Abstract
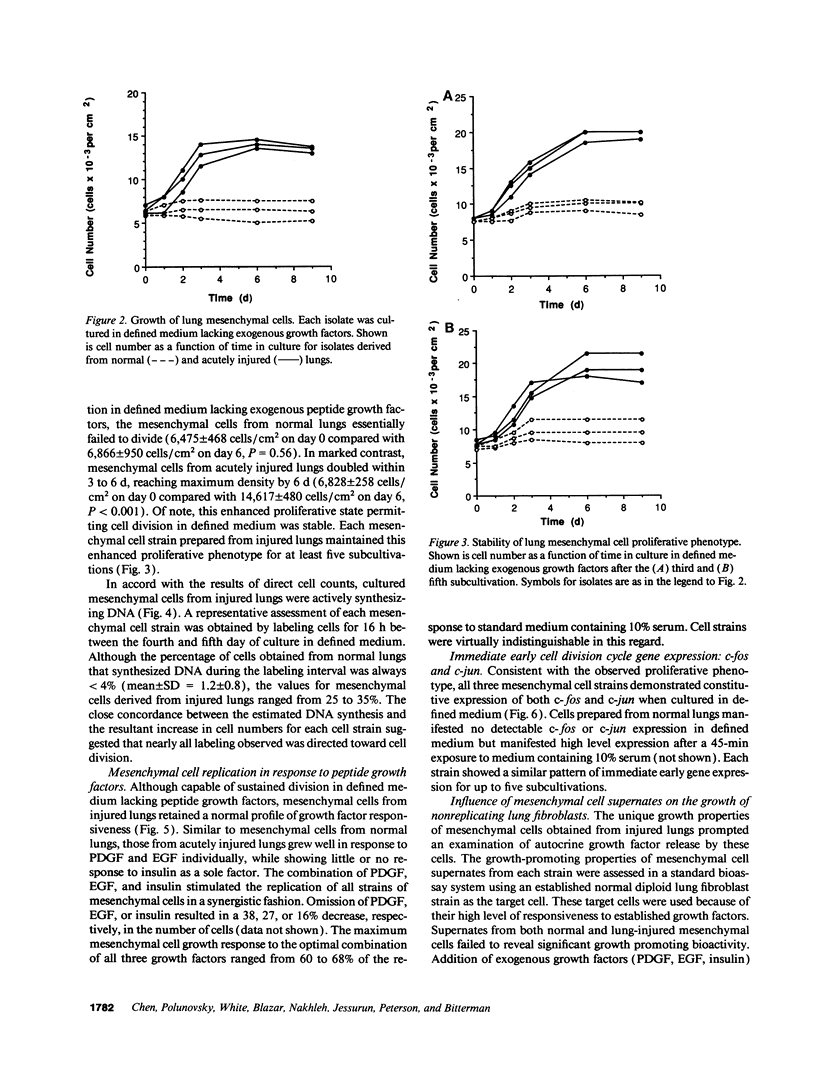
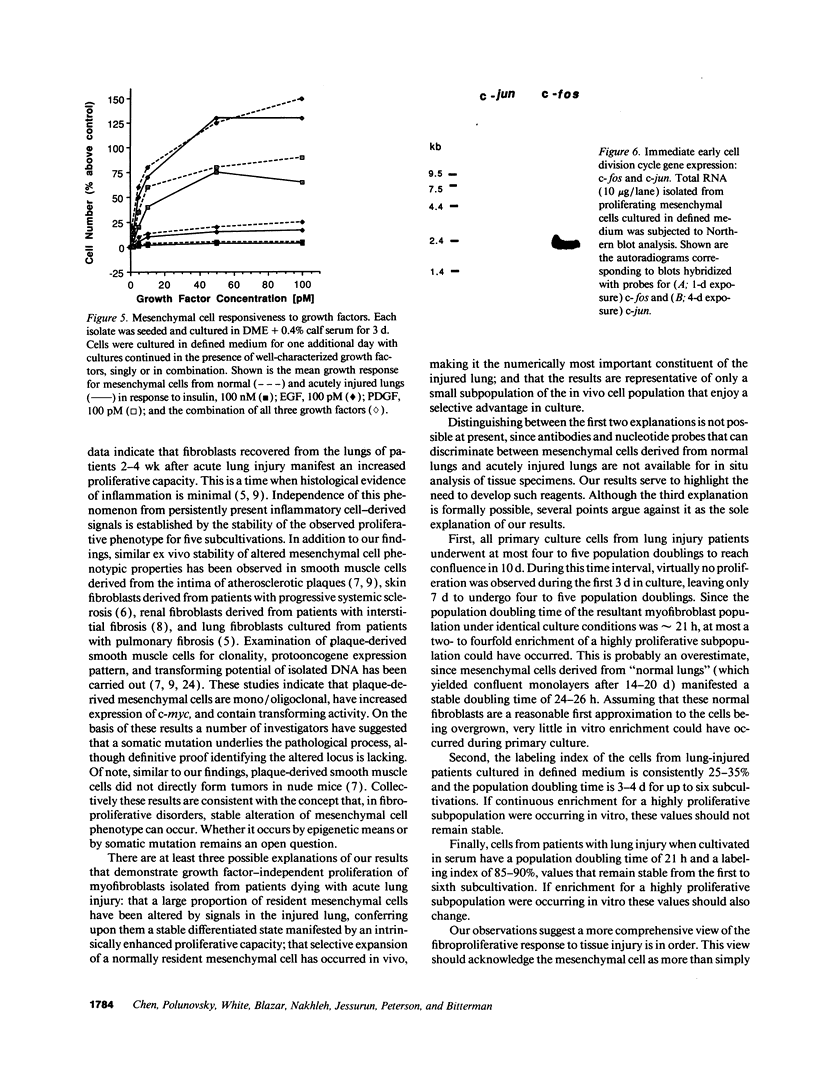
After acute lung injury, mesenchymal cells migrate into the alveolar airspace where they proliferate and deposit connective tissue macromolecules. Early in the disease process, inflammatory cell-derived trophic factors modulate these mesenchymal cell functions. However, in those patients who die, even as the inflammatory response abates, the fibroproliferative response continues, resulting in extensive intraalveolar fibrosis. We therefore hypothesized that lung mesenchymal cells obtained from individuals dying with acute alveolar fibrosis would manifest an enhanced proliferative capacity that was independent of persistent exogenous signals. To examine this hypothesis, the in vitro growth properties of mesenchymal cells prepared from patients dying with acute lung injury (n = 3) were analyzed in defined medium and compared with those of mesenchymal cells similarly prepared from patients dying with histologically normal lungs (n = 3). Isolates were characterized as mesenchymal cells by using morphological and immunohistochemical criteria. In accord with the hypothesis, mesenchymal cells isolated from lung-injured patients doubled within 3 d in the complete absence of exogenous peptide growth factors, reaching a saturation density of approximately 15 x 10(3) cells/cm2. As expected, lung mesenchymal cells from normal individuals failed to significantly increase in number. Consistent with this proliferative phenotype, the immediate early cell division cycle genes c-fos and c-jun were constitutively expressed in each cell strain prepared from injured lungs, but not in those from control lungs. The observed proliferative phenotype was stable through the fifth subcultivation of the cells. Despite these proliferative properties, three separate criteria indicated the mesenchymal cells from injured lungs were not transformed: normal karyotype; finite lifespan in vitro (9-10 subcultivations); and inability to disseminate in mice with severe combined immunodeficiency. These data support the hypothesis that mesenchymal cells manifest an enhanced proliferative state after acute lung injury.
Full text
PDF


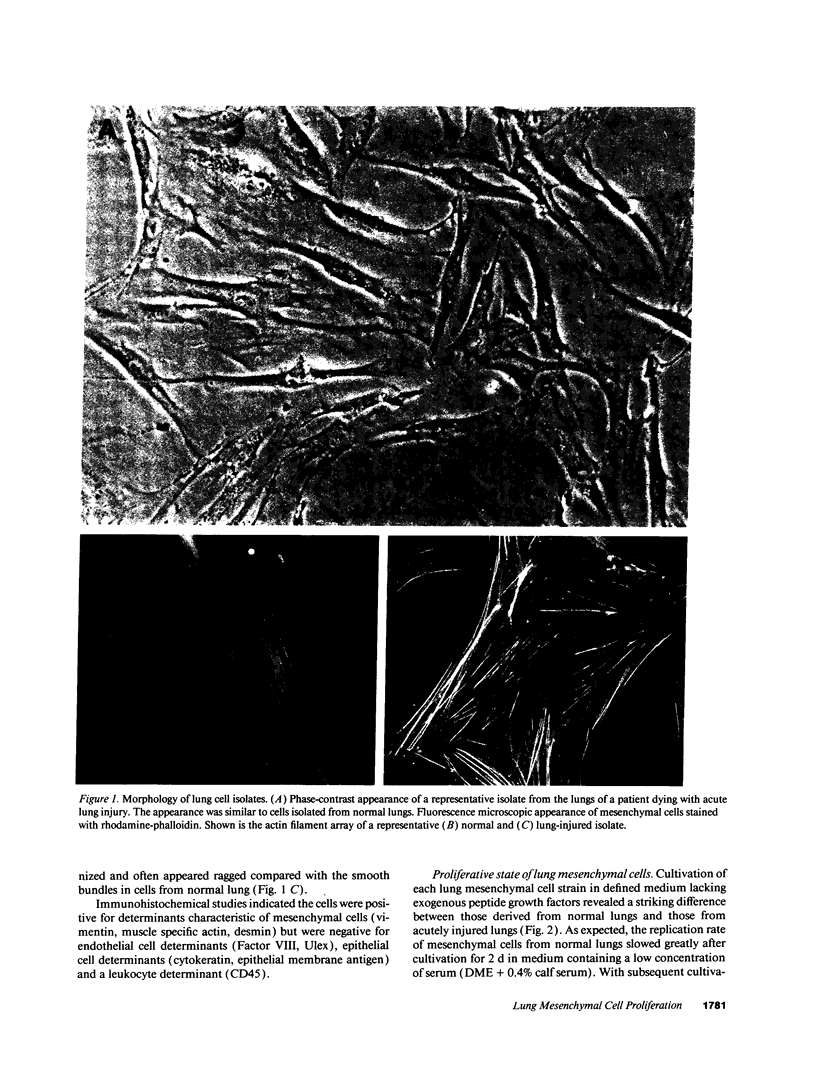


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Allegretto E. A., Okino S. T., Hattori K., Boyle W. J., Hunter T., Karin M. Oncogene jun encodes a sequence-specific trans-activator similar to AP-1. Nature. 1988 Mar 10;332(6160):166–171. doi: 10.1038/332166a0. [DOI] [PubMed] [Google Scholar]
- Benditt E. P., Benditt J. M. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Fowler A. A., Hamman R. F., Zerbe G. O., Benson K. N., Hyers T. M. Adult respiratory distress syndrome. Prognosis after onset. Am Rev Respir Dis. 1985 Sep;132(3):472–478. doi: 10.1164/arrd.1985.132.3.472. [DOI] [PubMed] [Google Scholar]
- Fukuda Y., Ishizaki M., Masuda Y., Kimura G., Kawanami O., Masugi Y. The role of intraalveolar fibrosis in the process of pulmonary structural remodeling in patients with diffuse alveolar damage. Am J Pathol. 1987 Jan;126(1):171–182. [PMC free article] [PubMed] [Google Scholar]
- Gerrard J. M., Phillips D. R., Rao G. H., Plow E. F., Walz D. A., Ross R., Harker L. A., White J. G. Biochemical studies of two patients with the gray platelet syndrome. Selective deficiency of platelet alpha granules. J Clin Invest. 1980 Jul;66(1):102–109. doi: 10.1172/JCI109823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke C., Fiegel V., Peterson M., Wick M., Knighton D., McCarthy J., Bitterman P. Identification and partial characterization of angiogenesis bioactivity in the lower respiratory tract after acute lung injury. J Clin Invest. 1991 Oct;88(4):1386–1395. doi: 10.1172/JCI115445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Jordana M., Schulman J., McSharry C., Irving L. B., Newhouse M. T., Jordana G., Gauldie J. Heterogeneous proliferative characteristics of human adult lung fibroblast lines and clonally derived fibroblasts from control and fibrotic tissue. Am Rev Respir Dis. 1988 Mar;137(3):579–584. doi: 10.1164/ajrccm/137.3.579. [DOI] [PubMed] [Google Scholar]
- Kamel-Reid S., Letarte M., Sirard C., Doedens M., Grunberger T., Fulop G., Freedman M. H., Phillips R. A., Dick J. E. A model of human acute lymphoblastic leukemia in immune-deficient SCID mice. Science. 1989 Dec 22;246(4937):1597–1600. doi: 10.1126/science.2595371. [DOI] [PubMed] [Google Scholar]
- Katzenstein A. L., Myers J. L., Mazur M. T. Acute interstitial pneumonia. A clinicopathologic, ultrastructural, and cell kinetic study. Am J Surg Pathol. 1986 Apr;10(4):256–267. [PubMed] [Google Scholar]
- LeRoy E. C. Increased collagen synthesis by scleroderma skin fibroblasts in vitro: a possible defect in the regulation or activation of the scleroderma fibroblast. J Clin Invest. 1974 Oct;54(4):880–889. doi: 10.1172/JCI107827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Curran T., Verma I. M. c-fos protein can induce cellular transformation: a novel mechanism of activation of a cellular oncogene. Cell. 1984 Jan;36(1):51–60. doi: 10.1016/0092-8674(84)90073-4. [DOI] [PubMed] [Google Scholar]
- Parkes J. L., Cardell R. R., Hubbard F. C., Jr, Hubbard D., Meltzer A., Penn A. Cultured human atherosclerotic plaque smooth muscle cells retain transforming potential and display enhanced expression of the myc protooncogene. Am J Pathol. 1991 Mar;138(3):765–775. [PMC free article] [PubMed] [Google Scholar]
- Petty T. L., Ashbaugh D. G. The adult respiratory distress syndrome. Clinical features, factors influencing prognosis and principles of management. Chest. 1971 Sep;60(3):233–239. doi: 10.1378/chest.60.3.233. [DOI] [PubMed] [Google Scholar]
- Rodemann H. P., Müller G. A. Abnormal growth and clonal proliferation of fibroblasts derived from kidneys with interstitial fibrosis. Proc Soc Exp Biol Med. 1990 Oct;195(1):57–63. doi: 10.3181/00379727-195-43118. [DOI] [PubMed] [Google Scholar]
- Skubitz K. M., Ehresmann D. D., Ducker T. P. Characterization of human neutrophil ecto-protein kinase activity released by kinase substrates. J Immunol. 1991 Jul 15;147(2):638–650. [PubMed] [Google Scholar]
- Snyder L. S., Hertz M. I., Peterson M. S., Harmon K. R., Marinelli W. A., Henke C. A., Greenheck J. R., Chen B., Bitterman P. B. Acute lung injury. Pathogenesis of intraalveolar fibrosis. J Clin Invest. 1991 Aug;88(2):663–673. doi: 10.1172/JCI115351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. G. Fine structural alterations induced in platelets by adenosine diphosphate. Blood. 1968 May;31(5):604–622. [PubMed] [Google Scholar]
- White J. G. Interaction of membrane systems in blood platelets. Am J Pathol. 1972 Feb;66(2):295–312. [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y., Mitsumata M., Yamane T., Tomikawa M., Nishida K. Morphology and increased growth rate of atherosclerotic intimal smooth-muscle cells. Arch Pathol Lab Med. 1988 Oct;112(10):987–996. [PubMed] [Google Scholar]