Abstract
Introduction:
Gender differences in smoking behavior have been proposed to account for poorer outcomes among women attempting to quit. Specifically, it has been suggested that women’s smoking behavior is less motivated by nicotine-seeking and more driven by environmental cues. To date, however, few real-world studies have examined the hypothesis that women’s smoking is under greater stimulus control.
Methods:
One hundred and ninety four daily smokers (men = 107; women = 87) completed 3 weeks of ecological momentary assessment (EMA) monitoring that provided data on real-world smoking behavior by reporting on situational contexts shown by previous research to influence smoking behavior (including social setting, cigarette availability, alcohol consumption, and mood).
Results:
Analyses of particular cues found few gender differences; however, men’s smoking increased to a greater extent compared with women’s when they were with others who were smoking. Idiographic analyses that allow individual subjects to have different directions of linkage to situational cues also were conducted to assess how predictable subjects’ smoking was from a range of contextual characteristics. Compared with women, men’s smoking was significantly more closely tied to food/alcohol consumption and tended to be more closely tied to social context. No other gender differences were found.
Conclusions:
EMA analyses suggest that men and women are similarly influenced by cues, including mood. Where there were gender differences, it was men rather than women whose smoking behavior was more influenced by cues. The data contradict the hypothesis that women’s smoking is more influenced by cues.
Introduction
While smoking rates are generally higher among men than women,1 the decline in smoking seen in Western countries in recent decades appears to be slower among females, particularly among teenage and older women (U.S. data; CDC1). Some researchers have suggested that this is due to women having greater difficulty quitting successfully, including when using available cessation treatments (Wetter et al.2; e.g., with Nicotine Replacement Therapy, Perkins3; West et al.4). It has also been suggested that female smokers are at greater risk of relapse following a quit attempt (e.g., Perkins3; Ward et al.5; Perkins, Donny, and Caggiula6). Ad libitum smoking patterns also appear to differ between males and females: Women tend to smoke fewer cigarettes per day than men and inhale less deeply.7,8 Understanding why such differences in smoking patterns exists may help to inform whether different cessation approaches may be needed for males and females.
Interest in gender differences in smoking patterns and motivation to smoke has been stimulated by Perkins’9 hypothesis that women’s smoking is less motivated by nicotine-seeking and driven more in response to cues (i.e., that it is under greater stimulus control). In particular, Perkins found that nicotine intake was less reinforcing in women than men (e.g., Perkins et al.6; Perkins, Grobe, Stiller, Fonte, and Goettler10), and, suggested that their smoking must therefore be motivated by non-nicotine factors, including environmental smoking cues.9 Laboratory cue-reactivity studies have examined men’s and women’s responses to cues, with mixed results. Some studies suggest that women exhibit greater craving in response to smoking cues,6,11,12,13 while a larger study of 207 smokers who completed over 1,000 reactivity sessions found no gender differences in cue effects on craving and smoking.14 However, many cue-reactivity studies have focused on cigarette craving as opposed to actual smoking in response to cues15 (although Shiffman et al.14 did examine cue effect on ab libitum smoking), and it is unclear how well laboratory-based cue-reactivity measures correspond to real-world smoking behavior.16 Thus, cue-reactivity studies may provide only limited insight into gender differences in smoking behavior in response to cues.
In addition to laboratory-based cue-reactivity studies, researchers have also used EMA17 methods to explore whether women’s smoking, compared to men’s, is driven more by cues in real-world smoking contexts. These methods allow data to be collected on smokers’ behavior in the real-world and in real-time, and across multiple occasions, thus reducing recall bias and ensuring ecological validity. EMA studies have demonstrated that smoking has systematic relationships with particular environmental contexts, prominent among them being alcohol consumption, other smokers, and smoking regulations (e.g., Shiffman et al.18). The few EMA studies, that have specifically analyzed gender differences in stimulus control of smoking found minimal gender differences. Research by Delfino and colleagues19 and McCarthy and colleagues20 found no gender differences in smoking behavior by social setting, or due to stressful events or seeing someone smoke. Shiffman and Rathbun21 found no gender differences in smoking patterns based on social setting (alone, versus with others) or in situations where smoking was allowed or discouraged, although women were less likely to smoke where smoking was prohibited.
One particular stimulus domain that has received attention as differentially affecting men and women’s smoking is mood. Since women often report smoking to reduce tension or for stress control,22–25 and negative affect has been shown to increase risk of relapse among smokers trying to quit (e.g., Shiffman and Waters26; Ferguson, Shiffman and Gwaltney27), it has been suggested that there are gender differences in the effect of mood on smoking behavior. In laboratory studies using stress/negative affect cues, both Perkins and colleagues28 and Saladin and colleagues29 reported that women’s craving level was more responsive to these cues than men’s. However, other laboratory studies using similar mood manipulations have found no gender differences on craving or desire to smoke,30–32 although gender differences in latency to smoke following a negative mood induction have been reported (with women being faster to smoke following a negative mood manipulation; Weinberger and McKee30). Field studies using EMA methods have also contradicted the hypothesis that women’s smoking is more responsive to mood, finding either no gender differences at all, or, unexpectedly, that negative affect was more associated with increased smoking among men, rather than women. Delfino et al.19, for example, found no gender differences in the relationship of smoking with anger, anxiety, and alertness, but found that fatigue and sadness were more positively associated with smoking urge in men, and happiness more so in women. The greater influence of negative affect and arousal on men’s smoking has been supported by more recent research, including studies using some of the largest samples in the EMA literature.21,33
An important limitation of the field-based studies conducted to date, however, is that they have focused on identifying gender differences in the stimuli whose association with smoking is shared across most smokers; that is, they have explored stimuli that might trigger craving or smoking across all or most women, versus all or most men. Such “aggregate” analyses are limited; they can miss meaningful within-subject associations and between-subject trends.34 For example, some women might be more likely to smoke when with other smokers, while some more likely to smoke when alone. Such patterns would result in little or no overall association, yet both groups of women would be demonstrating sensitivity to environmental cues. Further, analyzing gender differences one variable at a time may miss important effects. For example, with regard to mood, some women’s smoking might be more responsive to arousal, others’ to mood valence, possibly masking differences in sensitivity to mood. Analyses that examine entire domains of potential cues (e.g., “mood”) would be more sensitive to such effects. Here, we present data from a three-week EMA study of smoking behavior in a sample of daily smokers who were not interested in quitting. In addition to testing for gender differences in particular stimuli associated with smoking, we also quantify the degree of overall stimulus control—that is, the overall association between smoking and major multivariable domains of stimuli—by assessing these domain-level associations ideographically for each smoker, and then comparing the magnitude of stimulus control seen in men and women.
Methods
Participants
Participants comprised 194 daily smokers who volunteered to take part in the study advertised and promoted in Pittsburgh, PA, between November 2007 and April 2010. To be successfully screened, participants had to report being ≥21 years old, smoke between 5 and 30 cigarettes per day, report smoking for ≥3 years, had consistently been smoking at their current rate for ≥3 months, and not intend to quit within the next month. The final sample comprised 107 men and 87 women. These smokers are a sub-sample of a larger study examining differences between daily and non-daily smokers (for details see Shiffman et al.18 and Shiffman, Ferguson, Dunbar, and Scholl35). The demographic and smoking history characteristics—overall and separately for males and females—of the sample are presented in Table 1. With the exception of alcohol consumption—where males reported drinking significantly more than females —there were no significant differences between males and females. (Men were also more likely to report any alcohol use during the monitoring period, compared to women [p = .03].)
Table 1.
Subject Demographic and Smoking History Characteristics, Overall and by Gender
| Overall (n = 194) | Males (n = 107) | Females (n = 87) | |
|---|---|---|---|
| Age | 40.11 (11.68) | 40.40 (12.44) | 39.71 (10.73) |
| Race/ethnicity (%) | |||
| Black | 37.63% | 31.78% | 44.83% |
| Caucasian | 59.28% | 64.49% | 52.87% |
| Other | 3.09% | 3.74% | 2.30% |
| Education (% beyond high school) | 62.13% | 61.44% | 63.12% |
| Cigarettes per day | 16.62 (6.59) | 16.60 (6.63) | 16.65 (6.58) |
| Smoking days per month | |||
| Fagerström Test for Nicotine Dependence | 5.21±2.00 | 5.07 (2.25) | 5.41 (1.63) |
| Lifetime history of depression Ŧ | 28.39% | 25.74% | 31.64% |
| Average alcoholic consumption (drinks per week) | 7.91 (10.26) | 10.15 (11.75) | 4.65 (6.98)* |
Ŧ Measured using the Inventory to Diagnose Depression. 36 Descriptive analyses were weighted by race. Entries are M (SD), unless % is specified.
* p < .05.
Procedure
To collect real-time and real-world data on smoking patterns, participants were asked to engage in EMA monitoring by using a study provided electronic diary (ED) programmed with specially designed software (invivodata). Participants received detailed instructions and training on ED use, and data were downloaded at study visits during the 21 days of ED monitoring.
The EMA protocol and assessments have been described in detail elsewhere.18,37 Briefly, participants were asked to record each time they smoked a cigarette (event-based sampling); on a randomly-selected subset of occasions (4–5 per day), participants were asked a series of questions about the situation they were in. Further, to compare participants’ situations when they were not smoking (signal-based sampling), participants were also “beeped” 4–5 times a day at random and asked to complete parallel assessments of nonsmoking situations. Participants were to carry the ED with them at all times during their waking day.
Measures
Participants used the ED’s touchscreen interface to complete all assessments and answered questions using three response types depending on type of question (i.e., no open-ended questions): Visual Analog Scales (VAS; 0–100 scales), yes/no responses, or multiple selections from a list of options. Assessments were time-stamped and saved. The ED assessments surveyed participants’ situation in multiple domains, including location, activity, social setting, and mood. Each domain was analyzed for stimulus control. We focus our analysis of individual variables on the cues that have been consistently associated with smoking in prior studies26,39,40: smoking environment (cigarette availability [with ease, with difficulty, not available]; smoking restrictions [allowed, discouraged, forbidden: by own rule, by law, or not at all]; smoking allowed [controlling for presence of other smokers]); social setting (presence of other smokers [in group, in view, in group and in view, no one smoking]), and recent consumption (food/alcohol in the past 15min). Additionally, to assess mood, participants rated on a 0–100 VAS 14 mood adjectives (including ability to focus, angry/frustrated, happy, irritable, restless, sad) during each assessment, along with their overall mood and arousal levels. These mood data were characterized using factor analysis into the sub-scales: negative affect, positive affect, arousal and inattention.18 Factor scores thus derived are standardized T-scores (M = 50, SD = 10).
Data Reduction and Analytic Plan
GEE
We used Generalized Estimating Equations (GEE; SAS ProcGenmod, with logit link) to examine gender differences in the effects of situational variables on the probability of smoking (vs. nonsmoking). We assessed the interaction between gender and each predictor; if the interaction was significant, we subsequently examined main effects within gender. All analyses were weighted by race (to account for over-recruitment of Black smokers) and probability of cigarette assessment (that is, the probability of whether a cigarette was selected for full assessment vs. simply being logged), which varied day-to-day, depending on the number of cigarettes smoked the prior day. All predictors were examined in separate GEE models, unless otherwise noted.
Stimulus Control
Logistic analyses were conducted separately on each subject’s data, to gauge stimulus control while allowing for idiosyncratic effects on smoking.41 As a way of summarizing the degree to which each individual’s smoking was associated with variables from each domain, we used the area under the curve of the receiver operating characteristics curve (AUC-ROC). We computed AUC-ROC for each subject using logistic regression models to predict smoking versus nonsmoking events from situational variables in specific domains. Importantly, AUC-ROC values have a meaningful interpretation: they represent the probability of correctly identifying, given two situations, which one involved smoking and which did not, based on the covariates. Accordingly, AUC-ROC values can range from 0.5 (i.e., random guessing; no prediction) to 1.0 (perfect prediction). Separate sets of regression analyses were run for each of the domains assessed in EMA. To compare AUC values for each domain by gender, t tests were conducted. Tests were weighted by inverse standard error of the AUC-ROC and by race.
Results
Single Variable Directional Effects of Cues (GEE Analysis)
Situational Context
Gender did not interact with cigarette availability, presence of smoking restrictions, presence of others smoking (either in group or in view), or recent alcohol consumption (see Table 2). However, when participants reported that others were smoking both in view (e.g., a stranger smoking across the room) and in one’s immediate social group, there was a significant interaction with gender, such that the effect of other smokers being both in group and in view on probability of smoking was considerably stronger among men compared to women (OR = 2.99, 95% CI = 2.05–4.36; vs. OR = 1.64, 95% CI = 1.17–2.31). This pattern remained significant after controlling for being at a bar or restaurant.
Table 2.
Situation Interactions With Gender on Likelihood of Smoking
| Males | Females | Gender × stimulus × smoking | |||||
|---|---|---|---|---|---|---|---|
| Contextual Cue | Not smoking (%) | Smoking (%) | Not smoking (%) | Smoking (%) | OR | 95% CI | p |
| Alcohol consumption | 3.04 | 6.31 | 2.03 | 3.40 | 1.28 | 0.86–1.88 | .22 |
| Cigarette availability | |||||||
| With ease | 91.51 | 97.63 | 89.55 | 96.38 | 1.00 | 0.40–2.47 | .99 |
| With difficulty | 3.43 | 1.87 | 5.39 | 2.87 | 1.07 | 0.37–3.12 | .90 |
| Not available (ref) | 5.01 | 0.49 | 4.95 | 0.51 | – | – | – |
| Smoking restrictions | |||||||
| Forbidden (ref) | 18.90 | 8.25 | 23.49 | 10.98 | – | – | – |
| Discouraged | 7.44 | 5.17 | 8.49 | 7.63 | 0.80 | 0.36–1.79 | .58 |
| Allowed | 73.61 | 86.57 | 67.89 | 81.15 | 0.95 | 0.62–1.44 | .80 |
| Other smokers | |||||||
| In group | 9.78 | 16.96 | 10.19 | 12.95 | 1.43 | 0.87–2.37 | .16 |
| In view | 7.81 | 17.10 | 8.44 | 14.54 | 1.30 | 0.88–1.91 | .19 |
| In group and in view | 2.01 | 4.59 | 2.49 | 3.64 | 1.79 | 1.09–2.93 | .02 |
| No one smoking (ref) | 84.42 | 70.53 | 83.85 | 76.16 | – | – | – |
| Mood a | |||||||
| Negative affect | 51.58 | 51.10 | 50.91 | 51.80 | 0.99 | 0.97–1.00 | .14 |
| Quadratic | 1.00 | 1.00–1.00 | .15 | ||||
| Positive affect | 49.86 | 50.03 | 49.50 | 49.00 | 1.01 | 0.99–1.03 | .29 |
| Quadratic | 1.00 | 1.00–1.00 | .64 | ||||
| Arousal | 49.74 | 49.59 | 47.56 | 46.90 | 1.00 | 0.99–1.02 | .60 |
| Quadratic | 1.00 | 1.00–1.00 | .11 | ||||
| Inattention | 52.09 | 51.29 | 51.00 | 51.98 | 0.99 | 0.97–1.00 | .07 |
| Quadratic | 1.00 | 1.00–1.00 | .71 | ||||
OR = odds ratio; CI = confidence interval; ref = reference category for analysis and computation of the IR.
a Mood variables were multivariable continuous scores, standardized as t scores, with M = 50 and SD = 10. Each was tested for both linear and quadratic associations with smoking.
Mood
There were no significant gender by mood (negative affect, positive affect, arousal or inattention factors) interactions influencing probability of smoking.
Overall Stimulus Control by Cue Domain (AUC-ROC Analysis)
Figure 1 shows the AUC-ROC values for men and women by situational domains. Men had significantly higher AUC-ROC values for consumption (p < .05), indicating that their smoking was more closely tied to food and drink consumption (which included alcohol consumption); however, this difference disappeared when we controlled for baseline levels of alcohol consumption (see Table 1). When an AUC-ROC analysis was run focusing solely on alcohol consumption, the AUC-ROC values were reduced, but males again had higher AUC-ROC values (Males: 0.53, Females: 0.52; p = .022); again, however, this difference disappeared when baseline levels of alcohol consumption were included as a control. Men also had slightly higher AUC-ROC values for smoking context, but this difference was not significant (p < .10). No other gender differences were observed, including for mood (which included negative affect, positive affect, arousal and inattention factor scores as predictors).
Figure 1.
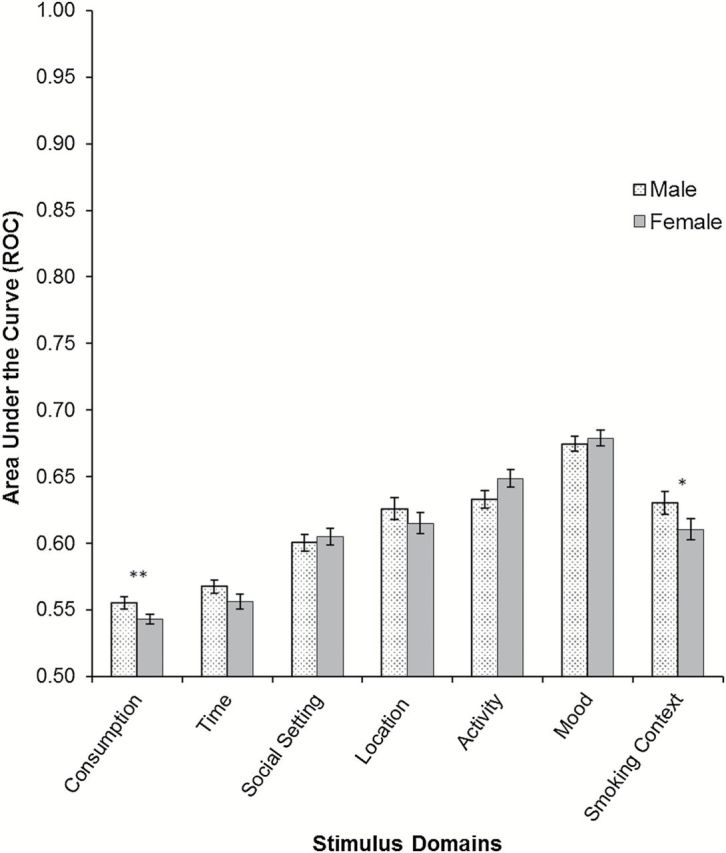
Average AUC-ROC by gender for domains of context. The ROC value indicates the probability of correct prediction that an observation is a smoking versus a nonsmoking situation. Thus a value of 0.50 indicates no association of smoking with the domain, while a value of 1.0 would represent perfect prediction. Gender differences were tested between subjects by t tests. (**p < .05, *p < .10). The mood model includes negative affect, positive affect, arousal, and inattention factor scores as predictors, including both linear and quadratic components.
Discussion
Gender differences in the antecedents of smoking have been proposed to account for the higher rates of unsuccessfully quit attempts and relapse among female smokers compared to men. Specifically, it has been suggested that women’s smoking behavior is more influenced by environmental stimuli and mood (e.g., Perkins9). This study used EMA data to examine gender differences in real-world smoking patterns. In addition to examining group-wise differences in how particular stimuli increased or decreased the likelihood of smoking, we also examined gender differences in stimulus control more broadly, by using within-subject analyses to examine whether men and women differed in overall sensitivity to context, without requiring that they necessarily respond to the same particular contextual cues. Examining several situational contexts, we found no cues that exercised greater influence on smoking among women. Indeed, the only significant stimulus by gender interaction indicated that men’s smoking was more likely to be associated with being with others who were smoking, both in their group and in view. Moreover, when examining the broader influence of cues, the trend was for men’s smoking, rather than women’s, to be under greater stimulus control (Figure 1). In particular, men’s smoking seemed to be more influenced by food and drink consumption, particularly alcohol consumption, and by others’ smoking. The relationship between smoking and consumption was no longer significant when we controlled for the different levels of average alcohol consumption. It makes sense that when drinking is more frequent, it has a greater opportunity to influence smoking. Even among men, who drink more, less than 4% of cigarettes were smoked while drinking. Given the male-female differences in the frequency of drinking, it is not surprising that adjusting for frequency eliminated the gender differences in the relationship of smoking to drinking. In any case, the unadjusted analyses suggested greater stimulus control among men, rather than the hypothesized greater stimulus control among women.
The finding that there was no differential effect of mood on men and women’s smoking behavior is consistent with results of previous EMA studies (e.g., Shiffman et al.40; Shiffman, Paty, Gwaltney, and Dang42) suggesting no differential effect of mood on smoking behavior overall between males and females, but is inconsistent with other studies suggesting that men show greater effects for mood (e.g., Delfino et al.19; Shiffman and Rathbun21; Todd33). Together, these mixed findings contradict the traditional view (based on smokers’ global self-reports of smoking patterns; e.g., Perkins3) that women’s smoking behavior is more influenced by mood. Indeed, it is important to note that no real-world study has reported that mood exerts a greater influence on women’s smoking than on men’s.
Our study also examined the role of other situational contexts on smoking. Contrary to hypothesis, social context was actually more strongly associated with men’s smoking than with women’s smoking: the effect of being in a group consisting of other smokers as well as having other smokers in view was significantly more influential on men’s smoking than women’s. There was, however, no differential gender effect of other social contexts (including cigarette availability, smoking restrictions, presence of others smoking in group or in view, or recent alcohol consumption). This is inconsistent with a recent study that found that women’s smoking was suppressed to a greater extent when smoking was prohibited or discouraged.21 These authors also found that women were more likely to smoke when they were alone compared to men, but there was no gender difference in smoking based on other social contexts (including, others smoking, discouraged/allowed). This inconsistency of study results may be accounted for by the study sample itself; the present study sample comprised non-quitting subjects while the Shiffman and Rathbun21 study consisted of smokers about to embark on a quit attempt. We consider the use of non-quitting smokers as a strength of this study, as here we could look for differences between male and female smoking without the potential confound that smokers may have been actively in the process of trying to modify—or reduce—their smoking in the lead up to a quit attempt. Nevertheless, these real-world findings, while mixed, collectively challenge the view that women’s smoking is influenced to a greater extent by situational contexts (e.g., Perkins9).
Above and beyond data on particular situational effects on men’s and women’s smoking, our analyses also assessed whether women’s smoking was more responsive to cues; that is, whether women’s smoking was under greater stimulus control. Whereas analyses of specific directional effects on smoking assess directional effects common to all subjects in a group (e.g., does drinking alcohol stimulate smoking?), the stimulus control analyses conducted here allowed individual subjects to have idiosyncratic associations between cues and smoking, even allowing for relationships in opposite directions (i.e., alcohol could increase smoking in some and decrease it in others). Such idiosyncratic or idiographic associations might be expected if the associations derive from conditioned learning, as has been proposed,43 because individuals may differ in their learning histories and exposures to various cues. With this broader analysis, evidence contradicted the hypothesis that women’s smoking is more cue-driven. In most cue domains, there were no gender differences. Where there were differences—food and drink consumption and smoking settings—it was men whose smoking seemed to be under greater stimulus control. Thus, from multiple perspectives, the data contradict the hypothesis that women’s smoking is more closely tied to cues.
In the absence of evidence that women are more influenced by contextual cues, what might account for women’s greater difficulty quitting? Factors such as differences in puffing patterns (e.g., Melikian et al.8), menstrual cycle effects (e.g., Gray et al.44; Carpenter, Saladin, Leinback, Larowe, and Upadhyaya45), which we did not assess, and differential motivation to use smoking as a means of weight control (e.g., Sieminska and Jassem46) have been suggested, but none of these have been definitively proven, and none are addressed by our data. A further limitation of the present study relate to the EMA methods employed and are thus similar to any study using this technique. There are two potential issues. Firstly, EMA protocol compliance may have biased the data, such that if participants failed to respond to prompts under certain conditions—for example, when arguing and under stress—their data would not accurately reflect their smoking behavior in such conditions. We do not believe, however, that such a potential bias would differ between men and women, and thus influence the results presented here. Secondly, participation in the study and engaging with the ED may have influenced participants’ smoking behavior (e.g., smoked less to avoid filling out survey), however, again, we have no reason to believe any potential reactivity to EMA procedures would vary by gender. Furthermore, participant reactivity to EMA protocol has been shown to have minimal influence over smoking behavior.47
It has been suggested that women’s smoking is less motivated by nicotine, and must therefore be more driven by cues, and that mood, and especially negative mood, is a particularly significant driver of women’s smoking. Our analyses do not support these hypotheses. The influence of cues on smoking was relatively similar for men and women and, when differences were observed, they showed that men’s smoking was actually more closely tied to cues. Treatment implications are unclear, since the data did not identify cues or contexts that are more important for women, and were obtained from smokers not currently trying to quit. Regardless, the present data do not support attending more closely to smoking cues in smoking cessation treatment for women.
Funding
This work was supported by grant R01-DA020742 (to SS) from the National Institutes of Health, National Institute on Drug Abuse. Additional support was provided by the National Science Foundation Graduate Research Fellowship (to MSD), National Cancer Institute grant R25-CA057703-15 (to MSD), and Cancer Council Tasmania (to MF). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declaration of Interests
SGF and SS have worked as consultants for GlaxoSmithKline Consumer Healthcare on matters relating to smoking cessation, and SGF has received researcher-initiated project grant funding from Pfizer (through the GRAND initiative). SS consults to and has an interest in eRT, which provides electronic diary services for clinical research.
Acknowledgments
The authors would like to thank S. Scholl for her comments and assistance with collecting data and preparing this manuscript.
Data from this paper were presented at a Pre-Conference Workshop at the Society for Research on Nicotine and Tobacco Annual Meeting; February 25, 2015; Philadelphia, Pennsylvania, USA.
References
- 1. Centers for Disease Control and Prevention. Current cigarette smoking among adults—United States 2011. MMWR Morb Mortal Wkly Rep. 2012;61:889–894. [PubMed] [Google Scholar]
- 2. Wetter DW, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB. Gender differences in smoking cessation. J Consult Clin Psychol. 1999;67:555–562. [DOI] [PubMed] [Google Scholar]
- 3. Perkins KA. Smoking cessation in women. Special considerations. CNS Drugs. 2001;15:391–411. [DOI] [PubMed] [Google Scholar]
- 4. West R, Hajek P, Nilsson F, Foulds J, May S, Meadows A. Individual differences in preferences for and responses to four nicotine replacement products. Psychopharmacology (Berl). 2001;153:225–230. [DOI] [PubMed] [Google Scholar]
- 5. Ward KD, Klesges RC, Zbikowski SM, Bliss RE, Garvey AJ. Gender differences in the outcome of an unaided smoking cessation attempt. Addict Behav. 1997;22:521–533. [DOI] [PubMed] [Google Scholar]
- 6. Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res. 1999;1:301–315. [DOI] [PubMed] [Google Scholar]
- 7. Zang EA, Wynder EL. Differences in lung cancer risk between men and women: examination of the evidence. J Natl Cancer Inst. 1996;88:183–192. [DOI] [PubMed] [Google Scholar]
- 8. Melikian AA, Djordjevic MV, Hosey J, et al. Gender differences relative to smoking behavior and emissions of toxins from mainstream cigarette smoke. Nicotine Tob Res. 2007;9:377–387. [DOI] [PubMed] [Google Scholar]
- 9. Perkins KA. Sex differences in nicotine versus nonnicotine reinforcement as determinants of tobacco smoking. Exp Clin Psychopharm. 1996;4:166–177. [Google Scholar]
- 10. Perkins KA, Grobe JE, Stiller RL, Fonte C, Goettler JE. Nasal spray nicotine replacement suppresses cigarette smoking desire and behavior. Clin Pharmacol Ther. 1992;52:627–634. [DOI] [PubMed] [Google Scholar]
- 11. Carpenter MJ, Saladin ME, Larowe SD, et al. Craving, cue reactivity, and stimulus control among early-stage young smokers: effects of smoking intensity and gender. Nicotine Tob Res. 2014;16:208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sayette MA, Hufford MR. Urge and affect: a facial coding analysis of smokers. Exp Clin Psychopharm. 1995;3:417–423. [Google Scholar]
- 13. Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Cue-provoked craving and nicotine replacement therapy in smoking cessation. J Consult Clin Psychol. 2004;72:1136–1143. [DOI] [PubMed] [Google Scholar]
- 14. Shiffman S, Dunbar M, Kirchner T, et al. Smoker reactivity to cues: effects on craving and on smoking behavior. J Abnorm Psychol. 2013;122: 264–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perkins KA. Does smoking cue-induced craving tell us anything important about nicotine dependence? Addiction. 2009;104:1620–1622. [DOI] [PubMed] [Google Scholar]
- 16. Wray JM, Gass JC, Tiffany ST. A systematic review of the relationships between craving and smoking cessation. Nicotine Tob Res. 2013;15:1167–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shiffman S. Real-time self-report of momentary states in the natural environment: computerized ecological momentary assessment. In Stone AA, Turkkan JS, Bachrach CA, Jobe JB, Kurtzman HS, Cain VS. (Eds.). The science of self-report, implications for research and practice. New Jersey: Lawrence Erlbaum Associates; 2000: 277–296. [Google Scholar]
- 18. Shiffman S, Dunbar MS, Li X, et al. Smoking patterns and stimulus control in intermittent and daily smokers. PLoS One. 2014;9:e89911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Delfino RJ, Jamner LD, Whalen CK. Temporal analysis of the relationship of smoking behavior and urges to mood states in men versus women. Nicotine Tob Res. 2001;3:235–248. [DOI] [PubMed] [Google Scholar]
- 20. McCarthy DE, Piasecki TM, Fiore MC, Baker TB. Life before and after quitting smoking: an electronic diary study. J Abnorm Psychol. 2006;115:454–466. [DOI] [PubMed] [Google Scholar]
- 21. Shiffman S, Rathbun SL. Point process analyses of variations in smoking rate by setting, mood, gender, and dependence. Psychol Addict Behav. 2011;25:501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pomerleau CS, Berman BA, Gritz ER, Marks JL, Goeters S. Why women smoke. In Watson RR. (Ed.). Drug and alcohol abuse reviews: Vol. 5. Addictive behaviors in women. Totowa, NJ: Humana; 1994: 39–70. [Google Scholar]
- 23. Berlin I, Singleton EG, Pedarriosse AM, et al. The Modified Reasons for Smoking Scale: factorial structure, gender effects and relationship with nicotine dependence and smoking cessation in French smokers. Addiction. 2003;98:1575–1583. [DOI] [PubMed] [Google Scholar]
- 24. McEwen A, West R, McRobbie H. Motives for smoking and their correlates in clients attending Stop Smoking treatment services. Nicotine Tob Res. 2008;10:843–850. [DOI] [PubMed] [Google Scholar]
- 25. Fidler JA, West R. Self-perceived smoking motives and their correlates in a general population sample. Nicotine Tob Res. 2009;11:1182–1188. [DOI] [PubMed] [Google Scholar]
- 26. Shiffman S, Waters AJ. Negative affect and smoking lapses: a prospective analysis. J Consult Clin Psychol. 2004;72:192–201. [DOI] [PubMed] [Google Scholar]
- 27. Ferguson SG, Shiffman S, Gwaltney CJ. Does reducing withdrawal severity mediate nicotine patch efficacy? A randomized clinical trial. J Consult Clin Psychol. 2006;74:1153–1161. [DOI] [PubMed] [Google Scholar]
- 28. Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA. Negative mood effects on craving to smoke in women versus men. Addict Behav. 2013;38: 1527–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saladin ME, Gray KM, Carpenter MJ, LaRowe SD, DeSantis SM, Upadhyaya HP. Gender differences in craving and cue reactivity to smoking and negative affect/stress cues. Am J Addict. 2012;21:210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weinberger AH, McKee SA. Gender differences in smoking following an implicit mood induction. Nicotine Tob Res. 2012;14:621–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fucito LM, Juliano LM. Depression moderates smoking behavior in response to a sad mood induction. Psychol Addict Behav. 2009;23:546–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Perkins KA, Grobe JE. Increased desire to smoke during acute stress. Br J Addict. 1992;87:1037–1040. [DOI] [PubMed] [Google Scholar]
- 33. Todd M. Daily processes in stress and smoking: effects of negative events, nicotine dependence, and gender. Psychol Addict Behav. 2004;18: 31–39. [DOI] [PubMed] [Google Scholar]
- 34. Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64:366–379. [DOI] [PubMed] [Google Scholar]
- 35. Shiffman S, Ferguson SG, Dunbar MS, Scholl SM. Tobacco dependence among intermittent smokers. Nicotine Tob Res. 2012;14:1372–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zimmerman M, Coryell W. The inventory to diagnose depression, lifetime version. Acta Psychiatr Scand. 1987;75:495–499. [DOI] [PubMed] [Google Scholar]
- 37. Shiffman S, Dunbar MS, Li X, et al. Craving in intermittent and daily smokers during ad libitum smoking. Nicotine Tob Res. 2014;16:1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lam CY, Businelle MS, Aigner CJ, et al. Individual and combined effects of multiple high-risk triggers on postcessation smoking urge and lapse. Nicotine Tob Res. 2014;16:569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thrul J, Buhler A, Ferguson SG. Situational and mood factors associated with smoking in young adult light and heavy smokers. Drug Alcohol Rev. 2014;33:420–427. [DOI] [PubMed] [Google Scholar]
- 40. Shiffman S, Gwaltney CJ, Balabanis MH, et al. Immediate antecedents of cigarette smoking: an analysis from ecological momentary assessment. J Abnorm Psychol. 2002;111:531–545. [DOI] [PubMed] [Google Scholar]
- 41. Shiffman S, Paty J. Smoking patterns and dependence: contrasting chippers and heavy smokers. J Abnorm Psychol. 2006;115:509–523. [DOI] [PubMed] [Google Scholar]
- 42. Shiffman S, Paty JA, Gwaltney CJ, Dang Q. Immediate antecedents of cigarette smoking: an analysis of unrestricted smoking patterns. J Abnorm Psychol. 2004;113:166–171. [DOI] [PubMed] [Google Scholar]
- 43. Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. J Abnorm Psychol. 1988;97:133–152. [DOI] [PubMed] [Google Scholar]
- 44. Gray KM, DeSantis SM, Carpenter MJ, Saladin ME, LaRowe SD, Upadhyaya HP. Menstrual cycle and cue reactivity in women smokers. Nicotine Tob Res. 2010;12:174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carpenter MJ, Saladin ME, Leinbach AS, Larowe SD, Upadhyaya HP. Menstrual phase effects on smoking cessation: a pilot feasibility study. J Womens Health. 2008;17:293–301. [DOI] [PubMed] [Google Scholar]
- 46. Sieminska A, Jassem E. The many faces of tobacco use among women. Med Sci Monit. 2014;20:153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. [DOI] [PubMed] [Google Scholar]


