Abstract
Background
We have recently demonstrated that hemodialysis (HD) patients have significantly higher levels of functional complement activity (FCA) in all three pathways, i.e. the classical pathway, alternative pathway and lectin pathway (LP), than in age-matched controls, though the role of FCA during HD still remains unknown.
Methods
Serial plasma or serum samples were obtained from five patients during HD in order to investigate the kinetics of complement components. The levels of the C5b-9 complex, the FCA of the three pathways, a derivative of C3a (C3a desArg) and a derivative of C5a (C5a desArg) in the samples were analyzed.
Results
The levels of the C5b-9 complex at 60 min were significantly increased when compared with those at 0 min. Functional activities for all three pathways showed different patterns so the same tendency between pathways was not observed. The levels of C3a desArg and C5a desArg at 60 min were markedly increased when compared with those at 0 min. A Spearman's rho test showed a strong positive correlation between functional LP activity and C5a desArg.
Conclusions
These findings lead to new insights into the FCA during HD and suggest that functional LP activity has an important role in C5 activation.
Keywords: complement, C3a desArg, C5a desArg, hemodialysis, lectin pathway
Introduction
Under normal physiological conditions, complement activation leads to a proteolytic cascade resulting in immune cell activation, rapid opsonization and the elimination of microorganisms. However, excessive complement activation, especially in the generation of the C3a, C5a and C5b-9 complexes, are life-threatening for patients on hemodialysis (HD) [1–6]. The C3a, C5a and C5b-9 complexes are generated from the activation of C3 and C5 via three complement activation pathways, the classical pathway (CP), alternative pathway (AP) and lectin pathway (LP). Therefore, although little is known about it, clarifying the activation process in the three pathways during HD sessions is of clinical importance.
Recent advances allow us to assess the functional complement activity (FCA) of the three pathways independently, and in parallel, by using the novel ELISA (Wielisa®-kit) instead of a hemolytic assay. Our recent study using this method has demonstrated that HD patients had significantly higher levels of FCA in all three pathways than in age-matched controls [7], although the role of the fluctuations in the FCA during HD is still unknown. Although measurements of C3a and C5a have been used as a monitoring parameter of complement activation during HD, C3a and C5a are immediately digested by carboxypeptidase N and processed to their more stable metabolites C3a desArg and C5a desArg in vivo [8, 9]. Thus, the measurement of C3a desArg and C5a desArg should reflect the physiological condition of C3a and C5a more accurately than by just measuring C3a and C5a.
In the present study, we first revealed the kinetic changes in the FCA of the three complement pathways during HD and also assessed the association between the three activation pathways and C3a desArg and C5a desArg.
Materials and methods
Patients and study design
The characteristics of the enrolled patients are listed in Table 1. In this study, two different HD membranes were used: a cellulose membrane (CL-EE®, Asahi Medical Co. Ltd., Tokyo, Japan) (n = 3) and a polysulfone membrane (APS®, Asahi Medical Co. Ltd.) (n = 2). Because patient No. 3 had residual renal function, the Kt/v-value was lower than in other patients. None of the patients manifested any infection or malignancy symptoms. Patients with a history of severe infection, unstable erythropoietin dosage or single-needle dialysis were excluded from this study. All of the patients gave their informed consent to participate in this study, which was performed in compliance with the Helsinki Declaration.
Table 1.
Patients characteristics
| Case | Age/gender | Renal disease | D.W. (kg) | History of HD (years) | Dialyzer | Hours/session | ΔB.W. (kg) | Kt/v | Anticoagulant |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 80/F | CGN | 36.3 | 13.2 | CL | 4 | 2.6 | 1.49 | Heparin, 3000 U/3 h |
| 2 | 59/M | DMN | 46.8 | 6.2 | CL | 4 | 3.7 | 1.29 | LMWH, 2000 U/one shot |
| 3 | 70/M | DMN | 58.5 | 1.9 | CL | 3 | 2.1 | 0.95 | Heparin, 2000 U/2 h |
| 4 | 69/M | Nephrosclerosis | 49.3 | 10.9 | PS | 4 | 4.1 | 1.46 | Heparin, 3000 U/3 h |
| 5 | 56/M | CGN | 66.5 | 14.8 | PS | 4 | 3.8 | 1.35 | Heparin, 3000 U/3 h |
CGN, chronic glomeruronephritis; CL, cellulose; ΔB.W., increased body weght from previous HD; DMN, diabetic nephropathy; PS, polysulfone; LMWH, low-molecular-weight heparin; D.W., dry weight.
Samples
Samples were obtained from the arterial side of the arteriovenous fistula before an anticoagulant injection (0 min). Subsequently, the samples were serially obtained from the arterial line of the dialyzer. After centrifugation, these samples were stored at −80°C prior to the processing.
Measurements
The measurement of FCA was performed using a Wielisa®-kit (Wieslab, Lund, Sweden) as described previously [7]. In brief, the wells of the microtiter strips were coated with specific activators for the classical, alternative or lectin pathways. Patient serum was diluted with different specific blockers to ensure that only a specific pathway was activated [10]. After activation, the C5b-9 complex was captured using an alkaline phosphatase-conjugated antibody, after which color development was performed. The negative control was given a value of 0%, and the positive control a value of 100%; subsequently, the values of the sera were expressed as a percentage of the positive control. The C3a desArg and C5a desArg concentrations were measured using ELISA kits (BD Bioscience, San Diego, CA, USA) and the C5b-9 complex was quantified using the EIA kit (Quidel, San Diego, CA, USA).
Statistical analysis
All statistical analyses were performed using Prism4 (GraphPad, La Jolla, CA, USA) for Windows. The Dunnett's test and Spearman's rho test were performed to investigate the significance of the differences and the correlation, respectively.
Results
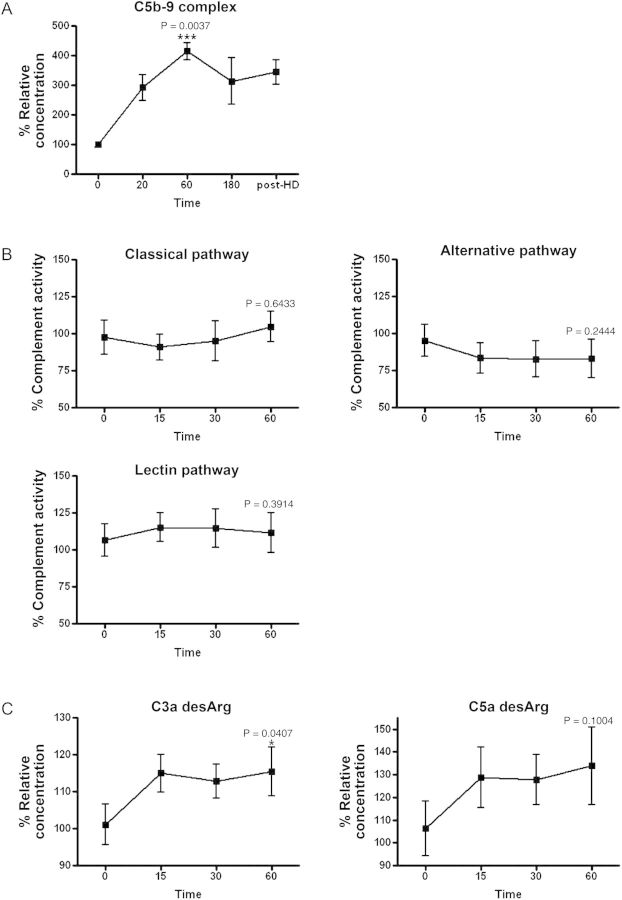
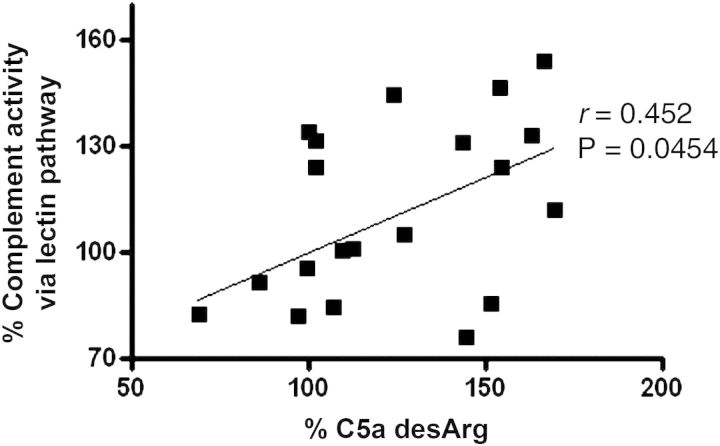
To confirm whether HD induces the formation of the C5b-9 complex during HD, the total levels of the C5b-9 complex in the plasma samples from each patient, obtained at 0, 20, 60 and 180 min and post-HD, were quantified (Figure 1A). The C5b-9 complex rapidly increased until 60 min and plateaued until the end of HD (100.0 ± 9.608, 291.8 ± 43.41, 414.5 ± 28.66, 313.3 ± 78.72, 344.3 ± 41.73), with the mean levels at 60 min significantly higher than those at 0 min (P= 0.0037). This result led us to focus on the early phase, up until 60 min, during HD. There was the same tendency in the total levels of the C5b-9 complex between HD patients who used a cellulosic membrane and those who used a polysulfone membrane (data not shown). To examine the kinetics change of the FCA in the three complement pathways during HD, the activity of each was measured in the serum samples at 0, 15, 30 and 60 min (Figure 1B). FCA via the CP was lower at 15 min and then increased as time passed (97.43 ± 28.07, 90.99 ± 21.29, 95.03 ± 33.43, 104.8 ± 24.96). FCA via the AP was lower at 15 min and reached a plateau until 60 min (95.21 ± 26.21, 83.37 ± 25.42, 82.82 ± 30.37, 83.15 ± 31.69). FCA via the LP was slightly higher at 15 min and slightly lower at 60 min (106.7 ± 24.74, 115.3 ± 21.65, 114.6 ± 28.79, 111.7 ± 30.04). There were no significant differences in any of the three pathways when comparison was made between 0 and 60 min. The same tendency was not observed in FCA in the three complement pathways. To evaluate the C3 and C5 activity during HD, the levels of C3a desArg and C5a desArg in the samples at 0, 15, 30 and 60 min were measured (Figure 1C). Both C3a desArg and C5a desArg tended to be rapidly increased until 15 min, and then reached a plateau (C3a desArg; 101.1 ± 5.519, 115.0 ± 5.152, 112.9 ± 4.62, 115.4 ± 6.612, C5a desArg; 106.3 ± 11.97, 128.6 ± 13.32, 127.8 ± 10.92, 133.8 ± 16.96). Especially, C3a desArg at 60 min was significantly higher than at 0 min (P= 0.047). Finally, we assessed the correlation between the FCA of three pathways and the levels of C3a desArg and C5a desArg in the total of 20 serum samples that were collected from these five patients at all points (0, 15, 30 and 60 min) in the early phase of HD. The analysis showed a strong positive correlation between only the FCA via LP activity and C5a desArg (Table 2 and Figure 2).
Fig. 1.
The kinetics of the complement system in the early phase of HD. (A) The levels of the C5b-9 complex in plasma samples obtained from patients during HD at 0, 20, 60, 180 min and post-HD. Statistical significance was assessed between 0 and 60 min. ***P < 0.005. (B) The three functional complement pathway activities in serum samples obtained from patients during HD at 0, 15, 30 and 60 min. Statistical significance was assessed between 0 and 60 min. (C) The levels of C3a desArg and C5a desArg in serum samples were obtained from patients during HD at 0, 15, 30 and 60 min. *P < 0.05. Statistical significance was assessed between 0 and 60 min. All data are shown as the mean ± standard error. n = 5 for each group.
Table 2.
Spearman rank correlation test between three complement activities and C3a and C5a
| Classical pathway | Alternative pathway | Lectin pathway | |
|---|---|---|---|
| C3a | N.S. | N.S. | N.S. |
| C5a | N.S. | N.S. | r = 0.452, P= 0.0454 |
N.S., not significant.
Fig. 2.
The association between LP activity and C5a desArg during HD. The complement activity via the LP is positively correlated with C5a desArg in identical sample sets that were obtained from five patients at 0, 15, 30 and 60 min.
Discussion
Our previous studies suggest that the complement system has important roles in HD patients [4, 7, 11–13]. This is the first study investigating the kinetics of the FCA of the three complement pathways simultaneously during HD. Total levels of the C5b-9 complex at 60 min were significantly higher than those at 0 min, and the three FCAs each showed a different pattern. These results suggest that the total levels of the C5b-9 complex may serve as a good marker for the evaluation of biocompatibility and that the FCA of the three complement pathways occurs independently during HD.
This study also showed that the FCA via the LP is correlated with the levels of C5a desArg, which is the more stable metabolite of C5a during HD. The result suggests that the LP is the main contributor in the generation of C5a. The LP, the most recently discovered of the three complement pathways, is triggered through recognition of mannose-binding lectin, L-ficolin and H-ficolin to carbohydrate [14, 15]. The activated LP results in activated C3, which acts as a C5 convertase. Eventually, the C5b-9 complex is formed through the terminal pathway activated by the cleavage of C5. The cleavage of C5 generates C5a, which not only contributes to the formation of the C5b-9 complex, leading to cell damage, but it also activates macrophages, helper T cells and B cells. Thus, the action of C5a results in the release of numerous proinflammatory cytokines and chemokines, such as IL-6, IL-8 and tumor necrosis factor [16], and therefore, the blocking of C5 activation is the key to anti-inflammatory treatment. Recently, Mares et al. [17, 18] analyzed proteins adsorbed to dialyzers by two-dimensional electrophoresis and the levels of complement components involved in the LP in plasma samples from patients during HD. They showed that enriched L-ficolin was observed in the eluates of dialyzers and that there is a strong association between L-ficolin and C5a in the early phase of HD, suggesting that L-ficolin adsorption to the dialyzer initiates the LP of complement activation. Our future studies should focus on the mechanism of L-ficolin activated by binding to the dialyzer in the early phase of HD.
In conclusion, our results could help in the understanding of the FCA during HD and suggest that the LP is a potential target in avoiding the generation of C5a and C5b-9 complexes.
Acknowledgements
We would like to express our gratitude to all those who work in Kasukabe Kisen Hospital for giving us the opportunity to use their clinical data to complete this thesis.
Conflict of interest statement. None declared.
References
- 1.Cheung AK, Parker CJ, Wilcox LA, et al. Activation of complement by hemodialysis membranes: polyacrylonitrile binds more C3a than cuprophan. Kidney Int. 1990;37:1055–1059. doi: 10.1038/ki.1990.85. [DOI] [PubMed] [Google Scholar]
- 2.Cheung AK, Faezi-Jenkin B, Leypoldt JK. Effect of thrombosis on complement activation and neutrophil degranulation during in vitro hemodialysis. J Am Soc Nephrol. 1994;5:110–115. doi: 10.1681/ASN.V51110. [DOI] [PubMed] [Google Scholar]
- 3.Cheung AK, Parker CJ, Hohnholt M. Soluble complement receptor type 1 inhibits complement activation induced by hemodialysis membranes in vitro. Kidney Int. 1994;46:1680–1687. doi: 10.1038/ki.1994.468. [DOI] [PubMed] [Google Scholar]
- 4.Tamano M, Ohi H, Sudo S, et al. Quantitative polymorphism of complement receptor type 1 (CR1) in patients undergoing haemodialysis. Nephrol Dial Transplant. 2004;19:1467–1473. doi: 10.1093/ndt/gfh184. [DOI] [PubMed] [Google Scholar]
- 5.Vaisar T, Pennathur S, Green PS, et al. Shotgun proteomics implicates protease inhibition and complement activation in the anti-inflammatory properties of HDL. J Clin Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kourtzelis I, Markiewski MM, Doumas M, et al. Complement anaphylatoxin C5a contributes to hemodialysis-associated thrombosis. Blood. 2010;116:631–639. doi: 10.1182/blood-2010-01-264051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoshita H, Ohsawa I, Kusaba G, et al. Complement in patients receiving maintenance hemodialysis: functional screening and quantitative analysis. BMC Nephrol. 2010;11:34. doi: 10.1186/1471-2369-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huey R, Bloor CM, Kawahara MS, et al. Potentiation of the anaphylatoxins in vivo using an inhibitor of serum carboxypeptidase N (SCPN). I. Lethality and pathologic effects on pulmonary tissue. Am J Pathol. 1983;112:48–60. [PMC free article] [PubMed] [Google Scholar]
- 9.Kreutzer DL, McCormick JR, Despins A, et al. Characterization of the anaphylatoxin inactivator and chemotactic factor inactivator activities during cardiopulmonary bypass. J Exp Pathol. 1984;1:183–187. [PubMed] [Google Scholar]
- 10.Inoshita H, Matsushita M, Koide S, et al. A novel measurement method for activation of the lectin complement pathway via both mannose-binding lectin (MBL) and L-ficolin. J Immunol Methods. 2009;349:9–17. doi: 10.1016/j.jim.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Wakabayashi H, Ohi H, Tamano M, et al. Acquired loss of erythrocyte complement receptor type 1 in patients with diabetic nephropathy undergoing hemodialysis. Nephron Exp Nephrol. 2006;104:e89–e95. doi: 10.1159/000094547. [DOI] [PubMed] [Google Scholar]
- 12.Ohsawa I, Ohi H, Maruyama T, et al. Leukocytapheresis (LCAP) for the treatment of rheumatoid arthritis on a maintenance hemodialysis patient. Clin Nephrol. 2007;68:121–124. doi: 10.5414/cnp68121. [DOI] [PubMed] [Google Scholar]
- 13.Ishii M, Ohsawa I, Inoshita H, et al. Serum concentration of complement components of the lectin pathway in maintenance hemodialysis patients, and relatively higher levels of L-Ficolin and MASP-2 in mannose-binding lectin deficiency. Ther Apher Dial. 2011;15:441–447. doi: 10.1111/j.1744-9987.2011.00936.x. [DOI] [PubMed] [Google Scholar]
- 14.Matsushita M, Endo Y, Taira S, et al. A novel human serum lectin with collagen- and fibrinogen-like domains that functions as an opsonin. J Biol Chem. 1996;271:2448–2454. doi: 10.1074/jbc.271.5.2448. [DOI] [PubMed] [Google Scholar]
- 15.Matsushita M, Endo Y, Fujita T. Cutting edge: complement-activating complex of ficolin and mannose-binding lectin-associated serine protease. J Immunol. 2000;164:2281–2284. doi: 10.4049/jimmunol.164.5.2281. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Kimura Y, Fang C, et al. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood. 2007;110:228–236. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mares J, Thongboonkerd V, Tuma Z, et al. Specific adsorption of some complement activation proteins to polysulfone dialysis membranes during hemodialysis. Kidney Int. 2009;76:404–413. doi: 10.1038/ki.2009.138. [DOI] [PubMed] [Google Scholar]
- 18.Mares J, Richtrova P, Hricinova A, et al. Proteomic profiling of blood-dialyzer interactome reveals involvement of lectin complement pathway in hemodialysis-induced inflammatory response. Proteomics Clin Appl. 2010;4:829–838. doi: 10.1002/prca.201000031. [DOI] [PubMed] [Google Scholar]