. It was speculated that Psd1p autocatalysis requires a mitochondrial-specific factor and that for Psd1p to function in vivo, it had to be embedded with the correct topology in the mitochondrial inner membrane. However, the identity of the mitochondrial factor required for Psd1p autocatalysis has not been identified. With the goal of defining molecular requirements for Psd1p autocatalysis, we demonstrate that: 1) despite the conservation of the LGST motif from bacteria to humans, only the serine residue is absolutely required for Psd1p autocatalysis and function; 2) yeast Psd1p does not require its substrate phosphatidylserine for autocatalysis; and 3) contrary to a prior report, yeast Psd1p autocatalysis does not require mitochondrial-specific phospholipids, proteins, or co-factors, because Psd1p re-directed to the secretory pathway undergoes autocatalysis normally and is fully functional in vivo.
Introduction
Phosphatidylserine decarboxylase 1 is a mitochondrial resident protein (1, 2) that decarboxylates phosphatidylserine (PS)2 within mitochondria to generate phosphatidylethanolamine (PE) (2). PE, the second most abundant phospholipid in eukaryotes, is a precursor to phosphatidylcholine (3–6), the major membrane phospholipid. In yeast, two enzymes mediate the production of PE from PS; Psd1p located in the mitochondrion (7–9), and Psd2p, a resident of endosomes (10). Yeast without PSD1 and PSD2 cannot grow unless supplemented with ethanolamine, which feeds production of PE via the cytidine diphosphate (CDP)-ethanolamine pathway (11–13). Although Psd2p is unique to yeast, Psd1p is an essential protein in mammals and has been evolutionarily conserved from bacteria to yeast to metazoans (14). The mitochondrial PS decarboxylation pathway and the endoplasmic reticulum (ER)-localized CDP-ethanolamine (Kennedy) pathways produce the majority of PE in cells. This compartmentalization suggests that the pools of PE made in these organelles may be functionally distinct. Indeed, disruption of either of the two major PE-producing pathways (the CDP-ethanolamine and Psd pathways) is embryonically lethal in mice (15, 16). Thus, the PE produced by each pathway has independent functions that are required during mammalian development.
The fact that one of the major PE producing pathways is localized to the mitochondrion suggests that PE produced within the mitochondrion is critical for normal mitochondrial functions. It further suggests that mechanisms to import PE produced in the ER into the mitochondrion are either lacking or inefficient. Indeed, PE produced by the CDP-ethanolamine pathway is poorly incorporated into mitochondrial membranes (11, 12, 17). The absence of Psd1p in yeast or mammalian cells affects mitochondrial morphology, impairs cell growth, and diminishes respiratory capacity (18–20). Furthermore, psd1Δ yeast lose their mitochondrial genome with a higher frequency (11, 21). PE physically associates with both respiratory complex III (22) and IV (23); however, of these two complexes, only the activity of complex IV is reduced in psd1Δ yeast (18). In addition, PE has recently been implicated in mitochondrial fusion (19) and import of proteins across the outer membrane (24). Even though Psd1p provides a privileged pool of PE that is crucial for normal mitochondrial physiology, our understanding of Psd1p itself is surprisingly limited.
Psd1p is synthesized as a zymogen and must be imported into the inner mitochondrial membrane where it is processed to achieve its functional state (7, 21). Upon import into the mitochondrion, the mitochondrial targeting sequence (MTS) of Psd1p is removed by the sequential action of two matrix peptidases, mitochondrial processing peptidase and Oct1p (7). Autocatalytic processing of Psd1p, an obligate step for its function (25), can occur before or after its integration into the mitochondrial inner membrane (IM) (7). Autocatalysis occurs within a conserved LGST motif between the glycine and the serine residues through a process of serinolysis (7, 25–29). Serinolysis separates the enzyme into mature α and β subunits, which remain non-covalently associated, and generates a pyruvoyl prosthetic group at the NH2 terminus of the α subunit that is absolutely required for enzymatic activity (25, 29, 30). The β subunit is integrated into the IM of the mitochondrion and serves to anchor the α subunit to the intermembrane space (IMS) side of the IM (7, 8). Although unrelated to Psd1p activity, the amino terminus of Psd1p is necessary and sufficient to induce multidrug resistance via Pdr5p signaling in yeast (27). Virtually nothing else is known about structural motifs required for Psd1p activity.
In Plasmodium knowlesi, pkPsd1 autocatalysis is accelerated by its substrate PS (31), raising the possibility that Psd1p autocatalysis is substrate-dependent. In the yeast Saccharomyces cerevisiae, Psd1p lacking an NH2-terminal transmembrane domain is mislocalized to the matrix but still undergoes autocatalysis (7). Interestingly, although this mutant Psd1p allele retains catalytic activity in vitro (7), it is non-functional in vivo (7, 27, 31). This was taken as evidence that for Psd1p to function in vivo, it must be properly integrated into the IM with its active site facing the IMS (7). However, the results derived from the matrix mislocalized Psd1p could simply reflect the absence of PS on the matrix side of the IM, which has not been demonstrated, or alternatively, an inability of Psd1p lacking a membrane anchor to position the α subunit at the membrane surface to decarboxylate PS. In the same study, in organello import studies demonstrated that radiolabeled Psd1p is readily imported and undergoes autocatalysis in mitochondria but not microsomes (7). As such, it was concluded that a mitochondrial-specific factor(s) is necessary for Psd1p autocatalysis and thus for Psd1p function. However, the failure of Psd1p to undergo autocatalysis when incubated with microsomes could simply reflect its inability to engage the ER translocation machinery.
Given the central importance of Psd1p in cellular and mitochondrial PE metabolism, it is crucial to define the molecular requirements for autocatalysis of Psd1p because this process is required for Psd1p to become functional. In this study, we demonstrate that although the entire LGST motif is widely conserved, only the serine residue is absolutely required for Psd1p autocatalysis, activity, and function. Further yeast Psd1p autocatalysis does not require its substrate (PS), nor does it require mitochondrial-specific lipids, proteins, or co-factors. Indeed, Psd1p targeted to the secretory pathway is autocatalytically competent and fully functional in vivo. Thus, for efficient autocatalysis, yeast Psd1p must have the capacity to engage a membrane-embedded translocon. Furthermore, to function in cells, processed Psd1p must have access to its substrate, PS.
EXPERIMENTAL PROCEDURES
Yeast Strains and Growth Conditions
All yeast strains used in this study were isogenic to GA74-1A (MATa, his3-11,15, leu2, ura3, trp1, ade8 [rho+, mit+]). psd1Δ, pss1Δ, and pgs1Δ were generated by replacing the entire open reading frames of PSD1, PSS1, and PGS1 with HIS3MX6. The psd1Δpsd2Δ was generated by replacing the entire open reading frames of PSD1 and PSD2 with TRP1 and HIS3MX6, respectively. Yeast were grown in either rich lactate (1% yeast extract, 2% tryptone, 0.05% dextrose, 2% lactic acid, 3.4 mm CaCl2-2H2O, 8.5 mm NaCl, 2.95 mm MgCl2-6H2O, 7.35 mm KH2PO4, 18.7 mm NH4Cl, pH 5.5) or YPD (1% yeast extract, 2% peptone, 2% dextrose). To assess the function of the assorted Psd1p constructs and mutants, overnight cultures grown in synthetic complete dextrose (SCD; 0.17% yeast nitrogen base, 0.5% ammonium sulfate, 0.2% complete amino acid mixture, 2% dextrose) supplemented with 2 mm ethanolamine were spotted on synthetic complete dextrose plates in the absence or presence of 2 mm ethanolamine, as indicated. Deletion strains were generated by PCR-mediated gene replacement of the entire open reading frame as previously described (32, 33).
PSD1 was amplified from genomic DNA isolated from GA74-1A yeast using primers that hybridized 418 bp 5′ of the predicted start codon and 185 bp 3′ of the predicted stop codon and subcloned into pRS315. Psd1p with a COOH-terminal 3× FLAG tag was generated by overlap extension (34) using pRS315PSD1 as template and subcloned into pRS305. PSD1 point mutations were also generated by overlap extension using pRS305Psd3XFLAG as template. To re-direct Psd1p to the secretory pathway, the first 57 amino acids of Psd1p, encompassing its MTS, was replaced by the NH2-terminal signal sequence (amino acids 1–23) of carboxypeptidase Y (CPY (35)). An NXS (X is an alanine here) N-glycosylation signal was included immediately downstream of the CPY leader sequence to determine the topology of CPY*mPsd1p. All psd1Δ and psd1Δpsd2Δ transformants utilized linearized pRS305-based constructs and genomic integrants were selected on synthetic dropout media (0.17% yeast nitrogen base, 0.5% ammonium sulfate, 0.2% dropout mixture synthetic −leu, 2% dextrose).
For the in vitro experiments, six methionine residues were added to the COOH terminus to allow detection of the α subunit post-autocatalysis. To monitor Psd1p and CPY*mPsd1p autocatalysis in vivo, the COOH-terminal six methionines were replaced by a 3× FLAG tag.
Subcellular Fractionation and Mitochondrial Isolation
Subcellular fractionation and isolation of crude mitochondria was performed as previously described (36). To isolate pure mitochondria, crude mitochondrial pellets were resuspended at 4 mg/ml in SEM buffer (250 mm sucrose, 1 mm EDTA, 10 mm MOPS, pH 7.2) by 10 strokes in a Teflon Dounce. Mitochondria were layered onto a sucrose step gradient composed of 1.5 ml of 15% sucrose, 1.5 ml of 23% sucrose, 4 ml of 32% sucrose, and 1.5 ml of 60% sucrose in EM buffer (1 mm EDTA, 10 mm MOPS, pH 7.2) to remove cytosolic and ER contaminants as described (37). Purified mitochondria were obtained from the 32–60% sucrose interface after the gradients were centrifuged at 134,000 × g for 1 h at 4 °C. Purified mitochondria were resuspended in SEM buffer to dilute the sucrose concentration and re-collected by centrifugation at 13,500 × g for 10 min at 4 °C. Mitochondria were washed in ice-cold BB7.4 (0.6 m sorbitol and 20 mm Hepes-KOH, pH 7.4), the amount of protein determined using the BCA assay, and aliquots were snap frozen in liquid nitrogen and stored at −80 °C.
Liposome Preparation
Liposomes were prepared as previously described (8). In brief, 1-palmitoyl-2-{12-[(7-nitro-2–1,3-benzoxadiazol-4-yl)amino]dodecanoyl}-sn-glycero-3-phosphoethanolamine (NBD-PE; catalogue number 810154), 1-palmitoyl-2-{12-[(7-nitro-2–1,3-benzoxadiazol-4-yl)amino]dodecanoyl}-sn-glycero-3-phosphoserine (NBD-PS; catalogue number 810193), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (catalogue number 850457), and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (catalogue number 850757) were purchased from Avanti Polar Lipids. 0.2 ml of a 3 mm liposome stock containing 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine:1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine:NBD-PS at a 77:20:3 molar ratio was prepared, dried under nitrogen gas, and resuspended in 800 μl of import buffer (300 mm sucrose, 150 mm KCl, 1 mm DTT, 10 mm Tris-HCl, pH 7.5). Following a brief vortex, the lipid solution was hydrated overnight at room temperature and extruded 30 times using a mini extruder (Avanti Polar Lipids) with 0.2-μm polycarbonate membranes to form unilamellar vesicles.
Psd1p Activity Assay
200–250 μg of mitochondria or microsomes was resuspended in 75 μl of import buffer and incubated with 25 μl of liposomes at the indicated temperature for 40 min. To stop the reaction, 400 μl of cold SEM buffer was added and spun at 12,000 × g for 5 min at 4 °C to isolate the mitochondria or 100,000 × g for 10 min at 4 °C (TLA120.1 rotor) to sediment the microsomes. Organellar lipids were extracted as previously reported (8) and resolved once using Silica Gel GHLF TLC plates (Analtech, Inc.) or ADAMANT TLC plates (Machery-Nagel) as described (36). NBD-PS and NBD-PE were imaged using a PharosFX molecular imager (Bio-Rad) and quantified using the affiliated Quantity One software.
In Organello Import
Radiolabeled precursors were produced using an SP6 Quick Coupled Transcription/Translation system (Promega) spiked with Easy-Tag l-[35S]methionine (PerkinElmer Life Sciences). In organello import into mitochondria was performed exactly as described previously (38) using mitochondria isolated from D273-10B yeast grown in rich lactate. The mitochondrial proton-motive force was collapsed where indicated by pre-incubating mitochondria for 5 min at 30 °C with 1 μm valinomycin and 5 μm carbonyl cyanide m-chlorophenyl hydrazine. At the indicated times, import was stopped, and non-imported precursor was degraded with an equal volume of ice-cold BB7.4 containing 40 μg/ml of trypsin. Trypsin was inhibited with 100 μg/ml of soybean trypsin inhibitor, and mitochondria were re-isolated by spinning at 21,000 × g for 5 min at 4 °C. 100% of each time point and 5% of imported precursors were resolved on 15% SDS-PAGE gels and analyzed by phosphorimaging. Targeting into the secretory pathway was tested by in vitro transcription and translation with and without canine pancreatic microsomes (Promega) essentially as described (39). Microsomes were collected by spinning at 21,000 × g for 5 min at 4 °C, resuspended in glycoprotein denaturing buffer (New England BioLabs), and incubated at 100 °C for 10 min. For removal of N-glycans, equal volumes of microsomes were digested at 37 °C for 2 h in the absence (mock) or presence of endoglycosidase H (EndoH; New England BioLabs). Samples were resolved on 15% SDS-PAGE gels and analyzed by phosphorimaging.
Antibodies
Most antibodies used in this study were generated in our laboratory or in the laboratories of J. Schatz (University of Basel, Basel, Switzerland) or C. Koehler (UCLA) and have been described previously (36, 40–44). Other antibodies used were mouse anti-Sec62p (kind gift of Dr. David Meyers, University of California, Los Angeles, CA) and horseradish peroxidase (Thermo Fisher Scientific)-conjugated secondary antibodies.
Miscellaneous
Sequence alignment of Psd across different species was performed using Clustal Omega software (45–47). Preparation of yeast cell extracts, phospholipid analysis, and immunoblotting were performed as described previously (36). For removal of N-glycans from whole cell extracts, yeast grown to saturation at 30 °C in YPD were digested at 37 °C for 4 h in the absence (mock) or presence of EndoH. Samples were resolved by 12% SDS-PAGE gel and analyzed by immunoblot. Statistical comparisons were performed by t test or one-way analysis of variance using SigmaPlot 11 software (Systat software). All graphs show the mean ± S.E.
RESULTS
Characterization of the Conserved LGST motif of Psd1p
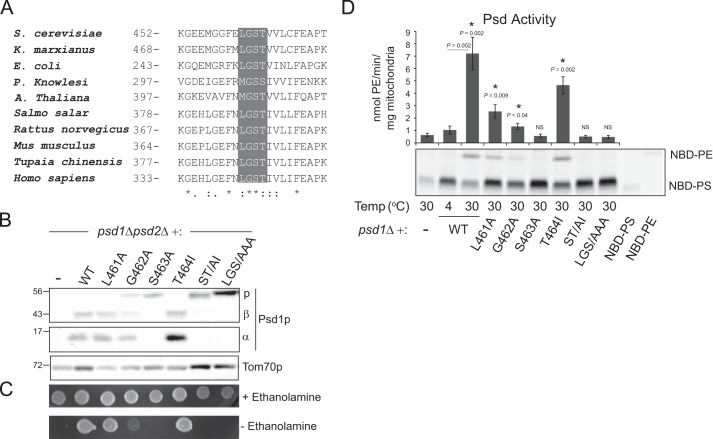
The LGST motif is widely conserved from bacteria to humans (25, 29) (Fig. 1A). Although the importance of this motif for Psd1p autocatalysis and/or function has been established in bacteria (29), Plasmodium falciparum (31), yeast (7), and mammals (28), to our knowledge there has been no systematic investigation of the individual role of each amino acid of the LGST motif. Therefore, to gain further insight into the function of the individual residues of the LGST motif, yeast Psd1p mutants harboring single and combined LGST mutations were expressed in a psd1Δpsd2Δ strain. In the absence of both Psd1p and Psd2p, yeast are ethanolamine auxotrophs (11, 12). Thus, the ability of a mutant Psd1p construct to support growth of a psd1Δpsd2Δ strain in the absence of ethanolamine reports on its functionality. As our Psd1p antiserum only detects the β subunit, a 3× FLAG tag was appended to the COOH terminus of each construct to allow detection of the α subunit post-autocatalysis. Importantly, wild type (wt) Psd1p with an added COOH-terminal 3× FLAG tag was fully functional (Fig. 1, B and C). Consistent with other studies (27), substitution of LGS for three alanines (LGS/AAA) completely prevented autocatalysis (Fig. 1B); furthermore, such a mutant failed to rescue the ethanolamine auxotrophy of the psd1Δpsd2Δ strain (Fig. 1C). Mutating the leucine (L461A) or threonine (T464I) residues did not cause any demonstrable autocatalytic defect (Fig. 1B). Only mutation of the serine residue (S463A), with or without additional mutation of the neighboring threonine (ST/AI), completely prevented any detectable autocatalysis. Mutation of the glycine (G462A) adjacent to the obligate serine significantly impaired autocatalysis; however, some processed Psd1p was still detected. Consistent with a low level of autocatalysis, the G462A Psd1p mutant supported some growth of the psd1Δpsd2Δ host strain in the absence of ethanolamine (Fig. 1C). In contrast, the S463A, ST/AI, and LGS/AAA mutants all failed to rescue the psd1Δpsd2Δ ethanolamine auxotrophy. The L461A and T464I Psd1p mutants fully promoted growth in the absence of ethanolamine supplementation. Next, the activity of each mutant was measured (Fig. 1D). Unilamellar liposomes containing PC, PE, and fluorescently labeled PS (NBD-PS) were incubated at 4 or 30 °C with mitochondria isolated from psd1Δ yeast (−) or psd1Δ yeast transformed as indicated. As expected based on the growth studies, mutation of the conserved serine residue singly (S463A) or in combination (ST/AI and LGS/AAA) completely abolished PS decarboxylase activity. In contrast, the L461A, G462A, and T464I mutants all retained some, albeit reduced, PS decarboxylase activity. Thus, the serine residue is the only amino acid of the highly conserved LGST motif that is absolutely required for Psd1p autocatalysis and activity.
FIGURE 1.
Only the serine residue of the conserved LGST motif is essential for yeast Psd1p autocatalysis and function. A, Clustal Omega sequence alignment of Psd from the indicated species. Gray box highlights the conserved LGST motif. Asterisks indicate identical residues. Single dots indicate lesser conserved residues, and double dots indicate highly conserved residues. B, psd1Δpsd2Δ yeast (−) and psd1Δpsd2Δ yeast transformed with the indicated Psd1p construct were grown overnight in synthetic complete dextrose (SCD) medium supplemented with 2 mm ethanolamine. The α and β subunits of Psd1p were detected in whole cell extracts by immunoblot; Tom70p served as a loading control. C, psd1Δpsd2Δ yeast (−) and psd1Δpsd2Δ yeast transformed with the indicated Psd1p constructs were spotted onto SCD plates ±2 mm ethanolamine and incubated at 30 °C for 48 h. D, mitochondria were isolated from psd1Δ yeast (−) and psd1Δ yeast transformed with the indicated Psd1p construct grown in rich lactate medium. Mitochondria were incubated with NBD-PS at the indicated temperature for 40 min, and phospholipids were separated by TLC. Psd activity was determined as the amount of NBD-PE formed per minute per mg of mitochondria (mean ± S.E., n = 6). 10 nmol of NBD-PS and NBD-PE were resolved by TLC as migration standards. Significant differences were determined using Student's t test. The asterisks indicate a significant difference compared with psd1Δ (−). NS, not significant relative to psd1Δ (−).
Psd1p Autocatalysis Does Not Require Its Substrate
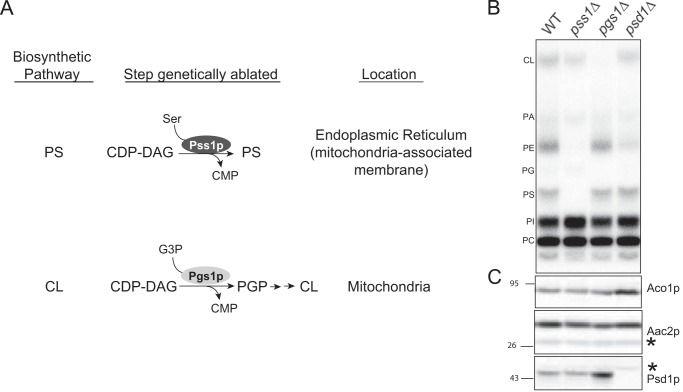
To test whether yeast Psd1p requires its substrate (PS) for autocatalysis, we adopted a genetic approach. In brief, PS is synthesized in the mitochondrial-associated membrane subcompartment of the ER by phosphatidylserine synthase-1 (Pss1p) (17, 48–52)) (Fig. 2A). Yeast lacking PSS1, the gene encoding Pss1p, are auxotrophic for ethanolamine (or choline) due to the absence of PS used by Psd1p and other PE biosynthetic pathways (53) (Fig. 2B). However, in pss1Δ yeast, the β subunit of endogenous Psd1p co-migrated with Psd1p in wt yeast indicating that autocatalysis still occurred (Fig. 2C). Therefore, the substrate PS is not needed for Psd1p autocatalysis in yeast. However, it is possible that in yeast, similar to P. knowlesi, PS may increase the autocatalytic rate of Psd1p (31).
FIGURE 2.
Autocatalytic processing of Psd1p does not require its substrate or mitochondrial-specific phospholipids. A, schematic showing the subcellular localization and steps genetically targeted in the PS and CL biosynthetic pathways, respectively. CDP-DAG, cytidine diphosphate-diacylglycerol; CMP, cytidine monophosphate; G3P, glycerol 3-phosphate; PGP, phosphatidylglycerol phosphate. B, the indicated yeast strains were grown overnight in YPD spiked with 10 μCi/ml of 32Pi to radiolabel mitochondrial phospholipids, which were extracted and then separated by TLC. PA, phosphatidic acid; PI, phosphatidylinositol. C, Psd1p was analyzed by immunoblot in whole cell extracts from the indicated strains following growth in YPD overnight; Aco1p and Aac2p served as loading controls. The asterisks indicate nonspecific bands that react with the antibodies.
A Mitochondrial-specific Lipid Is Not Obligatorily Required for Psd1p Autocatalysis
The failure of Psd1p to undergo autocatalysis when incubated with microsomes (7) suggests that there may exist some mitochondrial-specific factor that is required for this process. To interrogate whether Psd1p requires a unique mitochondrial lipid environment, we again took a genetic approach. Cardiolipin (CL) and its upstream intermediate, phosphatidylglycerol (PG), are unique mitochondrial lipids (54). Phosphatidylglycerolphosphate synthase (Pgs1p) functions at an early step in the CL biosynthetic pathway (55) (Fig. 2A). In pgs1Δ yeast, which lack both PG and CL (Fig. 2B), Psd1p autocatalysis still occurred (Fig. 2C). Thus, Psd1p autocatalysis does not require a phospholipid that is unique to mitochondria.
ER-targeted Psd1p Undergoes Autocatalysis in Vitro
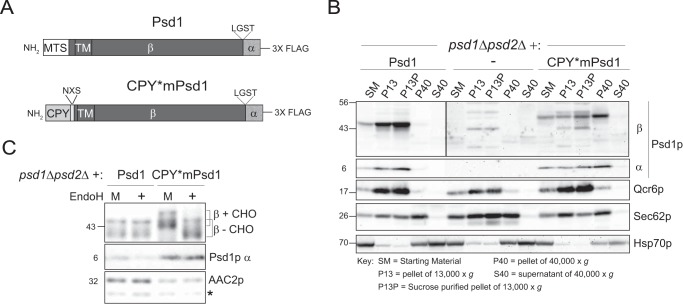
The inability of radiolabeled Psd1p to undergo autocatalysis when incubated with microsomes (7) could reflect a requirement for a mitochondrial-specific protein or co-factor, or alternatively, result from its inability to engage the ER translocation machinery due to the absence of a signal sequence in Psd1p. To distinguish between these two possibilities, we generated a chimeric in vitro construct (Fig. 3A). First, the MTS of Psd1p was replaced with the signal sequence of carboxypeptidase Y (35). An NXS N-glycosylation signal was also added immediately following the CPY signal sequence but upstream of the transmembrane domain in Psd1p. In addition, six methionine residues were added to the COOH terminus of Psd1p and CPY*mPsd1p, allowing us to detect both the α and β subunits post-autocatalysis. 35S-Labeled Psd1p or CPY*mPsd1p were separately incubated with purified mitochondria or canine microsomes. As expected (7), Psd1p was imported into mitochondria and underwent autocatalysis in a time- and membrane-potential dependent manner (Fig. 3B, the released α subunit is highlighted by the arrow). In contrast, CPY*mPsd1p was not imported into mitochondria and failed to undergo autocatalysis regardless of the presence or absence of a proton-motive force. However, when CPY*mPsd1p was incubated with microsomes, it now became autocatalytically competent, whereas Psd1p failed to undergo autocatalysis (Fig. 3C, the released α subunit is highlighted by the arrow). Moreover, the added N-glycosylation signal of CPY*mPsd1p accessed the microsomal lumen based on the increased mobility of CPY*mPsd1p upon treatment with EndoH, which removes immature N-glycans (Fig. 3D). Thus, Psd1p can undergo autocatalysis in a non-mitochondrial organelle as long as it contains suitable information allowing it to engage the translocation machinery of the organelle.
FIGURE 3.
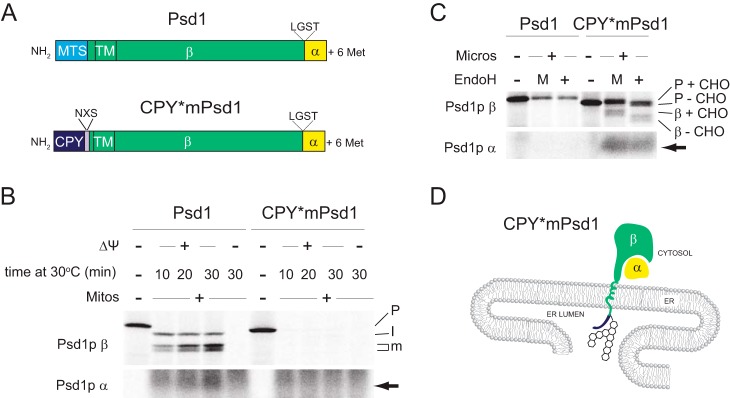
ER-targeted Psd1p undergoes autocatalysis in vitro. A, schematic of in vitro Psd1 and CPY*mPsd1 constructs. MTS, mitochondrial targeting sequence; TM, transmembrane domain; CPY, ER targeting signal of carboxypeptidase Y; NXS, inserted N-glycosylation signal. B, in vitro import of [35S]methionine-labeled Psd1 or CPY*mPsd1 into wt mitochondria in the presence (+ΔΨ) or absence (−ΔΨ) of a proton-motive force across the IM. Non-imported precursor was removed with trypsin. 5% of each precursor (−) and 100% of every time point were analyzed. P, precursor; I, import intermediate; m, mature β subunit. C, Psd1 or CPY*mPsd1 were [35S]methionine-labeled in vitro in the absence (−) or presence (+) of canine pancreatic microsomes. Microsomal membranes were harvested and mock-treated (M) or treated (+) with EndoH. For CPY*mPsd1, glycosylated (+CHO) and deglycosylated (−CHO) precursor (P) and β subunits are indicated. In B and C, the arrow indicates the position of the α subunit post-autocatalysis. D, a schematic depicting the topology of autocatalytically processed CPY*mPsd1 embedded in microsomes. The appended immature N-glycan is depicted.
ER-targeted Psd1p Is Functional in Vivo
Psd1p lacking its transmembrane domain is mistargeted to the mitochondrial matrix and retains residual catalytic activity in the presence of detergents, but fails to rescue PE levels in a psd1Δ host (7). This could reflect the fact that PS is not normally present on the matrix side of the IM (this is not known) or instead that the membrane anchor is required for the β subunit to hold the α subunit near the surface of the membrane where it decarboxylates PS to PE. As PS is made in the ER (Fig. 2A), substrate should not be limiting for Psd1p re-directed to this compartment. Therefore, a 3× FLAG tag was added to the COOH terminus of CPY*mPsd1p in place of the six methionines (Fig. 4A). Upon expression in psd1Δpsd2Δ yeast, CPY*mPsd1p co-fractionated with the ER, in contrast to Psd1p, which was enriched in the mitochondrion (Fig. 4B). Upon treatment with EndoH, only the mobility of CPY*mPsd1p was increased (Fig. 4C). As N-glycosylation occurs in the ER and not the mitochondrion, these results indicate that CPY*mPsd1p is targeted to the ER in vivo.
FIGURE 4.
Re-directing Psd1p to the ER in vivo. A, schematic of in vivo Psd1 and CPY*mPsd1 constructs. MTS, mitochondrial targeting sequence; TM, transmembrane domain; CPY, ER targeting signal of carboxypeptidase Y; NXS, inserted N-glycosylation signal. B, subcellular fractions were prepared from psd1Δpsd2Δ yeast (−) and psd1Δpsd2Δ yeast transformed with Psd1 or CPY*mPsd1 grown in YPD via a series of differential centrifugations. Thirty micrograms of each fraction were resolved by SDS-PAGE and immunoblotted for both subunits of Psd1p (α and β) and the following organelle markers; Qcr6p (mitochondria), Sec62p (ER), and Hsp70p (cytosol). C, whole cell extracts derived from psd1Δpsd2Δ yeast transformed with Psd1 or CPY*mPsd1 grown in YPD were mock-treated (M) or treated (+) with EndoH for 4 h at 37 °C. The β and α subunits of Psd1p were detected by immunoblot with Aac2p serving as a loading control. For CPY*mPsd1, glycosylated (+CHO) and deglycosylated (−CHO) β subunits are indicated.
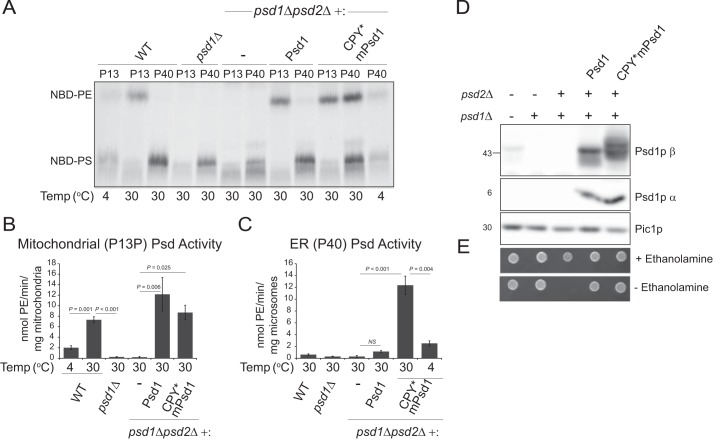
Importantly, ER-directed CPY*mPsd1p is functional. Mitochondria (P13) and ER (P40) derived from psd1Δ and psd1Δpsd2Δ yeast lacked any detectable Psd activity (Fig. 5, A–C). As expected, wt yeast or psd1Δpsd2Δ yeast transformed with Psd1p properly localized to the mitochondrial inner membrane had significant Psd activity in the P13/mitochondrial fraction (Fig. 5B) but not the P40/ER fraction (Fig. 5C). In contrast, psd1Δpsd2Δ yeast transformed with CPY*mPsd1p had significant PS decarboxylase activity in the P40/ER fraction (Fig. 5, A and C). As expected based on the PS decarboxylase activity, CPY*mPsd1p was autocatalytically competent just like its wt counterpart (Fig. 5D). Finally, CPY*mPsd1p rescued the ethanolamine auxotrophy of the psd1Δpsd2Δ strain similar to Psd1p (Fig. 5E). Thus, Psd1p does not need to be embedded in the mitochondrial IM facing the IMS to function to provide PE to the rest of the cell.
FIGURE 5.
Psd1p re-directed to the ER is functional in vivo. A, mitochondrial (P13P from Fig. 4B) and microsomal (P40) fractions from wt yeast (WT), psd1Δ yeast, psd1Δpsd2Δ yeast (−), and psd1Δpsd2Δ yeast transformed with Psd1 or CPY*mPsd1 grown in YPD were incubated with NBD-PS at the indicated temperature for 40 min. Lipids were extracted, separated by TLC, and NBD-labeled species detected with a PharosFX imager. B, mitochondrial and C, microsomal Psd activities were determined (mean ± S.E., n = 3). Significant differences were determined by one-way analysis of variance (psd1Δpsd2Δ transformed with Psd1p or CPY*mPsd1 compared with psd1Δpsd2Δ (−)) or Student's t test (30 versus 4 °C; wt versus psd1Δ). D, the β and α subunits of Psd1p were analyzed by immunoblot in whole cell extracts derived from the indicated strains grown overnight in YPD; Pic1p served as a loading control. E, the indicated strains were spotted onto SCD ± 2 mm ethanolamine and grown for 3 days at 30 °C.
DISCUSSION
Psd1p plays a pivotal role in the generation of PE across many species. To fulfill its vital role as a source of PE that is crucial at both the mitochondrial and cellular level, Psd1p undergoes an unusual self-processing event that generates a pyruvoyl prosthetic group required for the decarboxylase reaction (25, 29, 30). Our understanding of this autocatalytic event is incomplete and whether factors not provided by the Psd1p polypeptide itself are required is presently unknown. Therefore, in this study, we have performed a comprehensive analysis of every single amino acid residue of the conserved LGST motif. The S463A, ST/AI, and LGS/AAA mutants all failed to undergo autocatalysis or rescue the psd1Δpsd2Δ ethanolamine auxotrophy, whereas the L461A and T464I mutations did not cause any autocatalytic defect and were able to fully promote growth in the absence of ethanolamine supplementation. Mutation of the glycine adjacent to the obligate serine significantly impaired autocatalysis; however, some processed Psd1p was still detected and the G462A mutant retained some catalytic activity. Therefore, of the four residues in the conserved LGST motif, only the serine is absolutely required for autocatalysis. This is consistent with studies in both bacteria, where a mutation equivalent to yeast S463A abolishes enzyme activity (29), and eukaryotes (mammalian cells and yeast), where an S463A mutant is not fully processed and lacks activity (7, 28). Interestingly, whereas the G462A Psd1p mutant supported some growth of the psd1Δpsd2Δ host strain in the absence of ethanolamine on solid medium, in liquid-based media, the G462A mutant had a severe growth defect in the absence of ethanolamine relative to the other non-serine LGST mutants (data not shown). Combined with its partial autocatalytic defect, this suggests that the glycine residue of the LGST motif likely plays an important albeit not required structural role. Perhaps due to its flexibility, the glycine may allow the hydroxyl group in the neighboring serine to be positioned in such a manner that nucleophilic attack at the carbonyl carbon of glycine is enhanced. This positioning might be necessary for efficient autocatalysis.
Recently, using an in vitro transcription/translation-based assay, the importance of each residue in the LGST motif was reported for P. knowlesi (pk) Psd lacking an NH2-terminal membrane anchor (56). Similar to our results, the serine residue is required for autocatalysis of soluble pkPsd in vitro. However, contrary to our results, mutation of the adjacent glycine in pkPsd also completely prevents its autocatalysis. This same in vitro system also demonstrated that autocatalysis of soluble pkPsd is enhanced by the presence of its substrate, PS (31). In contrast, yeast Psd1p autocatalysis still occurs in the complete absence of its substrate (Fig. 2B). We speculate that these subtle differences result from the experimental paradigms used (in vitro versus in vivo) and the absence (soluble pkPsd) or presence (yeast Psd1) of a membrane-embedded domain.
Using a yeast strain devoid of the two known phospholipids unique to mitochondria, PG and CL, and a chimeric Psd1p construct that is re-directed to the secretory pathway, we demonstrated that in yeast, Psd1p autocatalysis does not depend on any mitochondrial-specific phospholipid, protein, or co-factor. Indeed, by simply swapping the mitochondrial targeting signal of Psd1p with the signal sequence from carboxypeptidase Y, CPY*mPsd1p was targeted to the ER where it was autocatalytically processed, enzymatically active, and fully functional in vivo.
These results allow us to re-interpret some of the conclusions made in a previous report (7). First, the failure of radiolabeled Psd1p to undergo autocatalysis when incubated with microsomes as reported (7) stems from the fact that Psd1p lacks a traditional signal sequence. When provided with such sorting information, CPY*mPsd1p can undergo autocatalysis in the context of microsomes but not mitochondria. Thus, it is likely that the ability to productively engage the translocation machinery of an organelle somehow promotes Psd1p autocatalysis, perhaps by allowing the formation of an autocatalytically competent tertiary fold. This is supported by the observation that Psd1p autocatalysis still occurs even when its import is arrested in the translocase of the outer membrane following depletion of the mitochondrial proton-motive force (7). Based on the autocatalysis-promoting effect of PS for P. knowlesi Psd lacking its predicted transmembrane domain (31), and the in vitro requirement of the glycine in the LGST motif for autocatalysis (56), it is tempting to speculate that such a conformation is promoted by the structural flexibility of the glycine residue and stabilized by the presence of substrate when pkPsd is not integrated in a membrane.
Second, the failure of matrix mislocalized Psd1p to restore PE levels when expressed in a psd1Δ strain despite retaining significant catalytic activity (7) does not indicate that Psd1p must be embedded in the mitochondrial IM with its active site facing the IMS to function in vivo. Instead, matrix mislocalized Psd1p retained activity as measured in detergent extracts simply because it still underwent autocatalysis. It remains unresolved whether its failure to restore PE levels in vivo reflects an inability of an anchorless β to properly position the α subunit near the surface of the membrane or alternatively, the lack of PS on the matrix side of the IM. However, what is clear is that when substrate access is not limiting, Psd1p targeted to a non-mitochondrial organelle can undergo autocatalysis, function, and rescue the ethanolamine auxotrophy of psd1Δpsd2Δ yeast.
The ability of CPY*mPsd1p to undergo autocatalysis in a non-mitochondrial organelle implies that once Psd1p is embedded in a membrane or engaged in a translocon, everything else that is needed for this self-activating process is provided by Psd1p itself. Moreover, the ability of CPY*mPsd1p to rescue the growth of psd1Δpsd2Δ yeast in the absence of ethanolamine indicates that PS is, as expected, a normal component of the cytosolic-facing leaflet of the ER. Thus, the ability of Psd1p targeted to different subcellular compartments to rescue the ethanolamine auxotrophy of psd1Δpsd2Δ yeast could be used as a general strategy to report on the presence of PS in a given membrane compartment.
Acknowledgments
We thank Drs. Jeff Schatz and Carla Koehler for antibodies.
This work was supported, in whole or in part, by National Institutes of Health Grant R01GM111548 (to S. M. C.).
- PS
- phosphatidylserine
- CL
- cardiolipin
- CDP
- cytidine diphosphate
- ER
- endoplasmic reticulum
- IM
- inner membrane
- IMS
- intermembrane space
- PE
- phosphatidylethanolamine
- PG
- phosphatidylglycerol
- Psd
- phosphatidylserine decarboxylase
- NBD-PE
- 1-palmitoyl-2-{12-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]dodecanoyl}-sn-glycero-3-phosphoethanolamine
- NBD-PS
- 1-palmitoyl-2-{12-[(7-nitro-2–1,3-benzoxadiazol-4-yl)amino]dodecanoyl}-sn-glycero-3-phosphoserine
- EndoH
- endoglycosidase H
- Pss1p
- phosphatidylserine synthase-1
- CPY
- carboxypeptidase Y
- MTS
- mitochondrial targeting sequence.
REFERENCES
- 1. Birner R., Nebauer R., Schneiter R., Daum G. (2003) Synthetic lethal interaction of the mitochondrial phosphatidylethanolamine biosynthetic machinery with the prohibitin complex of Saccharomyces cerevisiae. Mol. Biol. Cell 14, 370–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zborowski J., Dygas A., Wojtczak L. (1983) Phosphatidylserine decarboxylase is located on the external side of the inner mitochondrial membrane. FEBS Lett. 157, 179–182 [DOI] [PubMed] [Google Scholar]
- 3. Choi J.-Y., Martin W. E., Murphy R. C., Voelker D. R. (2004) Phosphatidylcholine and N-methylated phospholipids are nonessential in Saccharomyces cerevisiae. J. Biol. Chem. 279, 42321–42330 [DOI] [PubMed] [Google Scholar]
- 4. Cui Z., Vance J. E., Chen M. H., Voelker D. R., Vance D. E. (1993) Cloning and expression of a novel phosphatidylethanolamine N-methyltransferase: a specific biochemical and cytological marker for a unique membrane fraction in rat liver. J. Biol. Chem. 268, 16655–16663 [PubMed] [Google Scholar]
- 5. Summers E. F., Letts V. A., McGraw P., Henry S. A. (1988) Saccharomyces cerevisiae cho2 mutants are deficient in phospholipid methylation and cross-pathway regulation of inositol synthesis. Genetics 120, 909–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Waechter C. J., Lester R. L. (1973) Differential regulation of the N-methyl transferases responsible for phosphatidylcholine synthesis in Saccharomyces cerevisiae. Arch. Biochem. Biophys. 158, 401–410 [DOI] [PubMed] [Google Scholar]
- 7. Horvath S. E., Böttinger L., Vögtle F. N., Wiedemann N., Meisinger C., Becker T., Daum G. (2012) Processing and topology of the yeast mitochondrial phosphatidylserine decarboxylase 1. J. Biol. Chem. 287, 36744–36755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tamura Y., Onguka O., Itoh K., Endo T., Iijima M., Claypool S. M., Sesaki H. (2012) Phosphatidylethanolamine biosynthesis in mitochondria: phosphatidylserine (PS) trafficking is independent of a PS decarboxylase and intermembrane space proteins UPS1P and UPS2P. J. Biol. Chem. 287, 43961–43971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zinser E., Sperka-Gottlieb C. D., Fasch E. V., Kohlwein S. D., Paltauf F., Daum G. (1991) Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J. Bacteriol. 173, 2026–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trotter P. J., Voelker D. R. (1995) Identification of a non-mitochondrial phosphatidylserine decarboxylase activity (PSD2) in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 270, 6062–6070 [DOI] [PubMed] [Google Scholar]
- 11. Birner R., Bürgermeister M., Schneiter R., Daum G. (2001) Roles of phosphatidylethanolamine and of its several biosynthetic pathways in Saccharomyces cerevisiae. Mol. Biol. Cell 12, 997–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bürgermeister M., Birner-Grünberger R., Nebauer R., Daum G. (2004) Contribution of different pathways to the supply of phosphatidylethanolamine and phosphatidylcholine to mitochondrial membranes of the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 1686, 161–168 [DOI] [PubMed] [Google Scholar]
- 13. Carman G. M., Han G. S. (2011) Regulation of phospholipid synthesis in the yeast Saccharomyces cerevisiae. Annu. Rev. Biochem. 80, 859–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schuiki I., Daum G. (2009) Phosphatidylserine decarboxylase, key enzymes of lipid metabolism. IUBMB Life 61, 151–162 [DOI] [PubMed] [Google Scholar]
- 15. Fullerton M. D., Hakimuddin F., Bakovic M. (2007) Developmental and metabolic effects of disruption of the mouse CTP:phosphoethanolamine cytidylyltransferase gene (Pcyt2). Mol. Cell Biol. 27, 3327–3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Steenbergen R., Nanowski T. S., Beigneux A., Kulinski A., Young S. G., Vance J. E. (2005) Disruption of the phosphatidylserine decarboxylase gene in mice causes embryonic lethality and mitochondrial defects. J. Biol. Chem. 280, 40032–40040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shiao Y. J., Lupo G., Vance J. E. (1995) Evidence that phosphatidylserine is imported into mitochondria via a mitochondria-associated membrane and that the majority of mitochondrial phosphatidylethanolamine is derived from decarboxylation of phosphatidylserine. J. Biol. Chem. 270, 11190–11198 [DOI] [PubMed] [Google Scholar]
- 18. Böttinger L., Horvath S. E., Kleinschroth T., Hunte C., Daum G., Pfanner N., Becker T. (2012) Phosphatidylethanolamine and cardiolipin differentially affect the stability of mitochondrial respiratory chain supercomplexes. J. Mol. Biol. 423, 677–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Joshi A. S., Thompson M. N., Fei N., Hüttemann M., Greenberg M. L. (2012) Cardiolipin and mitochondrial phosphatidylethanolamine have overlapping functions in mitochondrial fusion in Saccharomyces cerevisiae. J. Biol. Chem. 287, 17589–17597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tasseva G., Bai H. D., Davidescu M., Haromy A., Michelakis E., Vance J. E. (2013) Phosphatidylethanolamine deficiency in mammalian mitochondria impairs oxidative phosphorylation and alters mitochondrial morphology. J. Biol. Chem. 288, 4158–4173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Trotter P. J., Pedretti J., Voelker D. R. (1993) Phosphatidylserine decarboxylase from Saccharomyces cerevisiae: isolation of mutants, cloning of the gene, and creation of a null allele. J. Biol. Chem. 268, 21416–21424 [PubMed] [Google Scholar]
- 22. Lange C., Nett J. H., Trumpower B. L., Hunte C. (2001) Specific roles of protein-phospholipid interactions in the yeast cytochrome bc1 complex structure. EMBO J. 20, 6591–6600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shinzawa-Itoh K., Aoyama H., Muramoto K., Terada H., Kurauchi T., Tadehara Y., Yamasaki A., Sugimura T., Kurono S., Tsujimoto K., Mizushima T., Yamashita E., Tsukihara T., Yoshikawa S. (2007) Structures and physiological roles of 13 integral lipids of bovine heart cytochrome c oxidase. EMBO J. 26, 1713–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Becker T., Horvath S. E., Böttinger L., Gebert N., Daum G., Pfanner N. (2013) Role of phosphatidylethanolamine in the biogenesis of mitochondrial outer membrane proteins. J. Biol. Chem. 288, 16451–16459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li Q. X., Dowhan W. (1990) Studies on the mechanism of formation of the pyruvate prosthetic group of phosphatidylserine decarboxylase from Escherichia coli. J. Biol. Chem. 265, 4111–4115 [PubMed] [Google Scholar]
- 26. Dowhan W., Wickner W. T., Kennedy E. P. (1974) Purification and properties of phosphatidylserine decarboxylase from Escherichia coli. J. Biol. Chem. 249, 3079–3084 [PubMed] [Google Scholar]
- 27. Gulshan K., Schmidt J. A., Shahi P., Moye-Rowley W. S. (2008) Evidence for the bifunctional nature of mitochondrial phosphatidylserine decarboxylase: role in Pdr3-dependent retrograde regulation of PDR5 expression. Mol. Cell Biol. 28, 5851–5864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuge O., Saito K., Kojima M., Akamatsu Y., Nishijima M. (1996) Post-translational processing of the phosphatidylserine decarboxylase gene product in Chinese hamster ovary cells. Biochem. J. 319, 33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li Q. X., Dowhan W. (1988) Structural characterization of Escherichia coli phosphatidylserine decarboxylase. J. Biol. Chem. 263, 11516–11522 [PubMed] [Google Scholar]
- 30. Satre M., Kennedy E. P. (1978) Identification of bound pyruvate essential for the activity of phosphatidylserine decarboxylase of Escherichia coli. J. Biol. Chem. 253, 479–483 [PubMed] [Google Scholar]
- 31. Choi J. Y., Augagneur Y., Ben Mamoun C., Voelker D. R. (2012) Identification of gene encoding Plasmodium knowlesi phosphatidylserine decarboxylase by genetic complementation in yeast and characterization of in vitro maturation of encoded enzyme. J. Biol. Chem. 287, 222–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baile M. G., Sathappa M., Lu Y. W., Pryce E., Whited K., McCaffery J. M., Han X., Alder N. N., Claypool S. M. (2014) Unremodeled and remodeled cardiolipin are functionally indistinguishable in yeast. J. Biol. Chem. 289, 1768–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wach A., Brachat A., Pöhlmann R., Philippsen P. (1994) New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10, 1793–1808 [DOI] [PubMed] [Google Scholar]
- 34. Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77, 51–59 [DOI] [PubMed] [Google Scholar]
- 35. Johnson L. M., Bankaitis V. A., Emr S. D. (1987) Distinct sequence determinants direct intracellular sorting and modification of a yeast vacuolar protease. Cell 48, 875–885 [DOI] [PubMed] [Google Scholar]
- 36. Claypool S. M., McCaffery J. M., Koehler C. M. (2006) Mitochondrial mislocalization and altered assembly of a cluster of Barth syndrome mutant tafazzins. J. Cell Biol. 174, 379–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meisinger C., Pfanner N., Truscott K. N. (2006) Isolation of yeast mitochondria. Methods Mol. Biol. 313, 33–39 [DOI] [PubMed] [Google Scholar]
- 38. Claypool S. M., Whited K., Srijumnong S., Han X., Koehler C. M. (2011) Barth syndrome mutations that cause tafazzin complex lability. J. Cell Biol. 192, 447–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Claypool S. M., Dickinson B. L., Yoshida M., Lencer W. I., Blumberg R. S. (2002) Functional reconstitution of human FcRn in Madin-Darby canine kidney cells requires co-expressed human β2-microglobulin. J. Biol. Chem. 277, 28038–28050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baile M. G., Whited K., Claypool S. M. (2013) Deacylation on the matrix side of the mitochondrial inner membrane regulates cardiolipin remodeling. Mol. Biol. Cell 24, 2008–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Claypool S. M., Oktay Y., Boontheung P., Loo J. A., Koehler C. M. (2008) Cardiolipin defines the interactome of the major ADP/ATP carrier protein of the mitochondrial inner membrane. J. Cell Biol. 182, 937–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Riezman H., Hay R., Gasser S., Daum G., Schneider G., Witte C., Schatz G. (1983) The outer membrane of yeast mitochondria: isolation of outside-out sealed vesicles. EMBO J. 2, 1105–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tamura Y., Onguka O., Hobbs A. E., Jensen R. E., Iijima M., Claypool S. M., Sesaki H. (2012) Role for two conserved intermembrane space proteins, Ups1p and Ups2p, [corrected] in intra-mitochondrial phospholipid trafficking. J. Biol. Chem. 287, 15205–15218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Whited K., Baile M. G., Currier P., Claypool S. M. (2013) Seven functional classes of Barth syndrome mutation. Hum. Mol. Genet. 22, 483–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goujon M., McWilliam H., Li W., Valentin F., Squizzato S., Paern J., Lopez R. (2010) A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res. 38, W695–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McWilliam H., Li W., Uludag M., Squizzato S., Park Y. M., Buso N., Cowley A. P., Lopez R. (2013) Analysis tool web services from the EMBL-EBI. Nucleic Acids Res. 41, W597–W600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sievers F., Wilm A., Dineen D., Gibson T. J., Karplus K., Li W., Lopez R., McWilliam H., Remmert M., Söding J., Thompson J. D., Higgins D. G. (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Atkinson K., Fogel S., Henry S. A. (1980) Yeast mutant defective in phosphatidylserine synthesis. J. Biol. Chem. 255, 6653–6661 [PubMed] [Google Scholar]
- 49. Hovius R., Faber B., Brigot B., Nicolay K., de Kruijff B. (1992) On the mechanism of the mitochondrial decarboxylation of phosphatidylserine. J. Biol. Chem. 267, 16790–16795 [PubMed] [Google Scholar]
- 50. Kohlwein S. D., Kuchler K., Sperka-Gottlieb C., Henry S. A., Paltauf F. (1988) Identification of mitochondrial and microsomal phosphatidylserine synthase in Saccharomyces cerevisiae as the gene product of the CHO1 structural gene. J. Bacteriol. 170, 3778–3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Letts V. A., Klig L. S., Bae-Lee M., Carman G. M., Henry S. A. (1983) Isolation of the yeast structural gene for the membrane-associated enzyme phosphatidylserine synthase. Proc. Natl. Acad. Sci. 80, 7279–7283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stone S. J., Vance J. E. (2000) Phosphatidylserine synthase-1 and -2 are localized to mitochondria-associated membranes. J. Biol. Chem. 275, 34534–34540 [DOI] [PubMed] [Google Scholar]
- 53. Atkinson K. D., Jensen B., Kolat A. I., Storm E. M., Henry S. A., Fogel S. (1980) Yeast mutants auxotrophic for choline or ethanolamine. J. Bacteriol. 141, 558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Claypool S. M., Koehler C. M. (2012) The complexity of cardiolipin in health and disease. Trends Biochem. Sci. 37, 32–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chang S. C., Heacock P. N., Clancey C. J., Dowhan W. (1998) The PEL1 gene (renamed PGS1) encodes the phosphatidylglycerophosphate synthase of Saccharomyces cerevisiae. J. Biol. Chem. 273, 9829–9836 [DOI] [PubMed] [Google Scholar]
- 56. Choi J. Y., Duraisingh M. T., Marti M., Ben Mamoun C., Voelker D. R. (2015) From protease to decarboxylase: the molecular metamorphosis of phosphatidylserine decarboxylase. J. Biol. Chem. 290, 10972–10980 [DOI] [PMC free article] [PubMed] [Google Scholar]