Abstract
Introduction:
Although the evidence is mixed, female smokers appear to have more difficulty quitting smoking than male smokers. Craving, stress, and negative affect have been hypothesized as potential factors underlying gender differences in quit rates.
Methods:
In the current study, the cue-reactivity paradigm was used to assess craving, stress, and negative affect in response to cues presented in the natural environment of cigarette smokers using ecological momentary assessment. Seventy-six daily smokers (42% female) responded to photographs (smoking, stress, and neutral) presented 4 times per day on an iPhone over the course of 2 weeks.
Results:
Both smoking and stress cues elicited stronger cigarette craving and stress responses compared to neutral cues. Compared with males, females reported higher levels of post-stress cue craving, stress, and negative affect, but response to smoking cues did not differ by gender.
Discussion:
Findings from this project were largely consistent with results from laboratory-based research and extend previous work by measuring response to cues in the natural environment of cigarette smokers. This study extends previous cue reactivity ecological momentary assessment research by using a new platform and by measuring response to stress cues outside of the laboratory. Findings from this project highlight the importance of addressing coping in response to stress cues in clinical settings, especially when working with female smokers.
Introduction
Most quit attempts end in relapse,1 and there is evidence that female smokers have more difficulty quitting smoking than male smokers.2–4 This gender difference has often been observed in clinical trials,5,6 but population-based evidence for gender differences in quit success has been mixed.7,8 An appeal for research identifying mechanisms related to these disparate cessation outcomes has been made in the smoking literature.9 Craving, stress, and negative affect (NA) have been hypothesized as potential factors underlying gender differences in quit rates.10
Previous research has demonstrated gender differences in reactivity to cues presented in the laboratory setting. Saladin et al.11 conducted a laboratory-based study examining gender differences in response to smoking cues (holding and viewing a cigarette), stress cues (listening to a description of a recent life event that the participant identified as stressful), and neutral cues (holding and viewing a pack of pencils and an eraser while listening to a description of a neutral event participants had recently experienced). Findings indicated that females reported more stress and higher levels of craving in response to “stress cues” compared to males. Females trended toward exhibiting more stress and higher craving levels after smoking cues than males, but this difference was not statistically significant. Few studies have examined gender differences in response to stress cues, but consistent with the Saladin11 results, Colamussi et al.12 reported that females displayed a greater change in craving from baseline to post-stress cues than males.
While more work has been done in the area of gender differences in response to “smoking cues,” this literature is mixed. Some studies have concluded that females report higher craving in response to smoking cues (vs. neutral cues) than males13,14 while others report equivalent levels of post smoking cue craving across genders.15,16 Taken together, the extant research on gender effects in cue reactivity suggests that, though females may be more reactive to stress cues than males, the evidence for gender differences in smoking cue reactivity is less consistent.
The cue-reactivity paradigm has been used extensively in the substance abuse literature to examine response to cues in laboratory settings. One limitation of cue-reactivity research is that most has been confined to the laboratory; as such, we do not know if findings translate well to the day-to-day experience of smokers. Ecological momentary assessment (EMA)17 has made it possible to bring the laboratory into the natural environment of participants. This methodology allows for data collection close in time to an event of interest and enhances the ecological validity of data collection. New procedures have used EMA to measure response to cues in the natural environment of cigarette smokers—this line of research demonstrates both feasibility of the procedure and robust cue-specific craving effects elicited by cues presented outside of the laboratory.18–20
In the present study, we evaluated responses to smoking and stress cues in the natural environment of smokers and examined whether these responses differed between males and females. Based on previous research, we hypothesized that female smokers would be more reactive to stress cues (i.e., higher craving, stress, and NA) than male smokers. Due to the mixed findings from laboratory-based studies, we did not make a priori predictions related to gender differences in response to smoking cues.
Methods
Procedures
Participants were recruited from the Charleston, SC area through the internet (i.e., Craigslist, broadcast emails), flyers, and friend referrals. A brief phone screen was followed by an initial assessment (baseline session). During this visit, participants provided informed consent, received a physical exam (including medical history), provided a carbon monoxide (CO) sample, and responded to self-report questionnaires. These measures included a smoking history form, a demographics questionnaire, and the Fagerström Test for Nicotine Dependence (FTND).21 The Mini-International Neuropsychiatric Interview22 and the substance use disorder modules of the structured clinical interview for DSM-IV23 were administered by trained interviewers.
Participants were included in the study if they were between the ages of 18–45, smoked ≥5 cigarettes per day for at least the past 6 months, had a CO level of ≥5 ppm at the baseline visit, and had regular menstrual cycles between 25–35 days (females only). Participants were excluded if they had a serious or unstable medical or psychiatric disorder, used tobacco products other than cigarettes, or were on medication that could interfere with psychophysiological monitoring. Females who were breast-feeding and those who were taking either birth control or hormone replacement medication that would affect menstrual cycle were excluded. Males were excluded if they were post orchiectomy. The current research project occurred in the context of a larger study examining gender and sex hormone influences on the relationships between stress, craving, and smoking behavior; the present results represent a limited subset of the findings to be derived from the larger study.
During the training session (Day 0), participants were given an iPhone 4S equipped with cue reactivity ecological momentary assessment (CREMA) software.18–20 Alternatively, participants could download the CREMA application to their personal iPhone. This is a second generation of software that was the result of a collaborative effort between the smoking research groups at the Medical University of South Carolina and the University at Buffalo; this software was developed for implementation on the Apple iPhone and therefore, overcame some of the technological obstacles associated with the previous hardware platform (Tungsten, Palm). Data were uploaded in real time using REDCap,24 which allowed research staff to periodically check on compliance and provide feedback to participants if they were not completing sessions regularly. Participants were trained on iPhone operation; this included a tutorial video with a practice CREMA session. Participants completed 14 days of CREMA sessions, returning to the lab on Day 7 for a check-in visit and again on Day 15 after having completed the CREMA portion of the study.
CREMA sessions began the day after the training visit and occurred 4 times per day over the course of 2 weeks. An alarm indicated the start of a study session. Sessions were administered pseudo-randomly; that is, one per each 3-hr block, with sessions occurring at least 30min apart. Three cue types were presented: smoking photographs (pictures with smoking behavior or objects such as lit cigarettes and people smoking), stress photographs (unpleasant images from the standardized International Affective Picture System [IAPS])25 and neutral photographs (images devoid of smoking or unpleasant content such as pencils and scissors). Stressful images from the IAPS database were selected by investigators based on ratings of valence (unpleasant to pleasant; 1–9 scale) and arousal (calm to excited; 1–9 scale). Images included in the current study had valence ratings ranging from 2–3 (mean rating = 2.4) and arousal ratings from 5–7 (mean rating = 6.1). Examples of pictures chosen include images of violence, natural disasters, and injury. Three smoking, three stress, and two neutral photographs were presented per day, with a different photograph presented during each study trial.
Participants had 15min to respond to a session after the initial alarm signaled the start of a trial. Participants were instructed to finish their cigarette if they were currently smoking and then provided baseline craving and mood ratings. The first photograph was presented for 10 s, and participants were then asked to respond to questions in relation to how they felt at the time they were viewing the photograph. During each session, two trials were administered; that is, participants saw two photographic cues and responded to a set of questionnaires after each cue.
Post cue craving was assessed on a 5-point scale using the 4-item craving questionnaire,26 with item order randomized during each trial. Post cue stress was assessed on a 5-point scale using the item, “How stressed did you feel?” Post cue NA was assessed using the items: “How sad did you feel?” “How worried/anxious did you feel?” “How angry/hostile did you feel?” and “How frustrated did you feel?”
Participants were compensated $1.25 for each CREMA session completed. In addition, they received $100 for completing the screening assessment, $50 for the training visit, $50 for the Day 7 check in visit, and $50 bonus for returning the iPhone and accessories.
Data Analysis
Trials were eliminated from analyses (1.8%) if participants indicated that they were unable to view the picture on the screen of the iPhone. A general NA score was derived using the five NA items listed above. Data of like trial types (i.e., smoking, stress, neutral) were collapsed within participant. The general data analytic framework for this study was repeated measures analysis of variance, with cue type as a within-subjects factor and gender as a between-subjects factor. A priori power analyses indicated that our sample size was sufficient to detect even small main effects and interactions.
Results
The sample included in the current analysis consisted of 76 participants (42% female) who were predominately Black (49%) or White (46%) and had completed at least a high school level of education (76%). Participants averaged 29 years of age (SD = 7.6), smoked 16 cigarettes per day (SD = 7.7), were moderately dependent on nicotine (FTND M = 5.07, SD = 1.91), had been smoking for 12 years (SD = 7.3), and had expired CO levels of 14.3 ppm (SD = 9.0) at the screening visit. There were no baseline differences between males and females on these or any other participant characteristic variables. Further, males and females did not differ on pre-cue (baseline) stress (male M = 1.67, SE = 0.10, female M = 1.82, SE = 0.13, t = 0.89, p = .38), craving (male M = 2.23, SE = 0.11, female M = 2.61, SE = 0.19, t = 1.76, p = .09), or NA (male M = 1.45, SE = 0.07, female M = 1.62, SE = 0.11, t = 1.48, p = .14) assessed in the natural environment or on percentage of sessions completed/missed (participants fully completed 81% of sessions, missed 17% of sessions, and failed to complete the entire assessment for 2% of sessions). There were no differences in time since last cigarette across cue type (p = .69), gender (p = .36), or cue type X gender (p = .42)
Responses to Stress Cues
Responses to stress cues are depicted in Figure 1. Stress cues elicited higher levels of stress than neutral cues, F (1, 74) = 79.28, p = .000, ηp 2 = .52, and females reported higher overall post cue stress than males, F (1, 74) = 4.30, p < .05, ηp 2 = .06. A cue type (stress vs. neutral cue) by sex interaction indicated that females reported higher levels of stress after stress cues than males, F (1, 74) = 6.58, p < .05, ηp 2 = .08.
Figure 1.
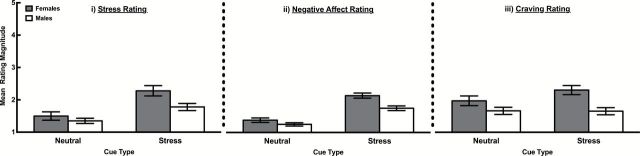
Stress (i), negative affect (ii), and craving (iii) ratings of female and male smokers in response to stress versus neutral cues.
Stress cues elicited higher levels of NA than neutral cues, F (1, 74) = 107.59, p = .000, ηp 2 = .59, and females reported higher overall post cue NA than males, F (1, 74) = 4.42, p ≤ .05, ηp 2 = .06. A cue type (smoking vs. neutral cue) by sex interaction indicated that females reported higher levels of NA after stress cues than males, F (1, 74) = 4.53, p < .05, ηp 2 = .06.
Stress cues elicited stronger craving than neutral cues, F (1, 74) = 11.07, p = .001, ηp 2 = .13, and females reported higher overall post-cue craving than males, F (1, 74) = 7.73, p < .01, ηp 2 = .10. A cue type (smoking vs. neutral cue) by sex interaction demonstrated that females reported higher craving after stress cues than males, F (1, 74) = 12.59, p = .001, ηp 2 = .15.
Responses to Smoking Cues
Smoking cues elicited higher levels of stress (M = 1.62, SE = 0.11) than neutral cues (M = 1.41, SE = 0.07), F (1, 74) = 30.63, p = .000, ηp 2 = .29. There was no cue type (smoking vs. neutral cue) by sex interaction (p = .17).
Smoking cues elicited higher levels of NA (M = 1.43, SE = 0.06) than neutral cues (M = 1.31, SE = 0.06), F (1, 74) = 21.88, p = .000, ηp 2 = .23. There was not a statistically significant cue type (smoking vs. neutral cue) by sex interaction (p = .06).
Smoking cues elicited stronger craving (M = 2.66, SE = 0.11) than neutral cues (M = 1.79, SE = 0.09), F (1, 74) = 97.34, p = .000, ηp 2 = .57. There was no cue type (smoking vs. neutral cue) by sex interaction (p = .82).
Discussion
This is the first study to examine gender differences in response to smoking and stress cues in the natural environment of cigarette smokers. Stress cues elicited stronger craving, higher stress, and greater NA than neutral cues. Females were more reactive to stress cues than males, reporting higher levels of post cue craving, stress, and NA. Smoking cues elicited stronger craving, higher stress, and greater NA than neutral cues, but response to smoking cues did not differ as a function of gender.
CREMA research has now consistently demonstrated that participants will complete cue-reactivity sessions outside of the laboratory over an extended period of time with good compliance. The current study advanced previous CREMA work by using a new hardware platform and real time data uploading, which allowed for feedback to participants close in time to the CREMA sessions. In addition, this is the first study to measure response to stress cues and to report gender differences outside of the laboratory. The convergence of findings between laboratory-based studies and the present CREMA study should bolster confidence about the authenticity of stress reactivity differences between male and female smokers.
The gender differences found in response to stress cues using CREMA methodology were consistent with the two laboratory studies investigating this question.11,12 These findings add to the body of literature identifying potential factors (e.g., differential response to nicotine, severity of withdrawal symptoms, response to nicotine replacement therapy) that contribute to gender differences in rates of smoking cessation.
Although there has been speculation around why females may be more responsive to stress cues than males, little experimental work has investigated this gender difference. Saladin et al.27 recently found that females in the luteal phase of the menstrual cycle experienced stronger post stress cue craving than males, while females in the follicular phase experienced stronger post stress cue stress than males. This preliminary evidence suggests that the moderating effects of gender may be at least partially explained by menstrual cycle phase; however, it is unlikely that this explains the gender effects found in the current study, given that most women would have crossed over from one phase to the next during the 2-week assessment period.
Previous research has explored potential reasons for gender differences in smoking quit success rates. Some work has suggested that females smoke more for nonpharmacological reasons than males, and that cues may be a more important contributor to relapse for females than males.28,29 In terms of types of cues that might be especially important, our findings suggest that stress cues may be particularly problematic for females as compared to males, while females and males may struggle equally with heightened craving, stress, and NA in response to smoking related cues.
Further, although this study did not examine the impact of response to cues on smoking behavior, past research indicates that smokers perceive stress to be a major contributor to relapse episodes,30 and quit rates have been shown to be lower among those who are experiencing psychological distress.31 In addition, negative mood induction has been associated with shorter latency to smoke and greater puff volume in females as compared to males.32,33 Taken together, these findings suggest that stressful cues or situations may make it difficult to resist smoking, and that this might be especially important for females.
The present study highlights the potential value of modified interventions for treatment seeking female smokers, focusing on management of stress and craving that arises during negative emotional experiences. Tailored interventions have been proposed to target aspects of cessation that may be especially difficult for females. For example, strategies such as exercise, coping skills, and stress management training have been used to help female smokers reduce and cope with stress, while cognitive behavioral strategies have been employed to help women effectively handle cues that have previously been paired with smoking behavior.34 Response to smoking cues may be important treatment targets across genders, as both craving and stress were enhanced after smoking cues were presented.
Several limitations of the current research should be noted. First, the clinical significance of the findings merits further investigation, especially given the relatively low levels of stress and craving elicited by cues.35 However, post-smoking and stress cue craving, stress, and NA were robustly elevated relative to these responses after neutral cues, which supports the potential clinical value of these changes. Further, it is notable that cues were able to increase craving, stress, and NA when participants were outside of the laboratory setting, where many environmental stimuli are present concurrent with cue presentation. Second, we did not measure the relationships between response to cues and smoking behavior, and thus we can only speculate about the potential impact that stress and craving response to cues in the natural environment may have on cigarette smoking. Finally, participants in this study were not seeking treatment; as such, further investigation is needed to determine if these results are generalizable to treatment-seeking smokers.
The current data extends laboratory findings in this area of research by examining cue-elicited responses in the natural environment of smokers. This project advances CREMA research and demonstrates that females are more responsive to cues in the natural environment than their male counterparts. These findings highlight the importance of addressing coping in response to stress cues in clinical settings, especially when working with female smokers.
Funding
This research was supported by NIDA/ORWH/FDA grant P50 DA016511, Specialized Center of Research (SCOR) on Sex and Gender Factors Affecting Women’s Health, and by the South Carolina Clinical and Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, through NIH grant numbers UL1 RR029882 and UL1 TR000062. KMG has received support from Merck, Inc, and Supernus Pharmaceuticals for unrelated research.
Declaration of Interests
None declared.
Acknowledgments
The authors thank A. McCullough, JO Hinton, D. Paquette, P. Muldrow, C. Horne, E. Klintworth, and LE Beech for their invaluable contributions to this research.
References
- 1. Caraballo RS, Kruger J, Asman K, et al. Relapse among cigarette smokers: the CARDIA longitudinal study—1985–2011. Addict Behav. 2014;39:101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perkins KA. Smoking cessation in women. Special considerations. CNS Drugs. 2001;15:391–411. [DOI] [PubMed] [Google Scholar]
- 3. Piper ME, Federman EB, McCarthy DE, et al. Efficacy of bupropion alone and in combination with nicotine gum. Nicotine Tob Res. 2007;9:947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scharf D, Shiffman S. Are there gender differences in smoking cessation, with and without bupropion? Pooled- and meta-analyses of clinical trials of Bupropion SR. Addiction. 2004;99:1462–1469. [DOI] [PubMed] [Google Scholar]
- 5. Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res. 2008;10:1245–1250. [DOI] [PubMed] [Google Scholar]
- 6. Piper ME, Cook JW, Schlam TR, et al. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine Tob Res. 2010;12:647–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bjornson W, Rand C, Connett JE, et al. Gender differences in smoking cessation after 3 years in the Lung Health Study. Am J Public Health. 1995;85:223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jarvis MJ, Cohen JE, Delnevo CD, Giovino GA. Dispelling myths about gender differences in smoking cessation: population data from the USA, Canada and Britain. Tob Control. 2013;22:356–360. [DOI] [PubMed] [Google Scholar]
- 9. Torchalla I, Okoli CTC, Hemsing N, Greaves L. Gender differences in smoking behaviour and cessation. J Smok Cessat. 2011;6:9–16. [Google Scholar]
- 10. Wetter DW, Fiore MC, Young TB, McClure JB, de Moor CA, Baker TB. Gender differences in response to nicotine replacement therapy: objective and subjective indexes of tobacco withdrawal. Exp Clin Psychopharmacol. 1999;7:135–144. [DOI] [PubMed] [Google Scholar]
- 11. Saladin ME, Gray KM, Carpenter MJ, LaRowe SD, DeSantis SM, Upadhyaya HP. Gender differences in craving and cue reactivity to smoking and negative affect/stress cues. Am J Addict. 2012;21:210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Colamussi L, Bovbjerg DH, Erblich J. Stress- and cue-induced cigarette craving: effects of a family history of smoking. Drug Alcohol Depend. 2007;88:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Field M, Duka T. Cue reactivity in smokers: the effects of perceived cigarette availability and gender. Pharmacol Biochem Behav. 2004;78:647–652. [DOI] [PubMed] [Google Scholar]
- 14. Knott VJ, Naccache L, Cyr E, et al. Craving-induced EEG reactivity in smokers: effects of mood induction, nicotine dependence and gender. Neuropsychobiology. 2008;58:187–199. [DOI] [PubMed] [Google Scholar]
- 15. Niaura R, Shadel WG, Abrams DB, Monti PM, Rohsenow DJ, Sirota A. Individual differences in cue reactivity among smokers trying to quit: effects of gender and cue type. Addict Behav. 1998;23:209–224. [DOI] [PubMed] [Google Scholar]
- 16. Tong C, Bovbjerg DH, Erblich J. Smoking-related videos for use in cue-induced craving paradigms. Addict Behav. 2007;32:3034–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. [DOI] [PubMed] [Google Scholar]
- 18. Gass JC, Wray JM, Hawk LW, Mahoney MC, Tiffany ST. Impact of varenicline on cue-specific craving assessed in the natural environment among treatment-seeking smokers. Psychopharmacology (Berl). 2012;223:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Warthen MW, Tiffany ST. Evaluation of cue reactivity in the natural environment of smokers using ecological momentary assessment. Exp Clin Psychopharmacol. 2009;17:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wray JM, Godleski SA, Tiffany ST. Cue-reactivity in the natural environment of cigarette smokers: the impact of photographic and in vivo smoking stimuli. Psychol Addict Behav. 2011;25:733–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. [DOI] [PubMed] [Google Scholar]
- 22. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33;quiz 34–57. [PubMed] [Google Scholar]
- 23. First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for Axis I disorders, patient edition. New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 24. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. Gainesville, FL: University of Florida.
- 26. Carter BL, Tiffany ST. The cue-availability paradigm: the effects of cigarette availability on cue reactivity in smokers. Exp Clin Psychopharmacol. 2001;9:183–190. [DOI] [PubMed] [Google Scholar]
- 27. Saladin ME, Wray JM, Carpenter MJ, et al. Menstrual cycle phase effects in the gender dimorphic stress cue reactivity of smokers. Nicotine Tobacco Res. 10.1093/ntr/ntu203. [DOI] [PMC free article] [PubMed]
- 28. Perkins KA. Sex differences in nicotine versus nonnicotine reinforcement as determinants of tobacco smoking. Exp Clin Psychopharm. 1996;4:166–177. [Google Scholar]
- 29. Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res. 1999;1:301–315. [DOI] [PubMed] [Google Scholar]
- 30. Brandon TH. Negative affect as motivation to smoke. Curr Dir Psychol Sci. 1994;3:33–37. [Google Scholar]
- 31. Sung HY, Prochaska JJ, Ong MK, Shi Y, Max W. Cigarette smoking and serious psychological distress: a population-based study of California adults. Nicotine Tob Res. 2011;13:1183–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Perkins KA, Giedgowd GE, Karelitz JL, Conklin CA, Lerman C. Smoking in response to negative mood in men versus women as a function of distress tolerance. Nicotine Tob Res. 2012;14:1418–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weinberger AH, McKee SA. Gender differences in smoking following an implicit mood induction. Nicotine Tob Res. 2012;14:621–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Torchalla I, Okoli CTC, Bottorff JL, Qu A, Poole N, Greaves L. Smoking cessation programs targeted to women: a systematic review. Women Health. 2012;52:32–54. [DOI] [PubMed] [Google Scholar]
- 35. Sayette MA, Tiffany ST. Peak provoked craving: an alternative to smoking cue-reactivity. Addiction. 2013;108:1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]


