Abstract
In humans and in several animal species, puberty results from changes in pulsatile gonadotropin-releasing hormone (GnRH) secretion in the hypothalamus. In particular, the frequency of pulsatile GnRH secretion increases at the onset of puberty, as can be shown by using hypothalamic explants of male rats of 15 and 25 d. Previous observations from us and others suggested that the initiation of puberty could involve a facilitatory effect of excitatory amino acids mediated through N-methyl-D-aspartate (NMDA) receptors. We found that GnRH secretion could be activated through NMDA receptors only around the time of onset of puberty (25 d). The aim of this study was to clarify why this activation did not occur earlier (at 15 d) and could no longer be observed by the end of puberty (at 50 d). We studied GnRH secretion in the presence of MK-801, a noncompetitive antagonist of NMDA receptors or AP-5, a competitive antagonist. We showed that, in the hypothalamus of immature male rats (15 d), a highly potent inhibitory control of pulsatile GnRH secretion in vitro was mediated through NMDA receptors. These data were confirmed in vivo because administration of the antagonist MK-801 (0.001 mg/kg) to immature male rats resulted in early pubertal development. Onset of puberty (25 d) was characterized by the disappearance of that NMDA receptor-mediated inhibition, thus unmasking a facilitatory effect also mediated through NMDA receptors. During puberty, there was a reduction in activity of this facilitatory control which was no longer opposed by its inhibitory counterpart. We conclude that a sequential reduction in activity of inhibitory and facilitatory NMDA receptors provides a developmental basis for the neuroendocrine mechanism of onset of puberty.
Full text
PDF

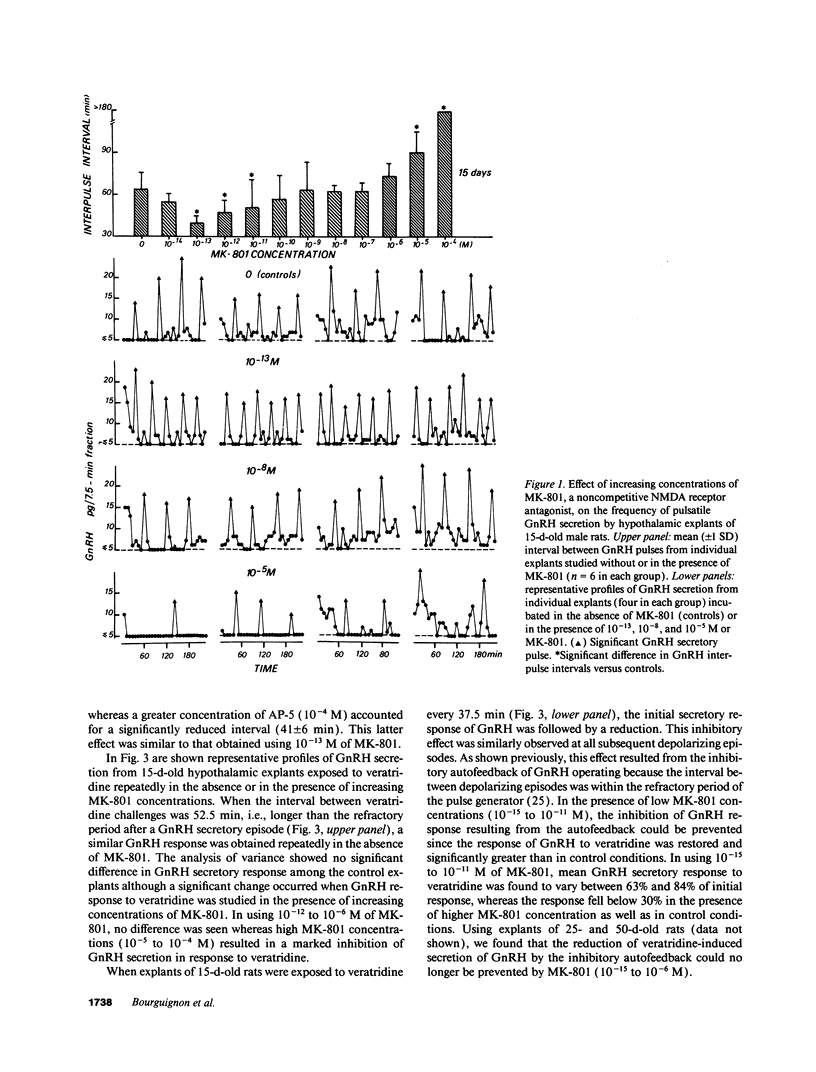
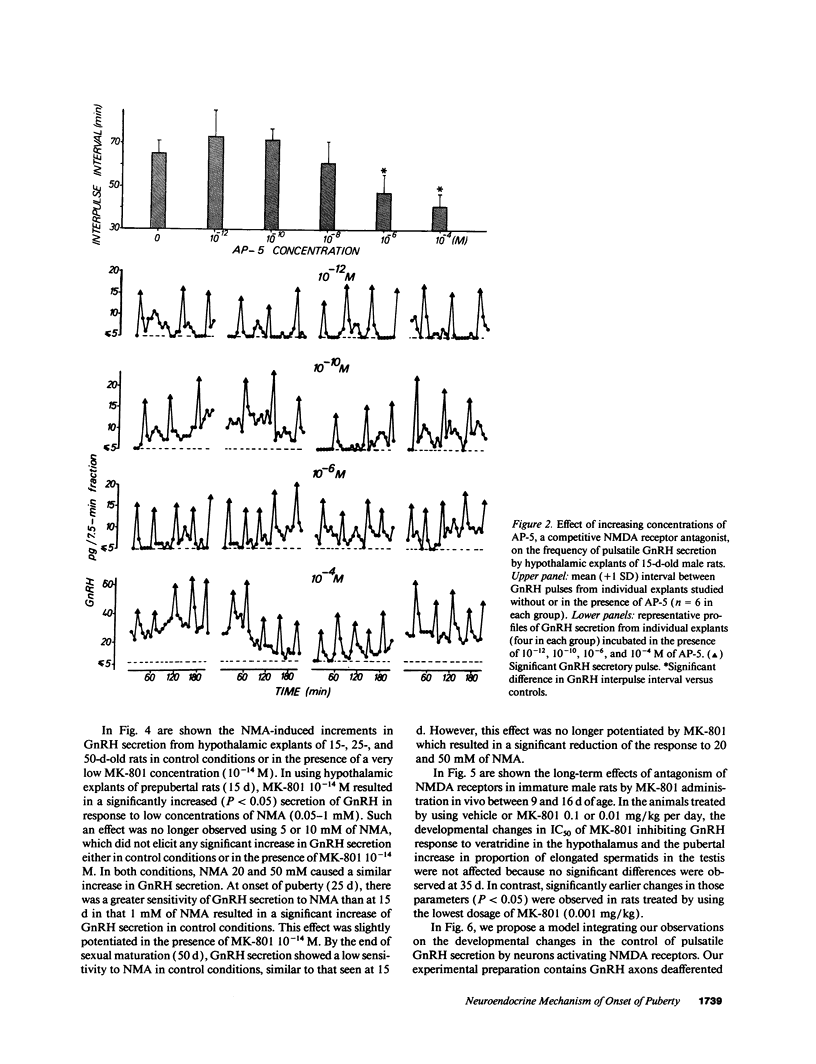
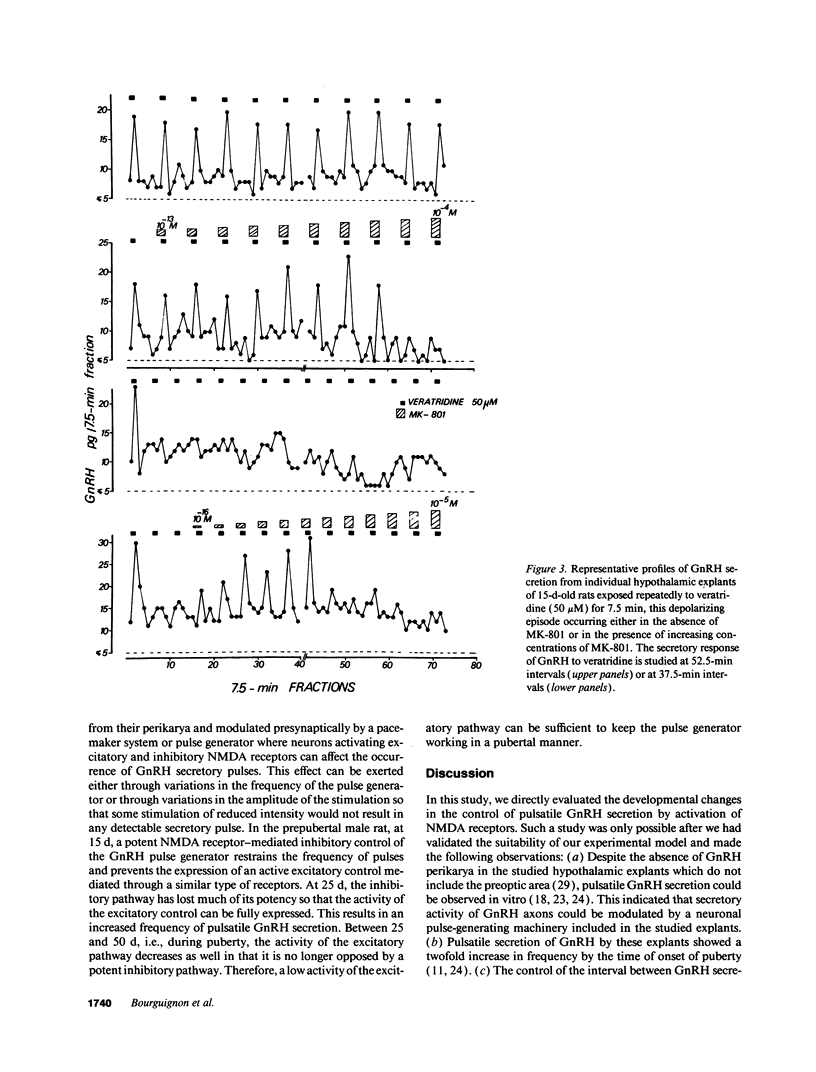
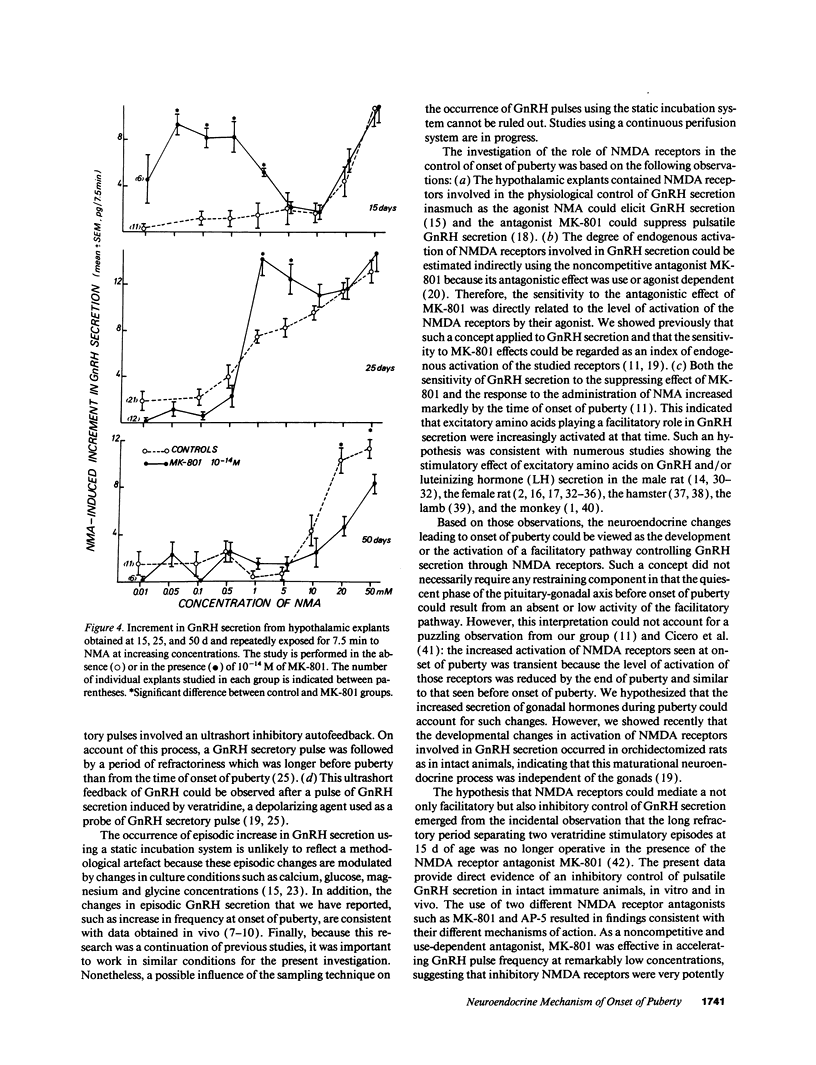
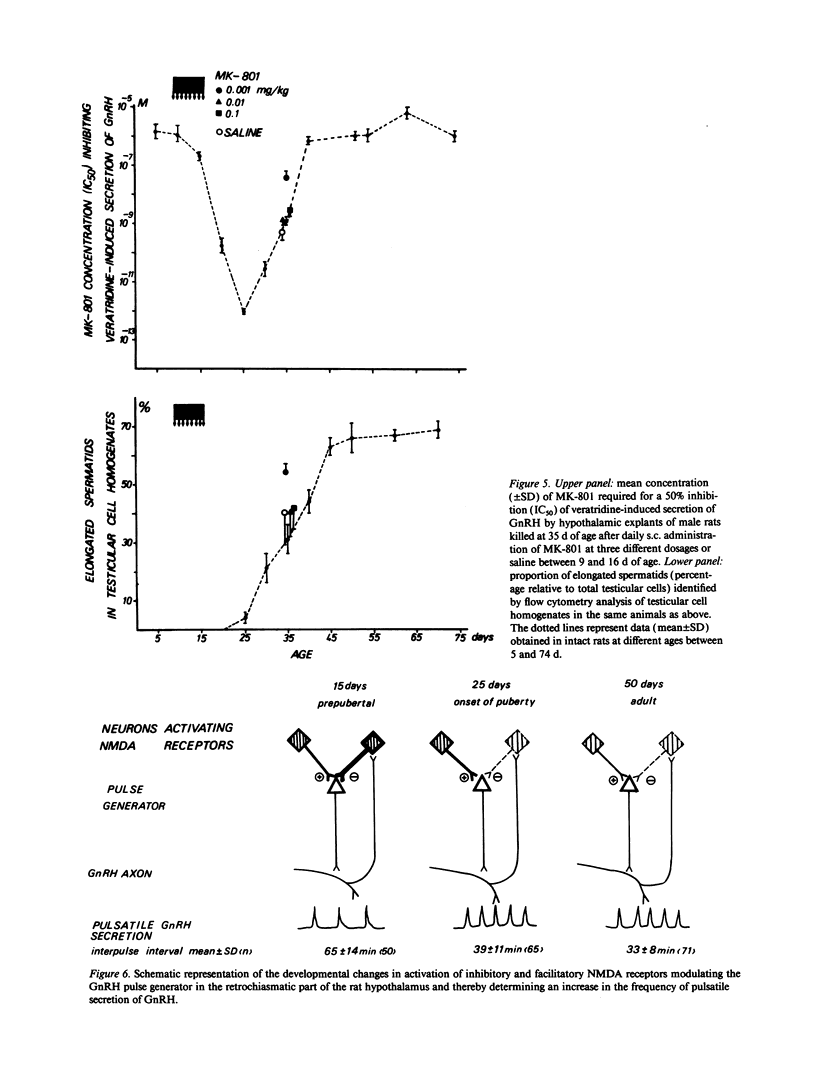


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arslan M., Pohl C. R., Plant T. M. DL-2-amino-5-phosphonopentanoic acid, a specific N-methyl-D-aspartic acid receptor antagonist, suppresses pulsatile LH release in the rat. Neuroendocrinology. 1988 May;47(5):465–468. doi: 10.1159/000124951. [DOI] [PubMed] [Google Scholar]
- Banki C. M., Arato M. Multiple hormonal responses to morphine: relationship to diagnosis and dexamethasone suppression. Psychoneuroendocrinology. 1987;12(1):3–11. doi: 10.1016/0306-4530(87)90016-3. [DOI] [PubMed] [Google Scholar]
- Belchetz P. E., Plant T. M., Nakai Y., Keogh E. J., Knobil E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978 Nov 10;202(4368):631–633. doi: 10.1126/science.100883. [DOI] [PubMed] [Google Scholar]
- Bhanot R., Wilkinson M. Opiatergic control of gonadotropin secretion during puberty in the rat: a neurochemical basis for the hypothalamic 'gonadostat'? Endocrinology. 1983 Aug;113(2):596–603. doi: 10.1210/endo-113-2-596. [DOI] [PubMed] [Google Scholar]
- Blank M. S., Panerai A. E., Friesen H. G. Opioid peptides modulate luteinizing hormone secretion during sexual maturation. Science. 1979 Mar 16;203(4385):1129–1131. doi: 10.1126/science.424743. [DOI] [PubMed] [Google Scholar]
- Bourguignon J. P., Franchimont P. Puberty-related increase in episodic LHRH release from rat hypothalamus in vitro. Endocrinology. 1984 May;114(5):1941–1943. doi: 10.1210/endo-114-5-1941. [DOI] [PubMed] [Google Scholar]
- Bourguignon J. P., Gerard A., Debougnoux G., Rose J., Franchimont P. Pulsatile release of gonadotropin-releasing hormone (GnRH) from the rat hypothalamus in vitro: calcium and glucose dependency and inhibition by superactive GnRH analogs. Endocrinology. 1987 Sep;121(3):993–999. doi: 10.1210/endo-121-3-993. [DOI] [PubMed] [Google Scholar]
- Bourguignon J. P., Gerard A., Fawe L., Alvarez-Gonzalez M. L., Franchimont P. Neuroendocrine control of the onset of puberty: secretion of gonadotrophin-releasing hormone from rat hypothalamic explants. Acta Paediatr Scand Suppl. 1991;372:19–25. doi: 10.1111/j.1651-2227.1991.tb17963.x. [DOI] [PubMed] [Google Scholar]
- Bourguignon J. P., Gerard A., Mathieu J., Mathieu A., Franchimont P. Maturation of the hypothalamic control of pulsatile gonadotropin-releasing hormone secretion at onset of puberty. I. Increased activation of N-methyl-D-aspartate receptors. Endocrinology. 1990 Aug;127(2):873–881. doi: 10.1210/endo-127-2-873. [DOI] [PubMed] [Google Scholar]
- Bourguignon J. P., Gerard A., Mathieu J., Simons J., Franchimont P. Pulsatile release of gonadotropin-releasing hormone from hypothalamic explants is restrained by blockade of N-methyl-D,L-aspartate receptors. Endocrinology. 1989 Aug;125(2):1090–1096. doi: 10.1210/endo-125-2-1090. [DOI] [PubMed] [Google Scholar]
- Bourguignon J. P., Gérard A., Alvarex-Gonzalez M. L., Fawe L., Franchimont P. Gonadal-independent developmental changes in activation of N-methyl-D-aspartate receptors involved in gonadotropin-releasing hormone secretion. Neuroendocrinology. 1992 Jun;55(6):634–641. doi: 10.1159/000126182. [DOI] [PubMed] [Google Scholar]
- Bourguignon J. P., Gérard A., Franchimont P. Direct activation of gonadotropin-releasing hormone secretion through different receptors to neuroexcitatory amino acids. Neuroendocrinology. 1989 Apr;49(4):402–408. doi: 10.1159/000125145. [DOI] [PubMed] [Google Scholar]
- Bourguignon J. P., Gérard A., Franchimont P. Maturation of the hypothalamic control of pulsatile gonadotropin-releasing hormone secretion at onset of puberty: II. Reduced potency of an inhibitory autofeedback. Endocrinology. 1990 Dec;127(6):2884–2890. doi: 10.1210/endo-127-6-2884. [DOI] [PubMed] [Google Scholar]
- Brann D. W., Mahesh V. B. Endogenous excitatory amino acid involvement in the preovulatory and steroid-induced surge of gonadotropins in the female rat. Endocrinology. 1991 Mar;128(3):1541–1547. doi: 10.1210/endo-128-3-1541. [DOI] [PubMed] [Google Scholar]
- Burde R. M., Schainker B., Kayes J. Acute effect of oral and subcutaneous administration of monosodium glutamate on the arcuate nucleus of the hypothalamus in mice and rats. Nature. 1971 Sep 3;233(5314):58–60. doi: 10.1038/233058a0. [DOI] [PubMed] [Google Scholar]
- Cicero T. J., Meyer E. R., Bell R. D. Characterization and possible opioid modulation of N-methyl-D-aspartic acid induced increases in serum luteinizing hormone levels in the developing male rat. Life Sci. 1988;42(18):1725–1732. doi: 10.1016/0024-3205(88)90038-0. [DOI] [PubMed] [Google Scholar]
- Claypool L. E., Watanabe G., Terasawa E. Effects of electrical stimulation of the medial basal hypothalamus on the in vivo release of luteinizing hormone-releasing hormone in the prepubertal and peripubertal female monkey. Endocrinology. 1990 Dec;127(6):3014–3022. doi: 10.1210/endo-127-6-3014. [DOI] [PubMed] [Google Scholar]
- Dada M. O., Blake C. A. Administration of monosodium glutamate to neonatal male rats: alterations in the gonadotrophs and in gonadotrophin secretion. Neuroendocrinology. 1984 Jun;38(6):490–497. doi: 10.1159/000123938. [DOI] [PubMed] [Google Scholar]
- Donoso A. O., López F. J., Negro-Vilar A. Glutamate receptors of the non-N-methyl-D-aspartic acid type mediate the increase in luteinizing hormone-releasing hormone release by excitatory amino acids in vitro. Endocrinology. 1990 Jan;126(1):414–420. doi: 10.1210/endo-126-1-414. [DOI] [PubMed] [Google Scholar]
- Dudek S. M., Bowen W. D., Bear M. F. Postnatal changes in glutamate stimulated phosphoinositide turnover in rat neocortical synaptoneurosomes. Brain Res Dev Brain Res. 1989 May 1;47(1):123–128. doi: 10.1016/0165-3806(89)90114-4. [DOI] [PubMed] [Google Scholar]
- Ebling F. J., Wood R. I., Karsch F. J., Vannerson L. A., Suttie J. M., Bucholtz D. C., Schall R. E., Foster D. L. Metabolic interfaces between growth and reproduction. III. Central mechanisms controlling pulsatile luteinizing hormone secretion in the nutritionally growth-limited female lamb. Endocrinology. 1990 May;126(5):2719–2727. doi: 10.1210/endo-126-5-2719. [DOI] [PubMed] [Google Scholar]
- Farah J. M., Jr, Rao T. S., Mick S. J., Coyne K. E., Iyengar S. N-methyl-D-aspartate treatment increases circulating adrenocorticotropin and luteinizing hormone in the rat. Endocrinology. 1991 Apr;128(4):1875–1880. doi: 10.1210/endo-128-4-1875. [DOI] [PubMed] [Google Scholar]
- Foster A. C., Wong E. H. The novel anticonvulsant MK-801 binds to the activated state of the N-methyl-D-aspartate receptor in rat brain. Br J Pharmacol. 1987 Jun;91(2):403–409. doi: 10.1111/j.1476-5381.1987.tb10295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin S. Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature. 1992 Jun 25;357(6380):686–689. doi: 10.1038/357686a0. [DOI] [PubMed] [Google Scholar]
- Huffman L. J., Inskeep E. K., Goodman R. L. Changes in episodic luteinizing hormone secretion leading to puberty in the lamb. Biol Reprod. 1987 Nov;37(4):755–761. doi: 10.1095/biolreprod37.4.755. [DOI] [PubMed] [Google Scholar]
- Kaplan S. L., Grumbach M. M. Clinical review 14: Pathophysiology and treatment of sexual precocity. J Clin Endocrinol Metab. 1990 Oct;71(4):785–789. doi: 10.1210/jcem-71-4-785. [DOI] [PubMed] [Google Scholar]
- López F. J., Donoso A. O., Negro-Vilar A. Endogenous excitatory amino acid neurotransmission regulates the estradiol-induced LH surge in ovariectomized rats. Endocrinology. 1990 Mar;126(3):1771–1773. doi: 10.1210/endo-126-3-1771. [DOI] [PubMed] [Google Scholar]
- MacDonald M. C., Wilkinson M. Peripubertal treatment with N-methyl-D-aspartic acid or neonatally with monosodium glutamate accelerates sexual maturation in female rats, an effect reversed by MK-801. Neuroendocrinology. 1990 Aug;52(2):143–149. doi: 10.1159/000125565. [DOI] [PubMed] [Google Scholar]
- McDonald J. W., Silverstein F. S., Johnston M. V. Neurotoxicity of N-methyl-D-aspartate is markedly enhanced in developing rat central nervous system. Brain Res. 1988 Aug 30;459(1):200–203. doi: 10.1016/0006-8993(88)90306-x. [DOI] [PubMed] [Google Scholar]
- Merriam G. R., Wachter K. W. Algorithms for the study of episodic hormone secretion. Am J Physiol. 1982 Oct;243(4):E310–E318. doi: 10.1152/ajpendo.1982.243.4.E310. [DOI] [PubMed] [Google Scholar]
- Nicoletti F., Iadarola M. J., Wroblewski J. T., Costa E. Excitatory amino acid recognition sites coupled with inositol phospholipid metabolism: developmental changes and interaction with alpha 1-adrenoceptors. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1931–1935. doi: 10.1073/pnas.83.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney J. W. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969 May 9;164(3880):719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- Ondo J. G., Wheeler D. D., Dom R. M. Hypothalamic site of action for N-methyl-D-aspartate (NMDA) on LH secretion. Life Sci. 1988;43(26):2283–2286. doi: 10.1016/0024-3205(88)90422-5. [DOI] [PubMed] [Google Scholar]
- Ota M., Narahashi T., Keeler R. F. Effects of veratrum alkaloids on membrane potential and conductance of squid and crayfish giant axons. J Pharmacol Exp Ther. 1973 Jan;184(1):143–154. [PubMed] [Google Scholar]
- Petersen S. L., McCrone S., Keller M., Gardner E. Rapid increase in LHRH mRNA levels following NMDA. Endocrinology. 1991 Sep;129(3):1679–1681. doi: 10.1210/endo-129-3-1679. [DOI] [PubMed] [Google Scholar]
- Plant T. M., Gay V. L., Marshall G. R., Arslan M. Puberty in monkeys is triggered by chemical stimulation of the hypothalamus. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2506–2510. doi: 10.1073/pnas.86.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A., Luckhaus J., Ferin M. Unexpected inhibitory action of N-methyl-D,L-aspartate or luteinizing hormone release in adult ovariectomized rhesus monkeys: a role of the hypothalamic-adrenal axis. Endocrinology. 1990 Aug;127(2):724–729. doi: 10.1210/endo-127-2-724. [DOI] [PubMed] [Google Scholar]
- Reyes A., Xia L. N., Ferin M. Modulation of the effects of N-methyl-D,L-aspartate on luteinizing hormone by the ovarian steroids in the adult rhesus monkey. Neuroendocrinology. 1991 Oct;54(4):405–411. doi: 10.1159/000125921. [DOI] [PubMed] [Google Scholar]
- Rodriguez R. E., Wise M. E. Ontogeny of pulsatile secretion of gonadotropin-releasing hormone in the bull calf during infantile and pubertal development. Endocrinology. 1989 Jan;124(1):248–256. doi: 10.1210/endo-124-1-248. [DOI] [PubMed] [Google Scholar]
- Ryan K. D., Robinson S. L. A rise in tonic luteinizing hormone secretion occurs during photoperiod-stimulated sexual maturation of the female ferret. Endocrinology. 1985 May;116(5):2013–2018. doi: 10.1210/endo-116-5-2013. [DOI] [PubMed] [Google Scholar]
- Saitoh Y., Silverman A. J., Gibson M. J. Effects of N-methyl-D,L-aspartic acid on luteinizing hormone secretion in normal mice and in hypogonadal mice with fetal preoptic area implants. Endocrinology. 1991 May;128(5):2432–2440. doi: 10.1210/endo-128-5-2432. [DOI] [PubMed] [Google Scholar]
- Tremblay E., Roisin M. P., Represa A., Charriaut-Marlangue C., Ben-Ari Y. Transient increased density of NMDA binding sites in the developing rat hippocampus. Brain Res. 1988 Oct 4;461(2):393–396. doi: 10.1016/0006-8993(88)90275-2. [DOI] [PubMed] [Google Scholar]
- Urbanski H. F. A role for N-methyl-D-aspartate receptors in the control of seasonal breeding. Endocrinology. 1990 Nov;127(5):2223–2228. doi: 10.1210/endo-127-5-2223. [DOI] [PubMed] [Google Scholar]
- Urbanski H. F., Ojeda S. R. A role for N-methyl-D-aspartate (NMDA) receptors in the control of LH secretion and initiation of female puberty. Endocrinology. 1990 Mar;126(3):1774–1776. doi: 10.1210/endo-126-3-1774. [DOI] [PubMed] [Google Scholar]
- Urbanski H. F., Ojeda S. R. Activation of luteinizing hormone-releasing hormone release advances the onset of female puberty. Neuroendocrinology. 1987 Sep;46(3):273–276. doi: 10.1159/000124831. [DOI] [PubMed] [Google Scholar]
- Wildt L., Marshall G., Knobil E. Experimental induction of puberty in the infantile female rhesus monkey. Science. 1980 Mar 21;207(4437):1373–1375. doi: 10.1126/science.6986658. [DOI] [PubMed] [Google Scholar]
- Wilson R. C., Knobil E. Acute effects of N-methyl-DL-aspartate on the release of pituitary gonadotropins and prolactin in the adult female rhesus monkey. Brain Res. 1982 Sep 23;248(1):177–179. doi: 10.1016/0006-8993(82)91160-x. [DOI] [PubMed] [Google Scholar]
- Wong E. H., Kemp J. A., Priestley T., Knight A. R., Woodruff G. N., Iversen L. L. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]