Abstract
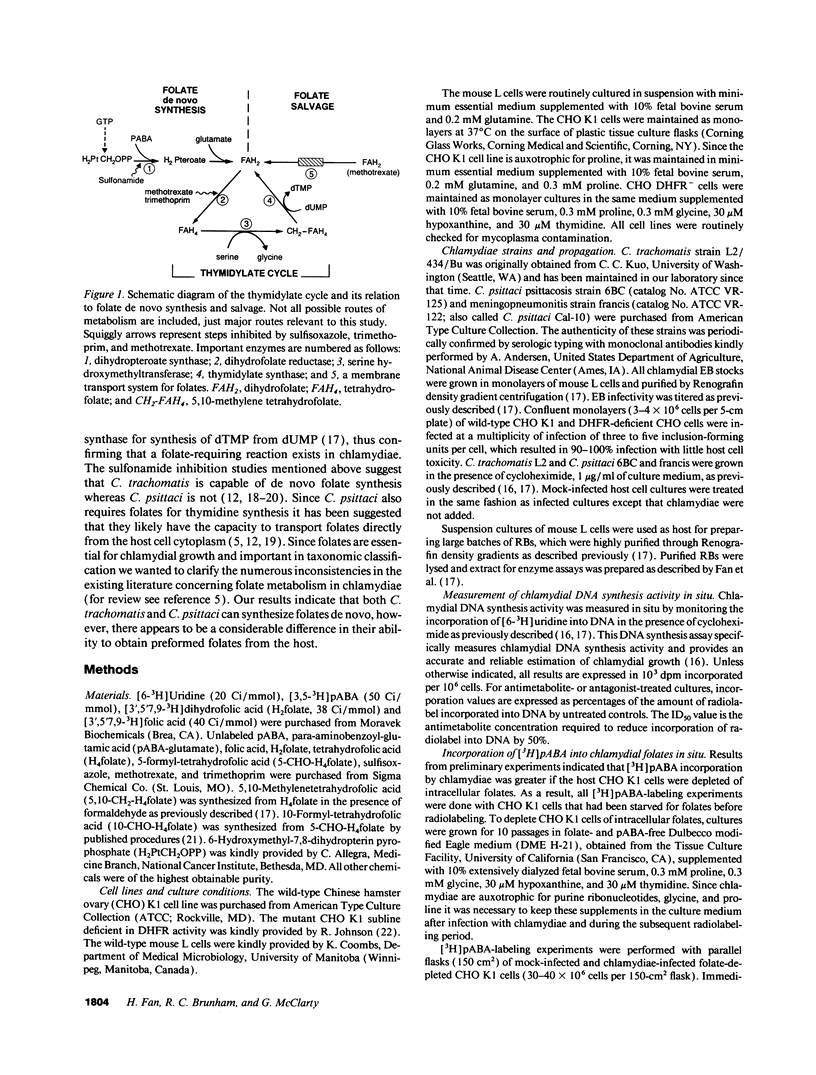
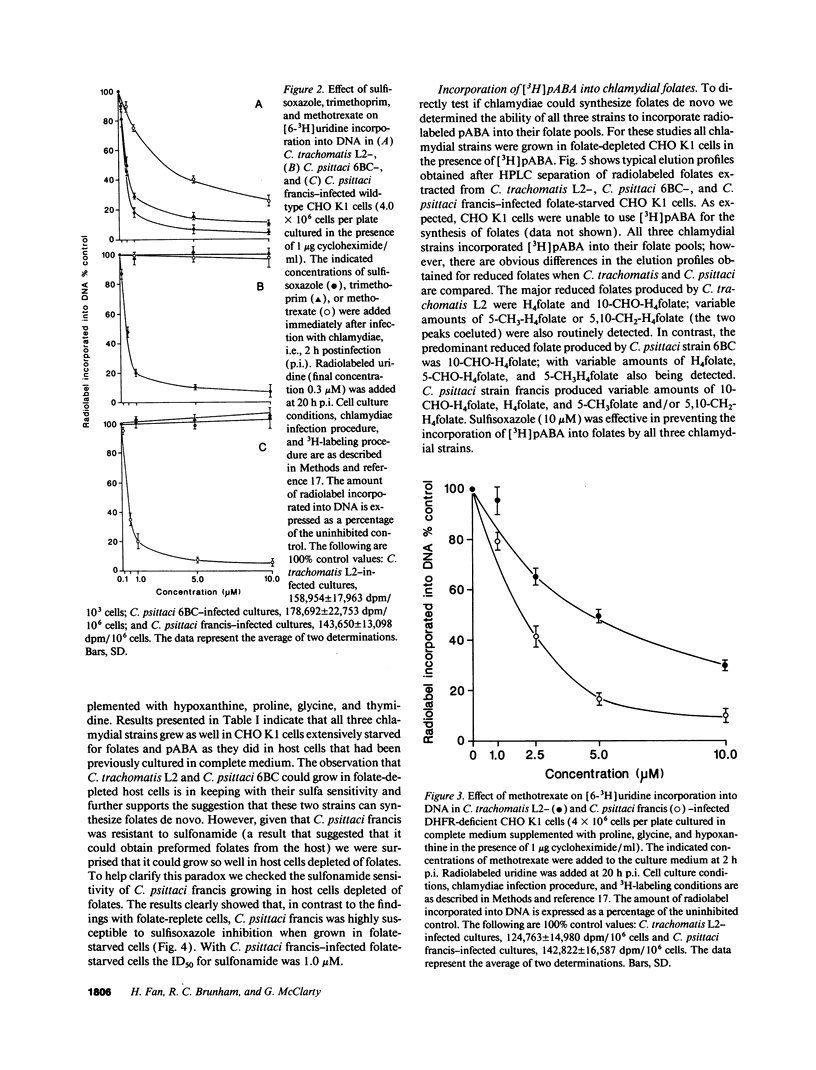
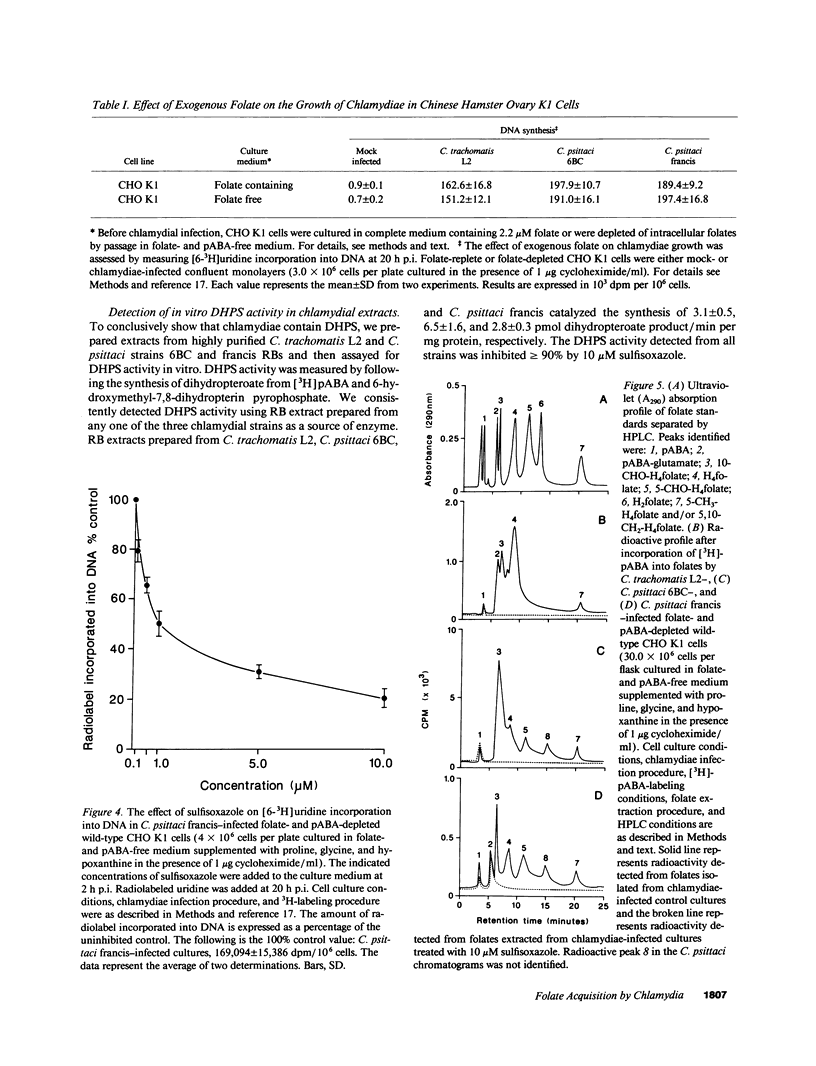
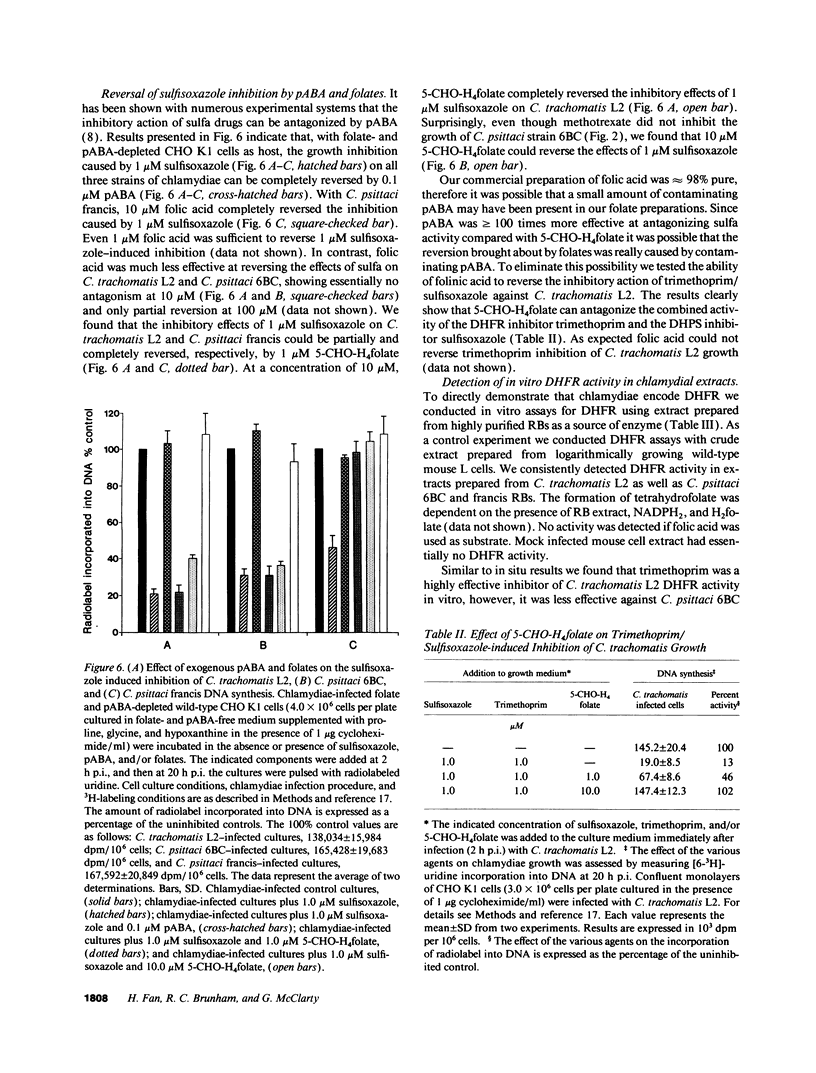
We undertook studies focused on folate acquisition by Chlamydia trachomatis L2, Chlamydia psittaci 6BC, and C. psittaci francis. Results from in situ studies, using wild-type host cells, confirmed that C. trachomatis L2 and C. psittaci 6BC are sensitive to sulfonamides whereas C. psittaci francis is resistant. In addition C. trachomatis L2 and C. psittaci francis were inhibited by methotrexate in situ whereas C. psittaci 6BC was not. In contrast to C. trachomatis, neither C. psittaci strain was affected by trimethoprim. Surprisingly our results indicate that all three strains are capable of efficient growth in folate-depleted host cells. When growing in folate-depleted cells C. psittaci francis becomes sensitive to sulfonamide. The ability of all three strains to carry out de novo folate synthesis was demonstrated by following the incorporation of exogenous [3H]pABA into intracellular folates and by detecting dihydropteroate synthase activity in reticulate body crude extract. Dihydrofolate reductase activity was also detected in reticulate body extract. In aggregate the results indicate that C. trachomatis L2, C. psittaci francis, and C. psittaci 6BC can all synthesize folates de novo, however, strains differ in their ability to transport preformed folates directly from the host cell.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allegra C. J., Fine R. L., Drake J. C., Chabner B. A. The effect of methotrexate on intracellular folate pools in human MCF-7 breast cancer cells. Evidence for direct inhibition of purine synthesis. J Biol Chem. 1986 May 15;261(14):6478–6485. [PubMed] [Google Scholar]
- Allegra C. J., Kovacs J. A., Drake J. C., Swan J. C., Chabner B. A., Masur H. Potent in vitro and in vivo antitoxoplasma activity of the lipid-soluble antifolate trimetrexate. J Clin Invest. 1987 Feb;79(2):478–482. doi: 10.1172/JCI112837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccanari D., Phillips A., Smith S., Sinski D., Burchall J. Purification and properties of Escherichia coli dihydrofolate reductase. Biochemistry. 1975 Dec 2;14(24):5267–5273. doi: 10.1021/bi00695a006. [DOI] [PubMed] [Google Scholar]
- COLON J. I., MOULDER J. W. Folic acid in purified preparations of members of the psittacosis group of micro-organisms. J Infect Dis. 1958 Sep-Oct;103(2):109–119. doi: 10.1093/infdis/103.2.109. [DOI] [PubMed] [Google Scholar]
- COLON J. I. The role of folic acid in the metabolism of members of the psittacosis group of microorganisms. Ann N Y Acad Sci. 1962 Mar 5;98:234–249. doi: 10.1111/j.1749-6632.1962.tb30548.x. [DOI] [PubMed] [Google Scholar]
- Ehret J. M., Judson F. N. Susceptibility testing of Chlamydia trachomatis: from eggs to monoclonal antibodies. Antimicrob Agents Chemother. 1988 Sep;32(9):1295–1299. doi: 10.1128/aac.32.9.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger T. E., Beverley S. M. Biochemistry and regulation of folate and methotrexate transport in Leishmania major. J Biol Chem. 1987 Jul 25;262(21):10053–10058. [PubMed] [Google Scholar]
- Fan H. Z., McClarty G., Brunham R. C. Biochemical evidence for the existence of thymidylate synthase in the obligate intracellular parasite Chlamydia trachomatis. J Bacteriol. 1991 Nov;173(21):6670–6677. doi: 10.1128/jb.173.21.6670-6677.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraiz J., Jones R. B. Chlamydial infections. Annu Rev Med. 1988;39:357–370. doi: 10.1146/annurev.me.39.020188.002041. [DOI] [PubMed] [Google Scholar]
- HOLTERMANN O. A., MERGENHAGEN S. E., MORGAN H. R. Factors related to psittacosis virus (strain 6BC) growth. V. Folic acid-like factor in infected cells. Proc Soc Exp Biol Med. 1959 Feb;100(2):370–372. doi: 10.3181/00379727-100-24630. [DOI] [PubMed] [Google Scholar]
- Hatch T. P. Utilization of exogenous thymidine by Chlamydia psittaci growing in the thymidine kinase-containing and thymidine kinase-deficient L cells. J Bacteriol. 1976 Feb;125(2):706–712. doi: 10.1128/jb.125.2.706-712.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. T., Eaton M. D. THE REVERSAL BY PABA OF SULFONAMIDE INHIBITION OF THE VIRUSES OF LYMPHOGRANULOMA VENEREUM AND MOUSE PNEUMONITIS. J Bacteriol. 1949 Jul;58(1):73–88. [PMC free article] [PubMed] [Google Scholar]
- Kaur K., Coons T., Emmett K., Ullman B. Methotrexate-resistant Leishmania donovani genetically deficient in the folate-methotrexate transporter. J Biol Chem. 1988 May 25;263(15):7020–7028. [PubMed] [Google Scholar]
- Kovacs J. A., Allegra C. J., Beaver J., Boarman D., Lewis M., Parrillo J. E., Chabner B., Masur H. Characterization of de novo folate synthesis in Pneumocystis carinii and Toxoplasma gondii: potential for screening therapeutic agents. J Infect Dis. 1989 Aug;160(2):312–320. doi: 10.1093/infdis/160.2.312. [DOI] [PubMed] [Google Scholar]
- Krungkrai J., Webster H. K., Yuthavong Y. De novo and salvage biosynthesis of pteroylpentaglutamates in the human malaria parasite, Plasmodium falciparum. Mol Biochem Parasitol. 1989 Jan 1;32(1):25–37. doi: 10.1016/0166-6851(89)90126-6. [DOI] [PubMed] [Google Scholar]
- MORGAN H. R. Factors related to the growth of psittacosis virus (strain 6BC). I. Pteroylglutamic acid, vitamin B12, and citrovorum factor. J Exp Med. 1952 Mar;95(3):269–276. doi: 10.1084/jem.95.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maley F., Maley G. F. A tale of two enzymes, deoxycytidylate deaminase and thymidylate synthase. Prog Nucleic Acid Res Mol Biol. 1990;39:49–80. doi: 10.1016/s0079-6603(08)60623-6. [DOI] [PubMed] [Google Scholar]
- Mandelbaum-Shavit F., Grossowicz N. Transport of folinate and related compounds in Pediococcus cerevisiae. J Bacteriol. 1970 Oct;104(1):1–7. doi: 10.1128/jb.104.1.1-7.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClarty G., Tipples G. In situ studies on incorporation of nucleic acid precursors into Chlamydia trachomatis DNA. J Bacteriol. 1991 Aug;173(16):4922–4931. doi: 10.1128/jb.173.16.4922-4931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMartin K. E., Virayotha V., Tephly T. R. High-pressure liquid chromatography separation and determination of rat liver folates. Arch Biochem Biophys. 1981 Jun;209(1):127–136. doi: 10.1016/0003-9861(81)90264-2. [DOI] [PubMed] [Google Scholar]
- Merali S., Zhang Y., Sloan D., Meshnick S. Inhibition of Pneumocystis carinii dihydropteroate synthetase by sulfa drugs. Antimicrob Agents Chemother. 1990 Jun;34(6):1075–1078. doi: 10.1128/aac.34.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991 Mar;55(1):143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve P., Taverne J., Bushby S. R. Inhibition by pyrimidine analogues of the synthesis of folic acid by trachoma agents. J Hyg (Lond) 1968 Jun;66(2):295–306. [PMC free article] [PubMed] [Google Scholar]
- Schachter J., Caldwell H. D. Chlamydiae. Annu Rev Microbiol. 1980;34:285–309. doi: 10.1146/annurev.mi.34.100180.001441. [DOI] [PubMed] [Google Scholar]
- Schachter J. The intracellular life of Chlamydia. Curr Top Microbiol Immunol. 1988;138:109–139. [PubMed] [Google Scholar]
- Shane B., Stokstad E. L. Vitamin B12-folate interrelationships. Annu Rev Nutr. 1985;5:115–141. doi: 10.1146/annurev.nu.05.070185.000555. [DOI] [PubMed] [Google Scholar]
- Tribby I. I., Moulder J. W. Availability of bases and nucleosides as precursors of nucleic acids in L cells and in the agent of meningopneumonitis. J Bacteriol. 1966 Jun;91(6):2362–2367. doi: 10.1128/jb.91.6.2362-2367.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urlaub G., Chasin L. A. Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4216–4220. doi: 10.1073/pnas.77.7.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]



