Abstract
Heavy-chain deposition disease (HCDD) is the least common of the monoclonal immunoglobulin deposition diseases with only 24 reported cases in English literature, including the present case. The rarity of this disease merits its documentation. We present a case of HCDD from our archival material, who presented with rapidly progressive renal failure and nephrotic syndrome and was found to have nodular glomerulosclerosis on renal biopsy which on immunofluorescence and electron microscopy confirmed HCDD of immunoglobulin G1 type without any light-chain deposition. We also present an in-depth literature review on HCDD.
Keywords: monoclonal immunoglobulin deposition disease, nodular glomerulosclerosis, rapidly progressive renal failure
Introduction
Heavy-chain deposition disease (HCDD) is the least common non-organized monoclonal immunoglobulin deposition disease (MIDD), with only 23 documented cases in world literature to date. The existence of this entity was postulated for many years until the first case was reported by Tubbs et al. [1] in 1982 followed by another report by Aucouturier et al. [2] in 1993. Unlike the more common light chain immunoglobulin deposition disease, an association of multiple myeloma or plasma cell dyscrasias with HCDD is less common, with only 7 of 23 (30%) reported cases being associated with development of demonstrable monoclonal plasmacytosis. Nodular glomerulosclerosis is the classic glomerular pattern of injury of all monoclonal immunoglobulin disorders, and the disease (though suspected on light microscopy) can be conclusively diagnosed only by an extended panel of immunofluorescence that includes antibodies against heavy-chain isotypes and heavy-chain constant domains. γ heavy-chain deposition is the most common among these; however, deposition of α- [3, 4] and µ- [5, 6] heavy chains is also reported.
Materials and methods
A single case of HCDD was identified from the archives of the Department of Histopathology, PGIMER, Chandigarh, India, during a 5-year period from 2007 to 2011. During this time, there were 5536 native and allograft kidney biopsies, among which there were 12 cases of light-chain deposition disease (LCDD). All cases were studied by light microscopy, immunofluorescence microscopy and transmission electron microscopy. A literature search on Pubmed showed 23 previously reported cases of HCDD.
Case
The present case was a 72-year-old woman, who presented with progressively increasing shortness of breath of 1-year duration associated with anasarca and intermittent fever with chills of 6-month duration and decreased urine output of 1-month duration. The patient was diagnosed with hypertension 6 months previously and was maintained on an alpha blocker (prazosin). There was no cough, jaundice or gastrointestinal symptoms. General physical examination showed pallor with anasarca and pitting edema, with a pulse of 94 bpm, blood pressure of 130/90 mm of mercury and respiratory rate of 18 breaths per minute. Systemic examination revealed pleural effusion, ascites and mild pericardial effusion. Pleural fluid analysis revealed a transudative fluid with SPAG of 1.9, sugar of 7.78 mmol/L (140 mg/dL) and adenosine deaminase levels of 7 U/L. Laboratory workup revealed abnormal renal function with serum BUN of 15.7 mmol/L (44 mg/dL) and creatinine of 167.96 µmol/L (1.9 mg/dL). Urine examination revealed nephrotic-range proteinuria and 30–35 white blood cells/high power field magnification. There were no dysmorphic red blood cells. Bence Jones proteins were absent. Twenty-four-hour urine proteins were 1.8 g/total volume (260 mL). Her serum protein was 51 g/L (5.1 g/dL) with serum albumin of 24 g/L (2.4 g/dL). Ultrasound examination revealed normal-sized kidneys (right kidney 10.5 cm and left kidney 9.5 cm) with normal echotexture along with mild hepatomegally. Hemogram showed a hemoglobin of 62 g/L (6.2 g/dL), total leukocyte count of 10 × 109/L with a normal differential count and platelets of 1.51 × 109/L. A review of her previous records revealed persistently low hemoglobin for which she was given two units of blood transfusion and also given erythropoietin twice weekly for 2 months prior to being referred to our center. Fasting and post-prandial blood sugars were within normal limits. Liver function tests were within normal limits and coagulation workup revealed a prothrombin index of 100%. Lipid profile showed a serum cholesterol of 3.34 mmol/L (129 mg/dL), triglycerides of 1.07 mmol/L (95 mg/dL), high-density lipoprotein of 0.8 mmol/L (31 mg/dL) and low-density lipoprotein of 1.66 mmol/L (64 mg/dL). Anti-nuclear antibody was strongly positive with a diffuse pattern. Abdominal fat pad biopsy for amyloid was negative and serum electrophoresis did not show an ‘M-band’. Urine electrophoresis showed a band in the albumin region and a faint band in the β region without any ‘M-band’. Human immunodeficiency virus, hepatitis B surface antigen and anti-hepatitis C virus antibodies were negative.
The renal biopsy performed showed nodular glomerulosclerosis and on immunofluorescence it showed strong positivity for polyclonal antibody against immunoglobulin G (IgG) without any positivity for light chains in the glomerular capillary walls, mesangium, Bowman's capsule, tubular basement membrane and blood vessels. Further immunofluorescence examination for IgG heavy-chain subtypes (IgG1–IgG4) showed positivity only for IgG1. Transmission electron microscopy showed powdery electron dense deposits in the lamina rara interna of the glomerular basement membrane, mesangium, tubules and blood vessels.
Bone marrow examination revealed 8% plasma cells. Radiological evaluation did not reveal any lytic lesions or any lymphadenopathy. The patient was started on thalidomide and dexamethasone as she could not afford bortezomib. At the last follow-up (8 months after diagnosis), there was no remittance and she had progressed with a serum creatinine of 565.76 µmol/L (6.4 mg/dL).
Discussion
Incidence and frequency
HCDD is one of the least frequent manifestations of MIDDs. Also, HCDD is the least common immunoglobulin deposition disease according to a review from the Presbyterian Hospital in New York with just 0.33% (six cases) among 7241 biopsies over a period of 19 years [7]. In the same study, LCDD accounted for 12 cases while 5 cases of LHCDD were seen. In our center, we have documented only one case of HCDD in comparison to 12 cases of LCDD in the last 5 years. Just 24 cases have been reported to date with only 8 of 24 (33.3%) patients having developed a plasma cell dyscrasia. In a recent review, HCDD was noted in just 1 of 30 cases in which renal histology was evaluated among 289 cases of paraproteinemias [8]. Analyzing all the HCDD cases reported to date (24, including the present case), the mean age of presentation was 58.41 ± 14.32 years with no significant sex predilection (F:M = 1.2:1). IgG1 (8 of 24 cases) was the most common heavy-chain isotype with IgG4 (4/24) and IgG3 (3/24) being the next common. IgG2 was reported in one case while three cases of IgA HCDD have been reported. Crescentic glomerulonephritis was consistently reported with IgA HCDD in addition to the nodular glomerulosclerosis pattern of the underlying capillary tufts. The clinical profile of HCDD is very similar to that of other MIDD, except for a higher incidence of hypertension and less-frequent association with either a demonstrable plasma cell dyscrasia (∼25% cases when compared with 50% cases of LCDD) or circulating monoclonal free light or heavy chain [7]. A summary of all reported cases of renal HCDD is presented in Table 1.
Table 1.
Clinico-pathological features of all reported cases of HCDDa
| Case | Clinical features and biochemistry |
Renal biopsy findings |
Missing domains in CH | Serum Ig | BM bx/aspirate | Other organ disease | Treatment | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Immumofluorscence |
EM deposits |
||||||||||||||||||
| Age/sex | Renal presentation | HTN | sCr (mg/dl) | C3 /C4 | Glomerulopathy | Ig | GBM | Mes | TBM | Ves | GBM | TBM | Ves | ||||||
| Tubbs-1 (1992) [1] | 69 M | RI, He, Pro | Ab | 4.7 | N | NSG | G4 | 3 | 3 | 3 | 3 | + | + | + | ND | IgG4ʎb | IgG4ʎb | ND | |
| Tubbs-2 (1992) [1] | 50 M | RI, NS, He | NO | 2.4 | N | NSG | G4 | 3 | 3 | 3 | 3 | + | + | + | ND | IgG4 ʎb | IgG4 Lb | ND | ND |
| Aucouturier-1 (1993) [2] | 53 F | RI, NS | NO | 1.5 | ND | NSG | ND | + | + | + | ND | + | – | – | CH1, CH2 and Hinge | IgG1ʎ | MM-IgGL | ND | Melphalan, MP |
| Aucouturier-2 (1993) [2] | 59 F | RI, NS | NO | 1.6 | ND | NSG | G4 | + | + | + | + | + | + | + | CH1 | OligoclonalIgb | nl | AIT, NIDDM, thrombocytopenia | Chlorambucil, MP, melphalan |
| Katz (1994) [35] | 51 M | RI | NO | 9.7 | ND | NSG | G4 | 3 | 3 | 3 | 3 | + | + | – | ND | IgG4 ʎ + ʎ | nl | ND | Cyclophos, MP |
| Yasuda (1995) [33] | 35 F | He, Pro | NO | 1 | L | NSG | G1 | + | + | + | ND | + | – | – | CH2 | IgG ʎ | IgGL | ND | MP |
| Cheng (1996) [3] | 62 M | RI, NS, He | Pr | 3.3 | ND | NSGC | A | 3 | 3 | ND | ND | + | + | + | ND | IgAκ | IgAκ | ND | Cyclophos, MP |
| Herzenberg (1996) [36] | 79 F | RI, NS, He | NO | 1.4 | L | NSG | G3 | 3 | 3 | 3 | 3 | + | + | ND | ND | N | nl | ND | None |
| Rott (1998) [24] | 73 M | NS, An, MP | Pr | 2.3 | ND | NSG | ND | 3 | 3 | 3 | 3 | + | + | ND | ND | IgGʎ | 14% plasma cells | Skin, muscle | mp, pred |
| Moulin-1 (1999) [32] | 58 M | RI, He, Pro | NO | 1.4 | N | NSG | G1 | + | + | ND | ND | + | – | – | CH1 | IgG1ʎ | MM-IgGʎ (14% plasma cells) | ND | VMCP followed by interferons |
| Moulin-2 (1999) [32] | 71 M | RI, He, NS | Pr | 2.8 | L | NSG | G1 | + | + | + | + | ND | ND | ND | CH1 | IgG1ʎ | N (2% plasma cells) | ND | NiL |
| Moulin-3 (1999) [32] | 51 M | RI, He, NS | Pr | 1.5 | L | NSG | G1 | + | + | + | ND | + | + | ND | CH1 | IgG1ʎ + ʎ + γ1 | Myeloma (20% plasma cells) | ND | VAD followed by ABSCG |
| Moulin-4 (1999) [32] | 35 F | RI, He, NS | Ab | 1.02 | L | NSG | G1 | + | + | + | ND | + | + | ND | CH1 | IgG1ʎ + γ1 | N (5.8% Plasma cells) | ND | Low-dose steroids |
| Kambham (1999) [37] | 45 F | RI, NS , He | NO | 4.3 | L | NSG | G3 | 3 | 2 | 2 | + | + | + | CH1 | IgG3 ʎ | nl | ND | MP | |
| Herzenberg-1 (2000) [34] | 26F | RI, NS , He | Pr | 2.26 | L | NSG | G1 | 1 | 3 | 3 | 3 | + | + | + | ND | – | N, 1% plasma cells | – | Melphalan, dexa |
| Herzenberg-2 (2000) [34] | 67F | RI, NS , He | Pr | 1.33 | N | NSG | G2 | 1 | 3 | 3 | 3 | + | + | + | ND | – | N, 3% plasma cells | – | MP, AZ |
| Liapis (2000)[6] | 68F | RI, HTN | Pr | ND | N | NSG | M | + | + | + | – | + | – | – | Neg | – | 1% plasma cells | nl | nil |
| Soma (2004) [29] | 54F | NS, He | Pr | 0.95 | L | NSG | G3 | 3 | 3 | 2 | ND | + | + | ND | CH1 | – | 4% plasma cells | ND | MP, melphala |
| Vedder (2004) [28] | 55 M | RI, NS | Pr | 6.73 | N | PGN | ND | 3 | 3 | 3 | 3 | + | + | + | ND | IgGκ | Monoclonal plasmacytosis-10% | ND | MP → VAD |
| Yuji oe (2010) [31] | 68F | NS, He | NO | 1.8 | L | NSG | G1 | 3 | 3 | + | ND | + | + | ND | ND | – | N, 1.2% plasma cells | ND | MP |
| Alexander-1 (2011) [4] | 29 M | RI | Pr | 2.5 | N | NSGC | A | + | – | – | – | + | + | + | ND | IgAk | MM IgAk | Skin-cutis laxa | Dexa, cyclo |
| Alexander-2 (2011) [4] | 78F | RI, NS , He | Pr | 2.5 | N | NSGC | A | + | – | – | – | + | + | + | ND | IgAk | 1% plasma cells/IgAk | ND | Thal, dexa, erythropoeitin |
| Alexander-3 (2011) [4] | 67F | RI, NS , He | ND | 4.8 | N | NSGC | A | + | – | – | – | + | + | – | ND | IgAk | MM/IgAk | ND | bortzomib + dexa |
| Present case (2012) | 72F | RI, NS | NO | 2.6 | ND | NSG | G1 | 3 | 3 | 3 | – | + | + | – | ND | _ | 4% plasma cells | – | Thal, dexa |
aRI, renal insufficiency; NS, nephrotic syndrome; He, hematuria; NSG, nodular glomerulosclerosis; NSGC, nodular glomerulosclerosis with crescentic pattern of injury; PGN, proliferative glomerulonephritis; ND, not described; N, normal; MM, multiple myeloma; CH, heavy chain; HTN, hypertension; GBM, glomerular basement membrane; Mes, mesangium; TBM, tubular basement membrane; Ves, vessel; EM, electron microscopy; MP, methyl prednisolone; Thal, thalidomide; VAD, vincristine, adriamycin and dexamethasone; dexa, dexamethasone, cyclo, cyclosporine; AZ, azathioprine; ABSCG, autologous blood stem cell graft; VMCP, vincristine, melphalan, cyclophosphamide and prednisone.
bConfirmed by immunofixation.
Aetiopathogenesis
Current evidence suggests that the loss of CH1 domain leads to secretion of heavy chains from the plasma cells prior to their association with the light chains, which under normal circumstances are held in the endoplasmic reticulum via the interaction between CH1 domain and heavy-chain-binding protein (BiP) and are released only after binding of light chains [9, 10]. In the presence of a normal CH1 domain and normal interaction with BiP, failure of association of the light and heavy chains leads to destruction of the unbound heavy chains within the endoplasmic reticulum and these are not secreted. However, what exactly causes the marked predisposition for tissue deposition and rapid clearance from the circulation is unclear. In a study by Khamlichi et al. [11], the authors demonstrate that in addition to the CH1 domain abnormalities, deletions of the variable regions of the heavy chains (VH domains) lead to alterations in the physicochemical properties of the immunoglobulin heavy chains altering the hydrophobicity and total charge and hence the tissue affinity. This mechanism is similar to the postulated cause of deposition of abnormal light chains in LCDD, in that mutations in the VL chain leads to accumulation of hydrophobic amino acids and alteration of the tertiary and quarternary structure of the protein, resulting in accentuation of the hydrophobicity of the abnormal light chains. In addition, contribution of abnormal N-glycosylation has already been suggested to be the cause of tissue deposition of the light chains as well as the reason for the absence of demonstrable circulating abnormal immunoglobulins in many cases [7, 12].
As in other MIDDs, the deposition of monoclonal immunoglobulins induces accumulation of extracellular matrix material, leading to glomerular and tubular basement membrane thickening, nodular glomerulosclerosis and interstitial fibrosis. Although there are no studies detailing the exact mechanisms of tissue injury in HCDD specifically, it is most likely similar to the mechanism of tissue injury in other MIDDs like LCDD and LHCDD. There is an excess accumulation of normal extracellular matrix proteins viz. fibronectin, collagen type IV, laminin and tenascin [13, 14], by enhancing their production in mesangial cells [15]. The same study attributes a significant role to tenascin for the irreversibility of glomerular lesions in MIDD. In vitro studies have also shown transformation of the mesangial cell to a myofibroblastic phenotype, with an increase in rough endoplasmic reticulum, increased production of cytokines viz. platelet-derived growth factor, transforming growth factor-β and monocyte chemotactic peptide-1, decrease in matrix metalloproteinases as well as increase in the proliferation markers like Ki-67 index, when mesangial cells are incubated with light chains obtained from patients with LCDD while no such effect was seen when they were incubated with tubulopathic light chains from patients with cast nephropathy. Furthermore, mesangial cells incubated with light chains of AL-amyloid show transformation to a macrophage phenotype with an increase in matrix metalloproteinases and a decrease in extracellular matrix production [16–18]. These divergent phenotypes result from the differential processing of the abnormal immunoglobulins through the receptors on mesangial cells which lead to internalization and delivery of the light chains of AL-amyloid to the lysosomes, where production of amyloid occurs while the abnormal immunoglobulins associated with MIDD are not internalized significantly [17, 19, 20]. However, there is no in vivo study that confirms these events.
Clinical presentation
Although HCDD as well as other MIDDs are systemic diseases with deposition of abnormal Igs in a variety of organs, it is the deposition of abnormal immunoglobulins in the renal parenchyma which most often leads to clinical dysfunction. Extra-renal deposits in HCDD are very uncommon; however, they have been reported in the heart [21], joints [21–23], skin, striated muscle [24], pancreas and thyroid as well as liver. Most of the non-renal visceral organ depositions are usually asymptomatic, and hence the incidence of these deposits is likely to be under-estimated. Skin is the next common organ to be affected by HCDD, with α-HCDD being the commonest and less commonly γ-HCDD presenting as cutis laxa [4, 25, 26]. Deposition of abnormal chains in LCDD and LHCDD has been documented in the liver and heart ∼25% cases [27]. Mild alterations in the liver function tests are common, but hepatic failure is distinctly rare. In the liver, deposition of abnormal immunoglobulins is commonly minimal and sinusoidal, but can be massive.
Renal manifestations
Renal involvement is a constant feature in HCDD with most patients presenting with renal failure (∼90% cases), recent onset hypertension (∼70% cases) and proteinuria (80%) most of whom had nephrotic range proteinuria (60%) (Table 1). Most patients present with rapidly progressive renal failure. Hematuria is variably present in 25% of cases reported to date. The manifestation of HCDD are very similar to other MIDD, except for a stronger association with hypertension, glomerulosclerosis and hematuria [7].
Renal pathology
Histopathology
Nodular glomerulosclerosis is the classical histological pattern (Figure 1), although other patterns like crescentic pattern of glomerular injury [3, 4] as well as a predominantly diffuse proliferative pattern of injury [28] is also reported. No cases have been reported of pure HCDD with either membranous pattern of injury or normal morphology on light microscopy. The glomeruli show nodular mesangial expansion by deposition of Periodic Acid Schiff (PAS) positive material which is Congo-red negative, can be fuschinophilic on trichrome stain and stains avidly with silver stains, unlike amyloid which is only weakly PAS positive and silver negative in addition to being congophilic and showing apple-green birefringence. Nodular glomerulosclerosis brings a histological differential diagnosis of diabetic nephropathy, membrano-proliferative glomerulonephritis (GN), amyloidosis and Congo-red-negative amyloid-like deposits (fibrillary GN, immunotactoid GN), MIDD of either LCDD or LHCDD type, idiopathic type I or III collagenofibrotic GN and fibronectin GN. Milder forms of the disease may show only a mild increase in mesangial matrix with basement membrane thickening. Although glomerular disease is the most common reason for clinical impairment, HCDD is not a pure glomerular disease. Tubular lesions are usually present in the form of PAS-positive, refractile thickening of the tubular basement membrane. There is some predominance of deposition in the distal tubules and loop of Henle. Advanced cases usually have significant fibrosis.
Fig. 1.
(a) Light microscopy shows nodular glomerulosclerosis with mesangial nodules, thickening of glomerular capillary walls and the Bowman's capsule (H&E, ×400 original magnification). (b) The mesangial expansion and basement membrane thickening is due to PAS-positive material (×200 original magnification) and (c) is not congophilic (×200 original magnification). (d) Similar material is also identified in the tubular basement membranes of some of the tubules (H&E, ×200 original magnification).
Immunofluorescence
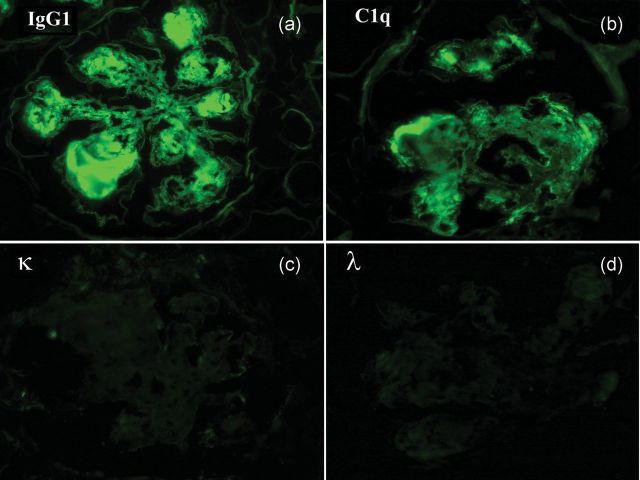
An appropriate immunofluorescence evaluation is essential in diagnosing HCDD and differentiating it from its other differential diagnoses. The diagnosis may be suspected in an initial panel which does not include the IgG/IgA subtypes, when anti-IgG/IgA are positive while there is no light-chain positivity. The definitive diagnosis however requires demonstration of monoclonality of the heavy chains by using a wider immunofluorescence panel of antibodies identifying distinctly the four IgG subtypes and/or two IgA subtypes. Since there is only one IgM subtype, the presence of IgM positivity without any light-chain positivity should lead to a diagnosis. Any of the IgG or IgA subtypes may be present. The most common IgG subtype implicated is the IgG1 (8 of 24 cases) among all the cases reported to date with IgG4 being the next most common (4 of 24). Positivity is usually linear with stronger positivity in the glomerular basement membrane when compared with the mesangial nodules (Figure 2). Positivity along the tubular basement membrane is a rule. Similar positivity may be seen within the blood vessels. Complement positivity is variable with maximum positivity being seen in cases with IgG1 and IgG3 (Table 1). Demonstration of deletion of CH1 domain of the IgG is however not necessary for the diagnosis. Deletion of the CH1 domain has been documented in all cases which were evaluated using antibodies specific to each CH domain (Table 1).
Fig. 2.
Direct immunofluorescence shows strong positivity for (a) IgG1 and (b) C1q without any (c and d) light-chain positivity in the mesangial nodules, GBM, TBM, Bowman's capsule and blood vessels. IgG2, IgG3, IgG4, IgA, IgM and C3 were also negative. (×400 original magnification, fluorescein isothiocyanate-tagged antibodies).
Ultrastructure
Transmission electron microscopy demonstrates the deposition of non-fibrillar, powdery, electron dense deposits along the tubular basement membrane, glomerular basement membrane and blood vessels. The deposits usually form a continuous band on the endothelial aspect of the glomerular basement membrane and on the outer aspect of the tubular basement membrane, facing the interstitum (Figure 3). But unlike amyloid deposits, they do not invade into the lamina densa. Deposits may also be found in the Bowman's capsule. Immunoelectron microscopy can be helpful in difficult cases.
Fig. 3.

(a) Transmission electron microscopy shows the presence of powdery, non-organized, electron dense deposits along the endothelial aspect of the GBM. (b) Similar deposits are also noted in the TBM (×2000 original magnification).
Treatment and outcome
Most patients reported have been treated with pulse methyl prednisolone or with a combination of methyl prednisolone and melphalan. Many patients also received other cytotoxic agents such as cyclophosphamide or chlorambucil, with just an occasional patient having received dexamethasone, thalidomide or bortezomib. Among all 24 cases recorded in the literature to date, just 3 cases responded apparently completely to the treatment given, which included melphalan and methyl prednisolone in 2 cases [29–31] and low-dose steroids in the third [32]. Only one of these cases [30] has been shown to be free of disease 2 years after the initial diagnosis on a follow-up biopsy. Another three cases showed partial improvement of symptoms and laboratory values, one of which was treated with melphalan along with cyclophosphamide [3], another with melphalan alone [33], while the third patient received combination chemotherapy with vincristine, adriamycin and dexamethasone, followed by autologous blood stem cell graft [32]. One patient [6] showed stable disease at 2 years of follow-up despite not receiving any therapy. The remaining 18 of 24 patients failed to show any response to treatment, most of whom received combination of methyl prednisolone with melphalan and/or other chemotherapeutic agents like cyclophosphamide, vincristine, adriamycin, chlorambucil etc. Our patient was treated with thalidomide with dexamethasone, but showed progressive worsening of renal failure and proteinuria at the last follow-up, 8 months after diagnosis. Significant long-term follow-up is unavailable in nearly all cases.
It is possible that the patients who responded completely (clinically as well as pathologically) or partially to treatment were in the initial stages of the disease as suggested by Soma et al. [30]. The response to treatment would probably also depend on the presence of an overt plasma cell dyscrasia. Whether patients with HCDD are poor candidates for renal transplantation is unknown; however, one patient who received renal graft, developed recurrent disease 2½ years post-transplant [34].
Conclusion
In conclusion, HCDD is a rare monoclonal immunoglobulin deposition disorder due to the deposition of abnormal heavy chains with CH1 and VH region abnormalities, which leads to their early secretion from the plasma cells prior to conjugation with light chains. Patients present with renal failure, hypertension and hematuria and have a considerably lesser association with an overt plasma cell dyscrasia. Early diagnosis and treatment might by the key to complete remission of disease with long-standing disease being practically incurable as of today.
Conflict of interest statement. None declared.
References
- 1.Tubbs RR, Berkley V, Valenzuela R, et al. Pseudo-gamma heavy chain (IgG4 lambda) deposition disease. Mod Pathol. 1992;5:185–190. [PubMed] [Google Scholar]
- 2.Aucouturier P, Khamlichi AA, Touchard G, et al. Brief report: heavy-chain deposition disease. N Engl J Med. 1993;329:1389–1393. doi: 10.1056/NEJM199311043291905. doi:10.1056/NEJM199311043291905. [DOI] [PubMed] [Google Scholar]
- 3.Cheng IK, Ho SK, Chan DT, et al. Crescentic nodular glomerulosclerosis secondary to truncated immunoglobulin alpha heavy chain deposition. Am J Kidney Dis. 1996;28:283–288. doi: 10.1016/s0272-6386(96)90315-7. doi:10.1016/S0272-6386(96)90315-7. [DOI] [PubMed] [Google Scholar]
- 4.Alexander MP, Nasr SH, Watson DC, et al. Renal crescentic alpha heavy chain deposition disease: a report of 3 cases and review of the literature. Am J Kidney Dis. 2011;58:621–625. doi: 10.1053/j.ajkd.2011.05.022. doi:10.1053/j.ajkd.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Feiner HD. Pathology of dysproteinemia: light chain amyloidosis, non-amyloid immunoglobulin deposition disease, cryoglobulinemia syndromes, and macroglobulinemia of Waldenstrom. Hum Pathol. 1988;19:1255–1272. doi: 10.1016/s0046-8177(88)80280-6. doi:10.1016/S0046-8177(88)80280-6. [DOI] [PubMed] [Google Scholar]
- 6.Liapis H, Papadakis I, Nakopoulou L. Nodular glomerulosclerosis secondary to mu heavy chain deposits. Hum Pathol. 2000;31:122–125. doi: 10.1016/s0046-8177(00)80209-9. doi:10.1016/S0046-8177(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 7.Ronco P, Plaisier E, Mougenot B, et al. Immunoglobulin light (heavy)-chain deposition disease: from molecular medicine to pathophysiology-driven therapy. Clin J Am Soc Nephrol. 2006;1:1342–1350. doi: 10.2215/CJN.01730506. doi:10.2215/CJN.01730506. [DOI] [PubMed] [Google Scholar]
- 8.Stratta P, Gravellone L, Cena T, et al. Renal outcome and monoclonal immunoglobulin deposition disease in 289 old patients with blood cell dyscrasias: a single center experience. Crit Rev Oncol Hematol. 2011;79:31–42. doi: 10.1016/j.critrevonc.2010.05.001. doi:10.1016/j.critrevonc.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Hendershot L, Bole D, Kohler G, et al. Assembly and secretion of heavy chains that do not associate posttranslationally with immunoglobulin heavy chain-binding protein. J Cell Biol. 1987;104:761–767. doi: 10.1083/jcb.104.3.761. doi:10.1083/jcb.104.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knittler MR, Haas IG. Interaction of BiP with newly synthesized immunoglobulin light chain molecules: cycles of sequential binding and release. EMBO J. 1992;11:1573–1581. doi: 10.1002/j.1460-2075.1992.tb05202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khamlichi AA, Aucouturier P, Preud'homme JL, et al. Structure of abnormal heavy chains in human heavy-chain-deposition disease. Eur J Biochem. 1995;229:54–60. doi: 10.1111/j.1432-1033.1995.tb20436.x. doi:10.1111/j.1432-1033.1995.tb20436.x. [DOI] [PubMed] [Google Scholar]
- 12.Denoroy L, Deret S, Aucouturier P. Overrepresentation of the V kappa IV subgroup in light chain deposition disease. Immunol Lett. 1994;42:63–66. doi: 10.1016/0165-2478(94)90036-1. doi:10.1016/0165-2478(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 13.Keeling J, Herrera GA. Matrix metalloproteinases and mesangial remodeling in light chain-related glomerular damage. Kidney Int. 2005;68:1590–1603. doi: 10.1111/j.1523-1755.2005.00571.x. doi:10.1111/j.1523-1755.2005.00571.x. [DOI] [PubMed] [Google Scholar]
- 14.Bruneval P, Foidart JM, Nochy D, et al. Glomerular matrix proteins in nodular glomerulosclerosis in association with light chain deposition disease and diabetes mellitus. Hum Pathol. 1985;16:477–484. doi: 10.1016/s0046-8177(85)80086-1. doi:10.1016/S0046-8177(85)80086-1. [DOI] [PubMed] [Google Scholar]
- 15.Herrera GA, Russell WJ, Isaac J, et al. Glomerulopathic light chain-mesangial cell interactions modulate in vitro extracellular matrix remodeling and reproduce mesangiopathic findings documented in vivo. Ultrastruct Pathol. 1999;23:107–126. doi:10.1080/019131299281752. [PubMed] [Google Scholar]
- 16.Zhu L, Herrera GA, Murphy-Ullrich JE, et al. Pathogenesis of glomerulosclerosis in light chain deposition disease. Role for transforming growth factor-beta . Am J Pathol. 1995;147:375–385. [PMC free article] [PubMed] [Google Scholar]
- 17.Keeling J, Teng J, Herrera GA. AL-amyloidosis and light-chain deposition disease light chains induce divergent phenotypic transformations of human mesangial cells. Lab Invest. 2004;84:1322–1338. doi: 10.1038/labinvest.3700161. doi:10.1038/labinvest.3700161. [DOI] [PubMed] [Google Scholar]
- 18.Russell WJ, Cardelli J, Harris E, et al. Monoclonal light chain—mesangial cell interactions: early signaling events and subsequent pathologic effects. Lab Invest. 2001;81:689–703. doi: 10.1038/labinvest.3780278. doi:10.1038/labinvest.3780278. [DOI] [PubMed] [Google Scholar]
- 19.Teng J, Russell WJ, Gu X, et al. Different types of glomerulopathic light chains interact with mesangial cells using a common receptor but exhibit different intracellular trafficking patterns. Lab Invest. 2004;84:440–451. doi: 10.1038/labinvest.3700069. doi:10.1038/labinvest.3700069. [DOI] [PubMed] [Google Scholar]
- 20.Demeule B, Gurny R, Arvinte T. Where disease pathogenesis meets protein formulation: renal deposition of immunoglobulin aggregates. Eur J Pharm Biopharm. 2006;62:121–130. doi: 10.1016/j.ejpb.2005.08.008. doi:10.1016/j.ejpb.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Husby G, Blichfeldt P, Brinch L, et al. Chronic arthritis and gamma heavy chain disease: coincidence or pathogenic link? Scand J Rheumatol. 1998;27:257–264. [PubMed] [Google Scholar]
- 22.Danevad M, Sletten K, Gaarder PI, et al. The amino acid sequence of a monoclonal gamma 3-heavy chain from a patient with articular gamma-heavy chain deposition disease. Scand J Immunol. 2000;51:602–606. doi: 10.1046/j.1365-3083.2000.00730.x. doi:10.1046/j.1365-3083.2000.00730.x. [DOI] [PubMed] [Google Scholar]
- 23.Husby G. Is there a pathogenic link between gamma heavy chain disease and chronic arthritis? Curr Opin Rheumatol. 2000;12:65–70. doi: 10.1097/00002281-200001000-00011. doi:10.1097/00002281-200001000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Rott T, Vizjak A, Lindic J, et al. IgG heavy-chain deposition disease affecting kidney, skin, and skeletal muscle. Nephrol Dial Transplant. 1998;13:1825–1828. doi: 10.1093/ndt/13.7.1825. doi:10.1093/ndt/13.7.1825. [DOI] [PubMed] [Google Scholar]
- 25.Harrington CR, Beswick TC, Susa JS, et al. Acquired cutis laxa associated with heavy chain deposition disease. J Am Acad Dermatol. 2008;59:S99–S101. doi: 10.1016/j.jaad.2008.05.007. doi:10.1016/j.jaad.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Tan S, Pon K, Bargman J, et al. Generalized cutis laxa associated with heavy chain deposition disease. J Cutan Med Surg. 2003;7:390–394. doi: 10.1007/s10227-002-0128-z. doi:10.1007/s10227-002-0128-z. [DOI] [PubMed] [Google Scholar]
- 27.Ganeval D, Noel LH, Preud'homme JL, et al. Light-chain deposition disease: its relation with AL-type amyloidosis. Kidney Int. 1984;26:1–9. doi: 10.1038/ki.1984.126. doi:10.1038/ki.1984.126. [DOI] [PubMed] [Google Scholar]
- 28.Vedder AC, Weening JJ, Krediet RT. Intracapillary proliferative glomerulonephritis due to heavy chain deposition disease. Nephrol Dial Transplant. 2004;19:1302–1304. doi: 10.1093/ndt/gfg575. doi:10.1093/ndt/gfg575. [DOI] [PubMed] [Google Scholar]
- 29.Soma J, Sato K, Sakuma T, et al. Immunoglobulin gamma3-heavy-chain deposition disease: report of a case and relationship with hypocomplementemia. Am J Kidney Dis. 2004;43:E10–E16. doi: 10.1053/j.ajkd.2003.09.024. doi:10.1053/j.ajkd.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 30.Soma J, Tsuchiya Y, Sakuma T, et al. Clinical remission and histopathological resolution of nodular lesions in a patient with gamma3 heavy-chain deposition disease. Clin Nephrol. 2008;69:383–386. doi: 10.5414/cnp69383. [DOI] [PubMed] [Google Scholar]
- 31.Oe Y, Nakaya I, Yahata M, et al. A case of gamma1-heavy chain deposition disease successfully treated with melphalan and prednisolone therapy. Intern Med. 2010;49:1411–1415. doi: 10.2169/internalmedicine.49.3499. doi:10.2169/internalmedicine.49.3499. [DOI] [PubMed] [Google Scholar]
- 32.Moulin B, Deret S, Mariette X, et al. Nodular glomerulosclerosis with deposition of monoclonal immunoglobulin heavy chains lacking C(H)1. J Am Soc Nephrol. 1999;10:519–528. doi: 10.1681/ASN.V103519. [DOI] [PubMed] [Google Scholar]
- 33.Yasuda T, Fujita K, Imai H, et al. Gamma-heavy chain deposition disease showing nodular glomerulosclerosis. Clin Nephrol. 1995;44:394–399. [PubMed] [Google Scholar]
- 34.Herzenberg AM, Kiaii M, Magil AB. Heavy chain deposition disease: recurrence in a renal transplant and report of IgG(2) subtype. Am J Kidney Dis. 2000;35:E25. doi: 10.1016/s0272-6386(00)70290-3. doi:10.1016/S0272-6386(00)70290-3. [DOI] [PubMed] [Google Scholar]
- 35.Katz A, Zent R, Bargman JM. IgG heavy-chain deposition disease. Mod Pathol. 1994;7:874–878. [PubMed] [Google Scholar]
- 36.Herzenberg AM, Lien J, Magil AB. Monoclonal heavy chain (immunoglobulin G3) deposition disease: report of a case. Am J Kidney Dis. 1996;28:128–131. doi: 10.1016/s0272-6386(96)90141-9. doi:10.1016/S0272-6386(96)90141-9. [DOI] [PubMed] [Google Scholar]
- 37.Kambham N, Markowitz GS, Appel GB, et al. Heavy chain deposition disease: the disease spectrum. Am J Kidney Dis. 1999;33:954–962. doi: 10.1016/s0272-6386(99)70432-4. doi:10.1016/S0272-6386(99)70432-4. [DOI] [PubMed] [Google Scholar]