Abstract
Introduction:
Previous work led to our hypothesis that sex differences produced by nicotine withdrawal are modulated by stress and dopamine systems in the nucleus accumbens (NAcc). We investigated our hypothesis by studying intact females to determine whether the mechanisms that promote withdrawal are ovarian-hormone mediated.
Methods:
Female rats were ovariectomized (OVX) or received sham surgery (intact) on postnatal day (PND 45–46). On PND 60, they received sham surgery (controls) or were prepared with nicotine pumps. Fourteen days later, half of the rats had their pumps removed (nicotine withdrawal) and the other half received sham surgery (nicotine exposure). Twenty-four hours later, the rats were tested for anxiety-like behavior using the elevated plus maze and light/dark transfer procedures. The NAcc was then dissected for analysis of several genes related to stress (CRF, UCN, CRF-R1, CRF-R2, CRF-BP, and Arrb2) or receptors for dopamine (Drd1 and Drd2) and estradiol (Esr2).
Results:
During withdrawal, intact females displayed an increase in anxiety-like behavior in both tests and CRF, UCN, and Drd1 gene expression. During nicotine exposure, intact females displayed a decrease in CRF-R1, CRF-R2, Drd3, and Esr2 gene expression and an increase in CRF-BP. This pattern of results was absent in OVX females.
Conclusions:
Nicotine withdrawal produced an increase in anxiety-like behavior and stress-associated genes in intact females that is distinct from changes produced by nicotine exposure. The latter effects were absent in OVX females, suggesting that stress produced by withdrawal is ovarian-hormone mediated. These findings have important implications towards understanding tobacco use liability among females.
Introduction
Tobacco use is the number one cause of preventable deaths in the United States.1 Of particular concern is the high rate of tobacco use among women, who are more susceptible to the negative health consequences of long-term smoking than men.2 As a result, tobacco use is believed to be a major contributing factor to health disparities in women. In spite of the magnitude of the problem, surprisingly little is known about the underlying biological factors that promote tobacco relapse in females.
Much work has suggested that stress promotes tobacco use in women. For example, women report more negative mood states, such as depression, anxiety, and intense craving during smoking abstinence than men.3–5 Women also use more tobacco products and display lower quit rates relative to men.6 Furthermore, women report that they maintain tobacco use to relieve intense withdrawal symptoms that emerge during abstinence, and they claim more often than men that the anxiety-reducing effects of cigarettes are the main reason for smoking.7–9
Preclinical studies have shown that the behavioral and biological consequences of nicotine withdrawal are greater in female versus male rodents. For example, female rats exhibit more physical signs of nicotine withdrawal than males.10 Female rats also display a larger aversion for an environment paired with nicotine withdrawal than males.11 During nicotine withdrawal, female rats also display higher levels of anxiety-like behavior, plasma corticosterone and corticotropin releasing factor (CRF) gene expression in the nucleus accumbens (NAcc) as compared to males.12 The latter study also showed that there were no sex differences in CRF gene expression in the amygdala or hypothalamus, suggesting that sex differences in withdrawal are modulated in the NAcc. Consistent with this, sex differences in various markers have been reported in the NAcc of rodents experiencing withdrawal from cocaine,13,14 ethanol,15,16 and opiates.17 Taken together, these studies provide converging lines of evidence to suggest that the intense negative symptoms produced by withdrawal from drugs of abuse in females is modulated within the local circuits of the NAcc.
Our previous research compared changes in CRF gene expression in the NAcc of female and male rats experiencing nicotine withdrawal.12 This work was important towards establishing that the NAcc plays a role in modulating sex differences produced by withdrawal. The present study expands this work by examining the role of ovarian hormones in promoting strong stress responses produced by nicotine withdrawal in females. Furthermore, we expand our previous work by examining various stress-associated genes and dopamine markers that are believed to promote withdrawal in females. Thus, the present study compared anxiety-like behavior and the expression of stress-associated genes in the NAcc of intact female and ovariectomized (OVX) female rats following nicotine exposure and withdrawal from this drug. The stress-associated genes that were examined include: CRF, urocortin (UCN), CRF receptor subtypes (CRF-R1 and CRF-R2), CRF binding protein (CRF-BP), and beta-arrestin2 (Arrb2). We included Arrb2 based on the finding that in the locus coeruleus, female rats display lower levels of Arrb2, an important protein involved in the internalization of CRF-R1 receptors.18,19
We recently presented a mechanistic hypothesis suggesting that nicotine withdrawal in females is mediated by an interaction between CRF, dopamine and estradiol systems in the NAcc.11 Previous work has shown that dopamine levels are decreased in the NAcc during nicotine withdrawal.20,21 We hypothesize that nicotine withdrawal activates stress systems in the NAcc, which decreases dopamine levels in this region. As an indirect assessment of changes in dopamine transmission in the NAcc, the present study compared changes in gene expression of dopamine receptors (Drd1, Drd2, and Drd3) during nicotine exposure and withdrawal. We also hypothesize that estradiol in the NAcc promotes the effects of CRF. Indeed, the highest levels of CRF are observed during proestrus when estradiol levels are highest.22 Furthermore, previous work has hypothesized that estradiol receptors (Esr2) are anatomically positioned in the striatum to promote dopamine release produced by administration of drugs of abuse, such as cocaine.23,24 Thus, the present study also compared changes in Esr2 gene expression in the NAcc across our experimental conditions.
Methods
Subjects
Female Wistar rats were purchased from Harlan, Inc. The rats were housed in groups of 2–3 per cage in a humidity- and temperature-controlled vivarium using a 12/12-hr light/dark cycle with lights off at 8:30 a.m. All procedures were approved by the UTEP Animal Care and Use Committee and followed the guidelines of the NIH Guide for the Care and Use of Laboratory Animals. This study consisted of six experimental groups, including intact and OVX female rats that received sham surgery (control) or implantation of a subcutaneous pump that delivered nicotine. The latter group was subdivided such that half of the rats were tested with nicotine present (nicotine exposure) or 24hr after pump removal (nicotine withdrawal). The experimental groups and a timeline of our procedures are depicted in Figure 1.
Figure 1.
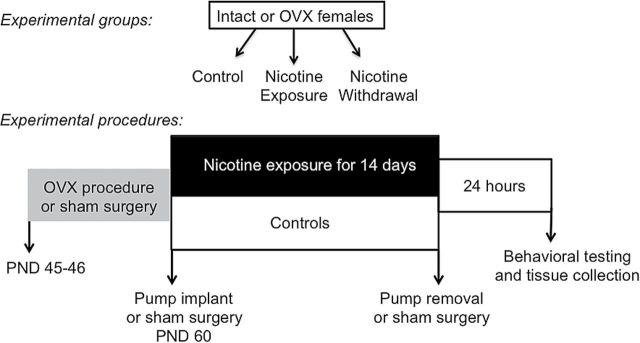
Experimental groups and the timeline of procedures.
Experimental Procedures
All surgical procedures were conducted using aseptic techniques under isoflurane/oxygen (1%–5%) gas anesthesia. Intact female rats received a sham surgery or OVX procedures, as described previously.25 The OVX surgery was conducted during an early stage of development (postnatal day [PND] 45–46) before the onset of adulthood (PND 50–60) when female rats begin to regularly cycle.26 This was done in order to eliminate the organizational effects of ovarian hormones believed to promote nicotine withdrawal in the NAcc. The present study did not compare our effects across the 4-day estrous cycle because repeated vaginal lavage procedures can induce stress, which might have influenced our results. Furthermore, previous work has shown that the behavioral effects of nicotine are not altered across estrous.25,27
On PND 60–65, the rats received a sham surgery (controls) or were surgically prepared with subcutaneous pumps that delivered nicotine continuously (3.2mg/kg/day/base; Alzet 2ML2). After 14 days of nicotine exposure, half of the rats had their nicotine pumps removed and the other half did not have their pumps removed. The pumps were surgically excised under isoflurane anesthesia. The group that did not have their pumps removed was exposed to isoflurane gas as a control procedure. This group of rats was included in order to better understand the effects of nicotine withdrawal versus those produced by chronic exposure to this drug. The nicotine exposure group was sacrificed on the same day as the nicotine withdrawal group, which had their pumps removed the previous day. In this way, only one group was tested during nicotine withdrawal.
Twenty-four hours after nicotine pump removal, anxiety-like behavior was assessed using the elevated plus maze (EPM) and light/dark transfer (LDT) procedures. The rats were tested individually in a series of steps. First, the rats were acclimated to the EPM test room in a rectangular Plexiglas® cage for 5min. The animals were then placed onto the EPM facing the open arm and time spent in open versus closed arm was recorded for 5min. Time spent in the center area was also recorded. After EPM testing, the rats were acclimated to a separate LDT test room for 5min. The rats were then placed in the dark side of the LDT apparatus and time spent in the light versus dark chambers was recorded for 5min. For each procedure, the apparatus was thoroughly cleaned with 70% ethanol and water between each animal.
After behavioral testing, the NAcc was rapidly dissected and placed on dry ice and stored at −80 °C. Total RNA was isolated using the All Prep DNA/RNA mini kit (QIAGEN, Inc.) for small tissue sections following to the manufacturer’s protocol. After isolation, RNA was quantified using a UV/V spectrophotometer (Beckman Coulter Inc.). The target ratio of 1.8–2.0 for A260/280 was used as an inclusion criterion for all RNA samples. The quality of the RNA was then visualized by MOPS 1% agarose gel (37% formaldehyde) using the Thermo Scientific easy cast electrophoresis system. The gels were verified for characteristic 18S and 28S ribosomal RNA bands using ethidium bromide staining and the Bio-Rad ChemiDoc XRS+ imaging system. Samples containing insufficient amounts of RNA were excluded from further analyses. One microgram of total RNA was then digested with DNaseI, Amp Grade (Invitrogen) prior to cDNA synthesis in order to remove any DNA contamination. The RNA was then reverse transcribed into cDNA with the Advantage® RT-for-PCR kit (Clontech) using Oligo (dT) primers, following the manufacturer’s instructions.
Table 1 lists the specific primers for stress-associated genes (CRF, UCN, CRF-R1, CRF-R2, CRF-BP, and Arrb2) and receptors for dopamine (Drd1, Drd2, and Drd3) and estradiol (Esr2). The primers were synthesized by Integrated DNA Technologies, Inc. Commercially available SYBR® Fast qPCR fluorescent labeling kits (Kapa Biosystems, Inc.) were used to perform qRT-PCR using the Mastercycler ep Realplex2 System (Eppendorf, Inc.). The amplification specificity for each primer was tested for a single-band product, as evidenced by TAE 1% gel electrophoresis and visualized on the Bio-Rad ChemiDoc XRS+ system. Total cDNA was first pooled from separate experimental groups and then diluted in serial fashion to calculate the PCR amplification efficiency, which averaged E = 1.88 across all of our primers. cDNA samples were run in duplicate for each gene. PCR values were normalized using RPL-13A based on our observation that this reference gene displayed the most stable expression in both intact and OVX females across the experimental conditions of this study (data not shown).
Table 1.
Primer Sequences 5′–3′
| Gene | Forward | Reverse |
|---|---|---|
| CRF | ATG CTG CTG GTG GCT CTG T | GGA TCA GAA TCG GCT GAG GT |
| UCN | TGG ATA GAC ACT CCG ATA AC | GGA TCC TGG ACC ACA TTC |
| CRF-R1 | CCA GAG CAA TGT GGC | CTG TGT GCA GGT AGC |
| CRF-R2 | TCA TTG GAT GGT GCA TAC | TTG ATG AGG AGC ACG AG |
| CRF-BP | TTG AAG AAA CCT GCG G | GGT AAC ACA GGT CCA CTA |
| Arrb2 | CTG AAA CCA CAC GCC | GTG ACG TGG ACG TTG |
| Drd1 | GA CAC AAG GTT GAG CA | CTG GGC AAT CCT GTA GAT A |
| Drd2 | GTG TGT TCA TCA TCT GCT | GAA CTC GAT GTT GAA GGT |
| Drd3 | ACC TCT GAG CCA GAT AAG | CAC AGC AGC ACA TAC CA |
| Esr2 | CTC AGC CTG TTG GAC CAA GT | CCT CAT CCC TGT CCA GAA CG |
| aRPL13a | GGA TCC CTC CAC CCT ATG ACA | CTG GTA CTT CCA CCC GAC CTC |
aReference gene.
Statistics
For the behavioral results, the dependent variable was time spent in the open versus closed arm of the EPM or the light versus dark side of the LDT apparatus. Anxiety-like behavior was operationally defined as a significant increase in time spent in the closed arm of the EPM or in the dark side of the LDT apparatus relative to controls. Accordingly, anxiety-like behavior was also evident as a significant decrease in time spent in the open arm of the EPM or in the light side of the LDT apparatus as compared to controls.
Our statistical approach involved an overall analysis to determine whether changes in anxiety-like behavior or gene expression were hormone dependent. Our analyses of gene expression were conducted in a similar manner as the behavioral studies. However, we began with an overall multivariate analysis of variance (MANOVA) that included subsets of gene families that are related (i.e., stress-associated genes or dopamine receptors) as dependent variables. This allowed us to explore whether there were differences within clusters of related gene families prior to our subsequent analyses on individual genes. Specifically, a two by three ANOVA was conducted with OVX condition (intact and OVX) and nicotine treatment (control, nicotine exposure, and nicotine withdrawal) as between subject factors. In instances where significant interaction effects were observed, individual group comparisons were conducted using Fisher’s LSD tests (p < .05). Given our a priori hypothesis regarding the unique contribution of stress in promoting nicotine withdrawal in intact females, we conducted separate analyses that only included intact females. Lastly, Pearson correlations (r) were conducted on UCN gene expression and time spent in the closed arms of the EPM and the dark side of the LDT apparatus in intact female controls and rats that were tested during nicotine withdrawal.
Results
Changes in Anxiety-Like Behavior
Figure 2 illustrates our assessment of anxiety-like behavior in the EPM (panel A and B) and LDT (panel C and D) procedures in female intact and OVX controls (white bars), nicotine exposed (grey bars) and nicotine withdrawal (black bars) groups of rats. Our analysis of time spent in the closed arm of the EPM revealed a significant interaction between nicotine treatment and OVX condition (F (2,52) = 7.89, p < .01). During nicotine withdrawal, intact females displayed a significant increase in time spent in the closed arms relative to controls (*p < .05) and nicotine-exposed rats (#p < .05). Similarly, our analysis of time spent in the open arms revealed an interaction between nicotine treatment and OVX condition (F (2,52) = 8.86, p < .01). During nicotine withdrawal, intact females displayed a significant reduction in time spent in the open arms relative to controls (*p < .05) and nicotine-exposed rats (#p < .05).
Figure 2.
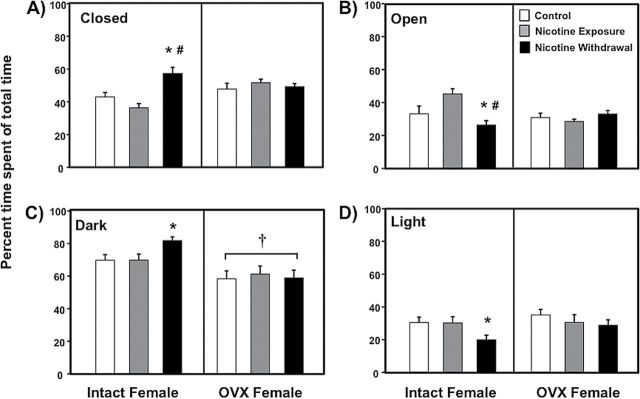
Percentage of time spent in the closed and open arms of the elevated plus maze (panel A and B) and in the light and in dark compartments of the light/dark transfer (panel C and D) in intact (control n = 8; nicotine exposure n = 6; nicotine withdrawal n = 9) and ovariectomized (OVX) (control n = 11; nicotine exposure n = 13; nicotine withdrawal n = 11) female rats. The asterisks denote a significant difference from controls, the number signs denote a difference between the nicotine exposure and withdrawal groups, and the dagger denotes a decrease across the OVX condition (p < .05).
Our analysis of time spent in the dark compartment of the LDT apparatus revealed that there was no significant interaction between nicotine treatment and OVX condition (F (2,52) = 1.03, p = .36). However, we did observe a significant main effect of OVX condition (F (1,52) = 12.73, p < .01). A separate analysis of intact females revealed a significant effect of nicotine treatment (F (2,20) = 3.8, p < .05), such that intact females displayed a significant increase in time spent in the dark compartment relative to controls during nicotine withdrawal (*p < .05). Similarly, our analysis of time spent on the light compartment revealed that intact females displayed a significant decrease in time spent in the light compartment relative to controls during nicotine withdrawal (*p < .05).
Changes in Gene Expression
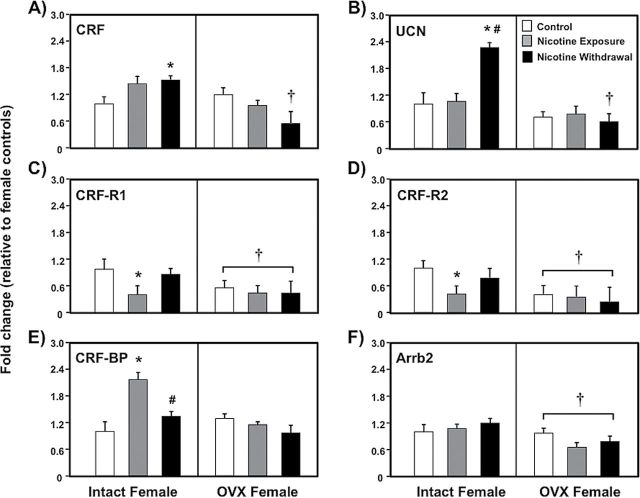
Figure 3 depicts changes in the expression of stress-related genes (CRF-panel A; UCN-panel B; CRF-R1-panel C; CRF-R2-panel D; CRF-BP-panel E; Arrb2-panel F) in the NAcc of intact and OVX female control (white bars), nicotine exposed (grey bars) and nicotine withdrawal (black bars) groups of rats. A MANOVA analyses that included all of the stress-associated genes depicted in Figure 3 revealed a significant overall interaction between nicotine treatment and OVX condition (F (4,48) = 3.90, p < .01).
Figure 3.
Gene expression of stress-associated targets in the nucleus accumbens of intact (control n = 8; nicotine exposure n = 6; nicotine withdrawal n = 9) and ovariectomized (OVX) (control n = 11; nicotine exposure n = 13; nicotine withdrawal n = 11) female rats. The data are presented as percent fold change (±SEM) from intact female controls. The asterisks denote a significant difference from controls, the number signs denote a difference between the nicotine exposure and withdrawal groups, and the daggers denote a decrease across the OVX condition (p < .05).
The subsequent analysis of CRF revealed a significant interaction between nicotine treatment and OVX condition (F (2,52) = 5.42, p < .01). The intact females that were tested during nicotine withdrawal displayed a significant increase in CRF gene expression relative to controls (*p < .05) and OVX rats (†p < .05). Similarly, the UCN results revealed a significant interaction between nicotine treatment and OVX condition (F (2,52) = 2.8, p < .05). The intact females that were tested during nicotine withdrawal displayed an increase in UCN gene expression relative to controls (*p < .05) and their nicotine-exposed counterparts (#p < .05). The OVX females that were tested during nicotine withdrawal displayed a decrease in UCN gene expression relative to their intact female counterparts (†p < .05).
The analysis of CRF-R1 revealed that there was no interaction between nicotine treatment and OVX condition (F (2,52) = 0.84, p = .43). However, there was a main effect of OVX condition (F (2,52) = 4.56, p < .05), with OVX females displaying lower levels of CRF-R1 gene expression as compared to intact females regardless of nicotine treatment (†p < .05). A separate analysis revealed that intact females that were tested during nicotine exposure displayed a significant decrease in CRF-R1 gene expression relative to controls (*p < .05). Similarly, the results from CRF-R2 revealed that there was no interaction between nicotine treatment and OVX condition (F (2,52) = 0.08, p = .44). However, there was a main effect of OVX condition (F (1,52) = 12.53, p < .05), with OVX females displaying lower levels of CRF-R2 gene expression as compared to intact females regardless of nicotine treatment (†p < .05). A separate analysis revealed that intact females that were tested during nicotine exposure displayed a significant decrease in CRF-R2 gene expression relative to controls (*p < .05).
The analysis of CRF-BP revealed a significant interaction between nicotine treatment and OVX condition (F (2,52) = 4.12, p < .05). The intact females that were tested during nicotine exposure displayed a significant increase in CRF-BP gene expression as compared to controls (*p < .05) and their female counterparts that were tested during withdrawal (#p < .05). The results from Arrb2 revealed that there were no interaction effects (F (2,52) =1.68, p = .19). However, there was a main effect of OVX condition (F (1,52) = 7.86, p < .05), with OVX females displaying lower levels of Arrb2 gene expression as compared to intact females regardless of nicotine treatment (†p < .05).
Figure 4 illustrates changes in gene expression of receptors for dopamine (Drd1-panel A; Drd2-panel B; Drd3-panel C) and estradiol (Esr2-panel D) in the NAcc of intact and OVX female control (white bars), nicotine exposed (grey bars) and nicotine withdrawal (black bars) groups of rats. A MANOVA analysis that included all of the genes depicted in Figure 4 revealed that there was no significant interaction between nicotine treatment and OVX condition (F (4,50) = 4, p = .06). Given our interest in examining the effects of nicotine withdrawal on dopamine and estrogen receptors, the following analyses were conducted on individual gene basis.
Figure 4.
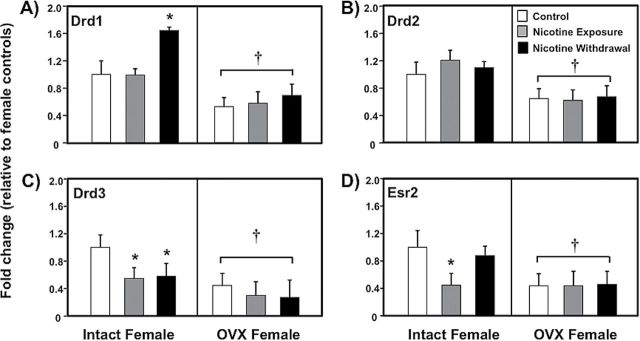
Gene expression of dopamine and Esr2 receptors in the nucleus accumbens of intact (control n = 8; nicotine exposure n = 6; nicotine withdrawal n = 9) and ovariectomized (OVX) (control n = 11; nicotine exposure n = 13; nicotine withdrawal n = 11) female rats. The data are presented as percent fold change (±SEM) from intact female controls. The asterisks (*) denote a significant difference from controls and the daggers (†) denote a decrease across the OVX condition (p < .05).
The subsequent analysis of Drd1 revealed that there was no interaction between nicotine treatment and OVX condition (F (2,52) = 0.32, p = .72). However, there was a main effect of OVX condition (F (1,52) = 21.6, p < .01), with OVX females displaying a significant decrease in Drd1 gene expression compared to intact females (†p < .05). A separate analysis revealed that intact females displayed an increase in Drd1 gene expression during nicotine withdrawal relative to controls (*p < .05). The results from Drd2 revealed that there was no interaction between nicotine treatment and OVX condition (F (2,52) = 0.24, p = .78). However, there was a main effect of OVX condition (F (1,52) = 14.3, p < .01), with OVX females displaying a decrease in Drd2 gene expression as compared to intact females regardless of nicotine treatment (†p < .05). The analysis of Drd3 revealed that there was no interaction between nicotine treatment and OVX condition (F (2,52) = 0.08, p = .91). However, there was a main effect of OVX condition (F (1,52) = 13.4, p < .01), with OVX females displaying lower levels of Drd3 gene expression compared to intact females regardless of nicotine treatment (†p < .05). A separate analysis revealed that intact females displayed a decrease in Drd3 gene expression during nicotine exposure and withdrawal relative to controls (*p < .05).
The results from Esr2 revealed that there was no interaction between nicotine treatment and OVX condition (F (2,52) = 1.3, p = .28). However, there was a main effect of OVX condition (F (1,52) = 7.86, p < .05), with OVX females displaying lower levels of Esr2 gene expression compared to intact females regardless of nicotine treatment (†p < .05). A separate analysis revealed that intact females displayed a decrease in Esr2 gene expression during nicotine exposure as compared to controls (*p < .05).
Figure 5 illustrates UCN gene expression plotted against our assessments of anxiety-like behavior (EPM-panel A; LDT-panel B) in intact female controls and rats that were tested during nicotine withdrawal. Our analysis of the EPM data revealed that there was no correlation between time spent on the closed arms and UCN gene expression in intact controls (r = −0.41, p = 0.16). However, there was a positive correlation between time spent on the closed arms and UCN gene expression in intact females that were tested during nicotine withdrawal (r = 0.62, p < .05). The correlation between anxiety-like behavior and UCN gene expression accounted for a larger proportion of the variance in the females that were tested during withdrawal (r 2 = 0.37) as compared to controls (r 2 = 0.16). Similarly, our analysis of UCN gene expression and the LDT data revealed that there was no correlation between time spent on the dark side of the LDT apparatus and UCN gene expression in intact controls (r = −0.13, p = .38). In contrast, there was only a trend for a positive correlation between these measures in intact females that were tested during nicotine withdrawal (r = 0.50, p = .08). The correlation between anxiety-like behavior and UCN gene expression accounted for a larger proportion of the variance in the females that were tested during withdrawal (r 2 = 0.23) as compared to controls (r 2 = 0.02).
Figure 5.
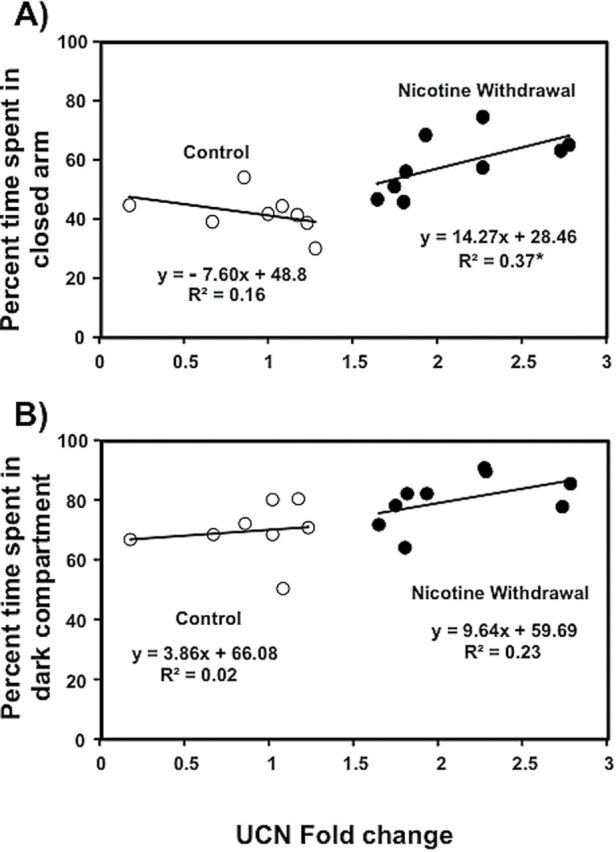
Correlational analysis between UCN gene expression and anxiety-like behavior (elevated plus maze EPM-panel A; LDT-panel B) in intact female controls (n = 8) and rats that were tested during nicotine withdrawal (n = 9). Time spent in the closed arms of the EPM was significantly correlated with UCN gene expression in intact females experiencing nicotine withdrawal (p < .05) but not in control rats.
Discussion
In summary, during nicotine withdrawal, intact females displayed an increase in anxiety-like behavior as compared to controls. This behavioral effect was accompanied by a withdrawal-induced increase in gene expression of the neuropeptides CRF and UCN. Intact females also displayed an increase in Drd1 gene expression during withdrawal. During nicotine exposure, a distinct pattern of changes in gene expression emerged as compared to withdrawal. Namely, intact females displayed a decrease in CRF-R1, CRF-R2, Drd3, and Esr2 relative to controls. There was also an increase in CRF-BP gene expression in intact females during nicotine exposure. The pattern of changes in anxiety-like behavior and gene expression were absent in OVX females.
A major finding of this report is that the pattern of changes in anxiety-like behavior and gene expression observed in intact females was absent in OVX females. This suggests that ovarian hormones play an important role in modulating strong stress responses produced by nicotine withdrawal in females. Our results also revealed that OVX females displayed a general suppression of gene expression across most of the targets that were examined (CRF-R1, CRF-R2, Arrb2, Drd1, Drd2, Drd3, and Esr2). These findings suggest that ovarian hormones are necessary for the normal induction of these genes in the NAcc. However, we recognize a previous report showing that estradiol replacement did not alter dopamine receptor gene expression in the NAcc of OVX females.28
The present study laid the groundwork for future research aimed at examining the role of ovarian hormones in promoting nicotine withdrawal in females. Our results suggest that estradiol systems are altered following nicotine exposure. This is based on our finding that Esr2 gene expression was reduced in a hormone-dependent manner following nicotine exposure. Future studies might also examine the role of other ovarian hormones, such as progesterone that have been posited to modulate tobacco use in females.29,30 In the present study, female rats received OVX procedures before puberty. Future studies might examine the activational effects of ovarian hormones on stress produced by nicotine withdrawal in females that receive OVX procedures during adulthood. This is important from a translational perspective with regard to the effects of menopause, hysterectomy or hormone treatments on tobacco use vulnerability in females.
Another important finding of this report is that nicotine withdrawal produced an increase in anxiety-like behavior that was accompanied by an activation of stress-associated neuropeptide genes in the NAcc of intact females. The finding that intact females display an increase in CRF gene expression in the NAcc during nicotine withdrawal is consistent with previous research in our laboratory.12 The present study extends prior work by showing that UCN is another important neuropeptide in the NAcc that promotes stress responses produced by nicotine withdrawal in females. Indeed, a correlational analysis revealed that there is a positive relationship between anxiety-like behavior exhibited on the EPM and UCN gene expression in the NAcc of intact females experiencing nicotine withdrawal. It is important to note that a significant correlation was only observed between our EPM data and changes in UCN gene expression. This is likely related to the robust group differences that were observed in the UCN gene data. Also, one potential limitation of our study is that we conducted multiple comparisons across several gene targets, which may lead to an increase in Type 1 error across multiple statistical tests. However, our finding that the general pattern of changes in UCN and CRF gene expression were similar suggests that stress-associated neuropeptides in the NAcc modulate sex differences during nicotine withdrawal. This is consistent with findings from other laboratories showing that female rodents display an increase in anxiety-like behavior and plasma corticosterone levels during nicotine withdrawal.31–34
The present study also revealed a different pattern of changes in CRF receptor gene expression during nicotine exposure and withdrawal. Specifically, both CRF-R1 and CRF-R2 gene expression was decreased during nicotine exposure in intact females. However, CRF-R1 and CRF-R2 gene expression returned to baseline values during withdrawal. Normalized levels of CRF-R1 and CRF-R2 during withdrawal may be important, given that these receptors are necessary for the expression of the behavioral effects of nicotine withdrawal.35,36 Future studies are warranted to examine posttranslational changes in neuropeptide receptor levels during withdrawal and the mechanisms by which these changes occur following chronic nicotine exposure.
The current study also demonstrated that nicotine exposure increased CRF-BP gene expression in the NAcc of intact females. CRF-BP has a high affinity for CRF and UCN and sequesters high levels of these neuropeptides as a protective mechanism to quell strong physiological responses during stress. Indeed, CRF-BP inhibits adrenocorticotrophic hormone (ACTH) release produced by CRF administration.37,38 CRF-BP knockout mice also display an increase in anxiety-like behavior.39 Intracerebroventricular administration a CRF-BP inhibitor has also been shown to blunt ACTH release and reverse the increases in weight gain and food intake produced by nicotine withdrawal.40 Taken together with the current findings, these studies suggest that CRF-BP may increase during nicotine exposure as a biological constraint against the excess levels of CRF produced by chronic nicotine exposure. However, during nicotine withdrawal CRF-BP normalizes, thus promoting a strong stress response in females.
We recently presented a hypothesis that stress systems interact with dopamine and estradiol in the NAcc to enhance nicotine withdrawal in females.11 Specifically, we posited that opponent processes are recruited to counteract the chronic over-activation of dopamine in the NAcc produced by repeated nicotine exposure. The emergence of these opponent processes is believed to be evident during withdrawal from chronic nicotine as an increase in stress-associated neuropeptides in the NAcc. This increase in neuropeptides is hypothesized to decrease dopamine during nicotine withdrawal. There is support for our hypothesis based on the finding that intact females displayed a robust increase in Drd1 gene expression during nicotine withdrawal. The increase in Drd1 gene expression is indirect evidence that dopamine levels were decreased in the NAcc of intact females experiencing nicotine withdrawal. The present study also revealed that Drd3 gene expression was decreased during nicotine exposure and withdrawal. Together these findings suggest that the different dopamine receptor subtypes play a unique role in modulating the effects of nicotine exposure and withdrawal.
We recognize that the role of stress in promoting drug abuse in females likely involves a complex interaction of several brain regions.41 The present study focused on the NAcc based on previous work in our laboratory that compared changes in CRF gene expression in the NAcc, amygdala, and hypothalamus of male and female rats experiencing nicotine withdrawal.12 The later study revealed that CRF gene expression is uniquely increased in the NAcc of female rats experiencing withdrawal. The present study expanded this work by including additional stress-associated genes as well as changes in dopamine receptor genes during nicotine withdrawal in females. Our focus on the local circuits of the NAcc allowed us to compare changes in multiple genes across a number of control groups to study role of ovarian hormones in nicotine exposure versus withdrawal conditions. Future studies are needed to elucidate the role of other brain regions in the complex circuitry that promotes tobacco use in females.
The present study reflects a first step toward addressing our hypothesis regarding enhanced vulnerability to tobacco use in females. We suggest that intense anxiety produced by nicotine withdrawal promotes tobacco use in women. Moreover, we propose that enhanced nicotine withdrawal in females is modulated via a complex interplay between stress-related neuropeptides and dopamine systems. In a clinical setting, most smoking cessation strategies focus on alleviating withdrawal. However, these treatment approaches are applied in a unilateral manner with limited success in women. The present study suggests that the most effective cessation medications for women might need to reduce intense stress produced by nicotine withdrawal. More research is needed at both the clinical and preclinical levels to help guide the development of more effective treatment approaches for tobacco use in females.
Funding
This research was supported by The National Institute on Drug Abuse (R01-DA021274, R24-DA029989 and R25-DA033613) and The National Institute of Minority Health Disparities (G12MD007592).
Declaration of Interests
None declared.
Acknowledgments
OVP and JAP are designated as first author given equal contributions to this work. The authors would like to thank C. Hinojosa and C. Tejeda for their excellent technical assistance on this project.
Data from this paper were presented at a Pre-Conference Workshop at the Society for Research on Nicotine and Tobacco Annual Meeting; February 25, 2015; Philadelphia, Pennsylvania, USA.
References
- 1. Centers for Disease Control and Prevention (CDC). Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000–2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226–1228. [PubMed] [Google Scholar]
- 2. Satcher D, Thompson TG, Koplan JP. Women and smoking: a report of the Surgeon General. Nicotine Tob Res. 2002;4:7–20. [DOI] [PubMed] [Google Scholar]
- 3. al’Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. Int J Psychophysiol. 2006;59:218–227. [DOI] [PubMed] [Google Scholar]
- 4. Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res. 2008;10:1245–1250. [DOI] [PubMed] [Google Scholar]
- 5. Schnoll RA, Patterson F, Lerman C. Treating tobacco dependence in women. J Womens Health. 2007;16:1211–1218. [DOI] [PubMed] [Google Scholar]
- 6. Cropsey K, Eldridge G, Weaver M, Villalobos G, Stitzer M, Best A. Smoking cessation intervention for female prisoners: addressing an urgent public health need. Am J Public Health. 2008;98:1894–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res. 1999;1:301–315. [DOI] [PubMed] [Google Scholar]
- 8. Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA, Sayette MA. Differences in negative mood-induced smoking reinforcement due to distress tolerance, anxiety sensitivity, and depression history. Psychopharmacology. 2010;210:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA. Negative mood effects on craving to smoke in women versus men. Addict Behav. 2013;38:1527–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hamilton KR, Berger SS, Perry ME, Grunberg NE. Behavioral effects of nicotine withdrawal in adult male and female rats. Pharmacol Biochem Behav. 2009;92:51–59. [DOI] [PubMed] [Google Scholar]
- 11. O’Dell LE, Torres OV. A mechanistic hypothesis of the factors that enhance vulnerability to nicotine use in females. Neuropharmacology. 2013;76:566–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Torres OV, Gentil LG, Natividad LA, Carcoba LM, O’Dell LE. Behavioral, biochemical, and molecular indices of stress are enhanced in female versus male rats experiencing nicotine withdrawal. Front Psychiatry. 2013;4:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ambrose-Lanci LM, Peiris NB, Unterwald EM, Van Bockstaele EJ. Cocaine withdrawal-induced trafficking of delta-opioid receptors in rat nucleus accumbens. Brain Res. 2008;1210:92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lynch WJ, Kiraly DD, Caldarone BJ, Picciotto MR, Taylor JR. Effect of cocaine self-administration on striatal PKA-regulated signaling in male and female rats. Psychopharmacology (Berl). 2007;191:263–271. [DOI] [PubMed] [Google Scholar]
- 15. Bell RL, Kimpel MW, Rodd ZA, et al. Protein expression changes in the nucleus accumbens and amygdala of inbred alcohol-preferring rats given either continuous or scheduled access to ethanol. Alcohol. 2006;40:3–17. [DOI] [PubMed] [Google Scholar]
- 16. Bell RL, Kimpel MW, McClintick JN, et al. Gene expression changes in the nucleus accumbens of alcohol-preferring rats following chronic ethanol consumption. Pharmacol Biochem Behav. 2009;94:131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Diaz SL, Barros VG, Antonelli MC, Rubio MC, Balerio GN. Morphine withdrawal syndrome and its prevention with baclofen: autoradiographic study of mu-opioid receptors in prepubertal male and female mice. Synapse. 2006;60:132–140. [DOI] [PubMed] [Google Scholar]
- 18. Bangasser DA, Curtis A, Reyes BA, et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15:877, 896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Valentino RJ, Bangasser D, Van Bockstaele EJ. Sex-biased stress signaling: the corticotropin-releasing factor receptor as a model. Mol Pharmacol. 2013;83:737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Natividad LA, Tejeda HA, Torres OV, O’Dell LE. Nicotine withdrawal produces a decrease in extracellular levels of dopamine in the nucleus accumbens that is lower in adolescent versus adult male rats. Synapse. 2010;64:136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rada P, Jensen K, Hoebel BG. Effects of nicotine and mecamylamine-induced withdrawal on extracellular dopamine and acetylcholine in the rat nucleus accumbens. Psychopharmacology. 2001;157:105–110. [DOI] [PubMed] [Google Scholar]
- 22. Bohler HC, Jr, Zoeller RT, King JC, Rubin BS, Weber R, Merriam GR. Corticotropin releasing hormone mRNA is elevated on the afternoon of proestrus in the parvocellular paraventricular nuclei of the female rat. Brain Res Mol Brain Res. 1990;8:259–262. [DOI] [PubMed] [Google Scholar]
- 23. Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci. 2011;8:73–96. [DOI] [PubMed] [Google Scholar]
- 24. Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O’Dell LE. Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology. 2009;206:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCormick CM. Effect of neonatal ovariectomy and estradiol treatment on corticosterone release in response to stress in the adult female rat. Stress. 2001;14:82–87. [DOI] [PubMed] [Google Scholar]
- 27. Donny EC, Caggiula AR, Rowell PP, et al. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology. 2000;151(4):392–405. [DOI] [PubMed] [Google Scholar]
- 28. Zhou W, Cunningham KA, Thomas ML. Estrogen regulation of gene expression in the brain: a possible mechanism altering the response to psychostimulants in female rats. Brain Res Mol Brain Res. 2002;100: 75–83. [DOI] [PubMed] [Google Scholar]
- 29. Anker JJ, Carroll ME. The role of progestins in the behavioral effects of cocaine and other drugs of abuse: human and animal research. Neurosci Biobehav Rev. 2010;35:315–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lynch WJ, Sofuoglu M. Role of progesterone in nicotine addiction: evidence from initiation to relapse. Exp Clin Psychopharmacol. 2010;18:451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Caldarone BJ, King SL, Picciotto MR. Sex differences in anxiety-like behavior and locomotor activity following chronic nicotine exposure in mice. Neurosci Lett. 2008;439:187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gentile NE, Andrekanic JD, Karwoski TE, Czambel RK, Rubin RT, Rhodes ME. Sexually diergic hypothalamic-pituitary-adrenal (HPA) responses to single-dose nicotine, continuous nicotine infusion, and nicotine withdrawal by mecamylamine in rats. Brain Res Bull. 2011;85:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther. 2007;322:399–407. [DOI] [PubMed] [Google Scholar]
- 34. Skwara AJ, Karwoski TE, Czambel RK, Rubin RT, Rhodes ME. Influence of environmental enrichment on hypothalamic-pituitary-adrenal (HPA) responses to single-dose nicotine, continuous nicotine by osmotic mini-pumps, and nicotine withdrawal by mecamylamine in male and female rats. Behav Brain Res. 2012;234:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bruijnzeel AW, Prado M, Isaac S. Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse. Biol Psychiatry. 2009;66:110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marcinkiewcz CA, Prado MM, Isaac SK, Marshall A, Rylkova D, Bruijnzeel AW. Corticotropin-releasing factor within the central nucleus of the amygdala and the nucleus accumbens shell mediates the negative affective state of nicotine withdrawal in rats. Neuropsychopharmacology. 2009;34:1743–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Potter E, Behan DP, Fischer WH, Linton EA, Lowry PJ, Vale WW. Cloning and characterization of the cDNAs for human and rat corticotropin releasing factor-binding proteins. Nature. 1991;349:423–426. [DOI] [PubMed] [Google Scholar]
- 38. Seasholtz AF, Burrows HL, Karolyi IJ, Camper SA. Mouse models of altered CRH-binding protein expression. Peptides. 2001;22:743–751. [DOI] [PubMed] [Google Scholar]
- 39. Karolyi IJ, Burrows HL, Ramesh TM, et al. Altered anxiety and weight gain in corticotropin-releasing hormone-binding protein-deficient mice. Proc Natl Acad Sci U S A. 1999;96:11595–11600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heinrichs SC, Lapsansky J, Behan DP, et al. Corticotropin-releasing factor-binding protein ligand inhibitor blunts excessive weight gain in genetically obese Zucker rats and rats during nicotine withdrawal. Proc Natl Acad Sci U S A. 1996;93:15475–15480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bisagno V, Cadet JL. Stress, sex, and addiction: potential roles of corticotropin-releasing factor, oxytocin, and arginine-vasopressin. Behav Pharmacol. 2014;25:445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]