Abstract
IgA nephropathy is the commonest cause of glomerulonephritis worldwide, and is usually a renal-limited disease. In rare cases, IgA nephropathy may also present with a pulmonary–renal syndrome in which pulmonary hemorrhage is a critical feature. Patients presenting with IgA nephropathy and pulmonary hemorrhage have high morbidity and are at high risk for mortality unless rapid immunosuppressive therapy is instituted. We present a case of IgA nephropathy complicated by pulmonary hemorrhage in which immunosuppressive therapy led to a good outcome, and review the literature on similar cases and the outcome of therapy.
Keywords: IgA nephropathy, immunosuppressive therapy, pulmonary hemorrhage
Background
IgA nephropathy is the commonest cause of glomerulonephritis worldwide. Patients with IgA nephropathy seldom show evidence of extra renal disease. It may present with asymptomatic microscopic or macroscopic hematuria, nephritic syndrome, nephrotic syndrome, with or without acute kidney injury. Pulmonary hemorrhage as a presentation of IgA nephropathy is distinctly rare, with only a handful of cases reported so far. These cases display disparate clinical and prognostic features. In this report, we describe a 44-year-old man who presented to the hospital with severe pulmonary hemorrhage and hematuria. His renal biopsy showed IgA nephropathy. This patient had an excellent response to immunosuppressive therapy.
Case report
A 44-year-old Bangladeshi man was admitted to hospital with a history of cough, hemoptysis and dyspnea of 1-day duration. He had previously been healthy until he developed coryza symptoms about 1 week prior to presentation. There was no history of chronic cough, weight loss, night sweats or recent travel outside the USA. The patient had smoked half a pack of cigarettes per day for 15 years but denied a history of exposure to any environmental or industrial exposure to any agents with known pulmonary toxicity. He denied passage of cola-colored urine or gross hematuria. His medications were bismuth subsalicylate for gastroesophageal reflux disease and daily multivitamins.
On examination, his vital signs showed a blood pressure of 143/81 mmHg, pulse of 90 beats/min, temperature of 37°C and respiratory rate of 20. Cardiac examination was normal, but diffuse pulmonary rales were heard in both lung fields on chest auscultation. The rest of his physical examination was normal. The hematocrit was 44% and hemoglobin was 12 g/dL. The white cell count was 4.5 k/mm3, platelet of 196 k/mm3 and a prothrombin time with a normalized international ratio of 0.96. His blood urea nitrogen was 19 mg/dL (6.78 mmol/L) and serum creatinine was 0.96 mg/dL (84.48 mmol/L). Urinalysis was significant for 3+ blood, and >50 rbc/hpf with dysmorphic red cells and 2+ protein. Serologies for antineutrophil cytoplasmic antibodies (ANCA), antinuclear antibodies (ANA), rheumatoid factor, antiglomerular basement membrane antibody (anti-GBM), hepatitis B and C and viruses influenza A and B, adenovirus, enteroviruses, herpes simples I and II, cytomegalovirus, Coxiella burnetti, Mycoplasma pneumonia were all negative. A tuberculin skin test was negative. Sputum cultures for bacterial, fungal and mycobacteria were negative.
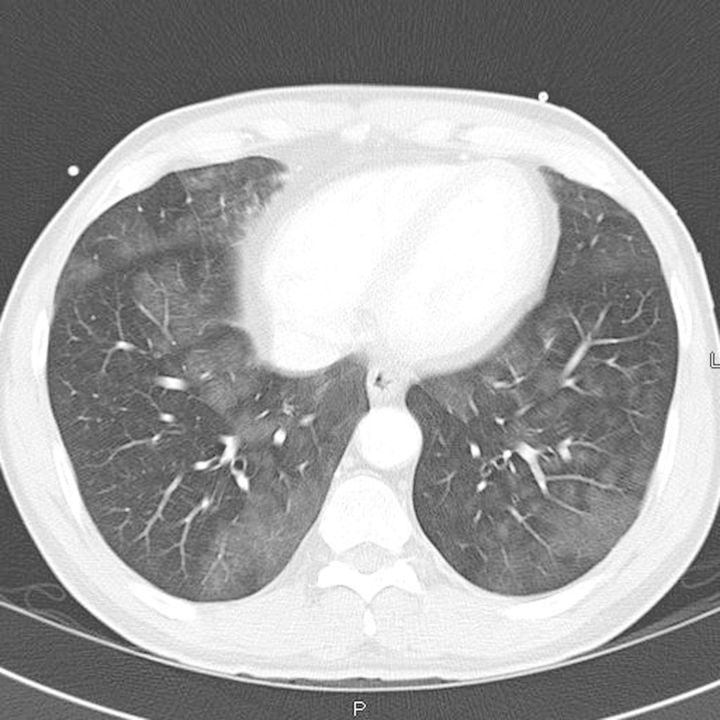
A CT scan of the chest with contrast (Figure 1) showed diffuse alveolar bilateral patchy infiltrates, with some emphysematous changes. The lung infiltrates had a ground glass appearance. The patient was admitted to the intensive care unit for closer monitoring, and started on empiric antibiotic therapy for possible pneumonia to exclude Goodpasture's syndrome in view of hemoptysis and hematuria. Further laboratory tests including ANA, anti-GBM IgG, complements, viral hepatitis screen, ESR, CRP, VWF, ANCA, sputum AFB ×3 and coagulation panel, were all within normal limits. An upper gastrointestinal endoscopy to investigate hemocult-positive stool did not reveal any active evidence of gastro-intestinal bleeding.
Fig. 1.
CT scan of chest with contrast, showing bilateral ground glass infiltrates
He continued to have massive (>500 mL/day) hemoptysis and hematuria with subsequent decline in the hematocrit necessitating transfusion of a total of 3 units of packed red cells. He underwent bronchoscopy and bronchoalveolar lavage which showed the presence of hemosiderin-laden macrophages and bloody exudate. To establish the cause of the pulmonary–renal presentation of this patient, a kidney biopsy was done.
Renal biopsy (Figure 2) showed mesangial proliferative glomerulonephritis with IgA deposition. The patient was started on intravenous cyclophosphamide 1 g and prednisone 60 mg daily. Over a 4-day period, his hemoptysis gradually resolved, and hemoglobin and hematocrit stabilized at 10.9 gm/dL and 32.1%, respectively. The patient was then started on intravenous cyclophosphamide infusion 1 gm monthly with daily oral prednisone tapered by 10 mg every month. He completed six cycles of monthly cyclophosphamide. Prednisone was tapered off over an additional 2-month interval. There has been no recurrence of hemoptysis and the patient has since stopped smoking (Table 1).
Fig. 2.
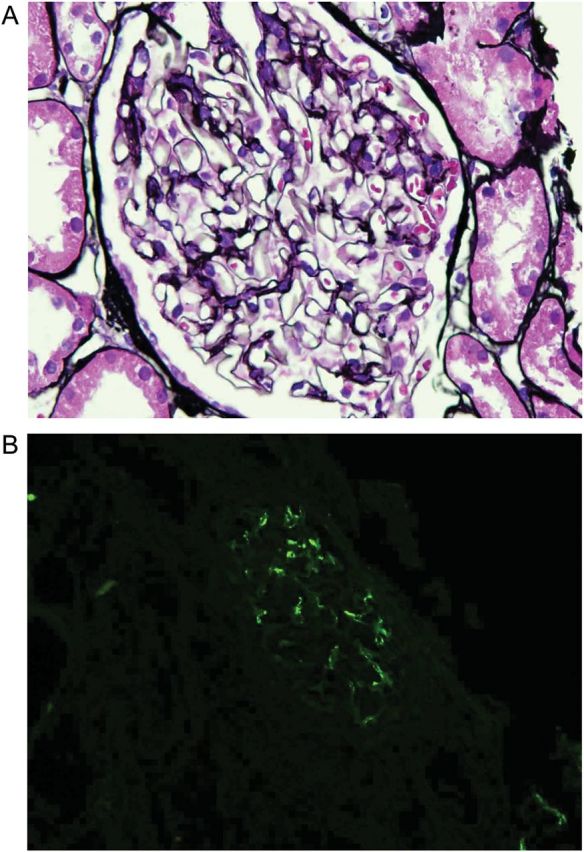
(A) Light microscopy of glomerulus with mild increase in mesangial matrix, ×600 Jones Methenamine. (B) Immunofluorescence of renal biopsy showing mesangial IgA deposition.
Table 1.
Laboratory values
| Variable | Admission | Day 2 | Day 3 | Day 4 | Discharge |
|---|---|---|---|---|---|
| White cell count (K/mm3) | 4500 | 5800 | 15 600 | 13 800 | 11 000 |
| Hemoglobin (g/dL) | 14.2 | 11.6 | 11.4 | 11.1 | 10.9 |
| Hematocrit (%) | 44.3 | 35.9 | 34.4 | 33.1 | 32.1 |
| Platelets (K/mm3) | 196 | 143 | 164 | 180 | 197 |
| Prothrombin time | 0.96 | 1.16 | 1.12 | — | 1.13 |
| Sedimentation rate | 4 | — | — | 2 | — |
| ANA | — | Negative | — | — | — |
| ANCA | — | <4 | — | — | — |
| Anti-GBM | — | 0 | — | — | — |
| Sodium (mmol/L) | 141 | 140 | 140 | 137 | 139 |
| Potassium (mmol/L) | 3.6 | 4.1 | 3.9 | 3.8 | 3.8 |
| Urea nitrogen (mmol/L) | 6.78 | 5.36 | 6.1 | 6.4 | 7.14 |
| Creatinine (umol/L) | 84.9 | 70.7 | 70.7 | 61.9 | 79.6 |
| Hepatitis B | — | Negative | — | — | — |
| Hepatitis C | — | Negative | — | — | — |
Discussion
While IgA nephropathy is the commonest cause of primary glomerulonephritis worldwide, pulmonary hemorrhage occurring in patients with IgA nephropathy is distinctly rare, and only 13 cases have so far been reported [1–9]. Our patient's clinical presentation was comparable with the other 13 cases of IgA nephropathy with pulmonary hemorrhage reported previously in the literature, as shown in Table 2. These cases differ in terms of patient survival, renal function, use of immunosuppressive therapy and whether or not plasmapheresis was used. Interestingly, until recently, most of the reports have been in adults, but a recent report shows that this clinical entity can also occur in children, in a form distinct from Henoch–Schönlein purpura [2].
Table 2.
Case reports of other cases of pulmonary hemorrhage in patients with IgA nephropathy
| Case reports | Age, sex at Dx | Renal function at diagnosis | Pulmonary hemorrhage diagnosis | Treatment received/duration | Outcome |
|---|---|---|---|---|---|
| 1. Medcalf et al. [1] | 20 M | Hematuria | Clinical | No treatment received |
|
| 2. Medcalf et al. [1] | 67 M | ARF | Clinical | Methylprednisone/cyclophosphamide of unknown duration |
|
| 3. Medcalf et al. [1] | 75 M | ESRD | Clinical | Methylprednisone/cyclophosphamide | Death |
| 4. Poyyapakkam Srivaths et al. [2] | 14 M | ARF Hematuria |
|
|
|
| 5. Devanand Anatham et al. [3] | 20 M | ESRD |
|
|
|
| 6. Bekele Afessa et al. [4] | 66 M | ARF Hematuria |
Clinical |
|
|
| 7. Mac-Moune Lai et al. [5] | 45 M | Hematuria |
|
Methylprednisone ×1 week | Death |
| 8. Mac-Moune Lai et al. [5] | 29 F | CRF |
|
None | Death |
| 9. Mac-Moune Lai et al. [5] | 43 F | Normal |
|
None | Death |
| 10. Yuichiro Endo et al. [6] | 59 M | Normal | Bronchoalveolar lavage |
Methylprednisone and cyclosporine for unknown duration | Unknown |
| 11. Chatchai Kreepala MD et al. [7] | 53 M | ARF Hematuria |
Clinical | Empiric antibiotics for systemic infection | Complete resolution of hemoptysis, hematuria and renal failure |
| 12. Fung and Churchill et al. [8] | 36 M | ARF Hematuria |
Bronchoalveolar lavage | Methylprednisone and cyclophosphamide for unknown duration | Complete resolution of hemoptysis, hematuria and renal failure |
| 13. Travis et al. [9] | Unknown | Unknown | Unknown | Unknown |
When pulmonary hemorrhage occurs with IgA nephropathy, other entities that cause pulmonary–renal syndrome such as vasculitides (especially Henoch–Schönlein purpura), Goodpasture's syndrome, systemic lupus erythematosus as well as co-existing pulmonary tuberculosis must be excluded.
Of the treated patients, four received intravenous methylprenisolone and oral cyclophosphamide [1–6, 8]. Two patients received plasmapheresis [3, 4]. The precise duration of immunosuppressive therapy remains to be determined. While Medcalf et al. [1] suggest that immunosuppressive therapy is of no benefit, our case and others [1–6, 8] suggest that immunosuppressive therapy with or without plasmapheresis may be life-saving. Our patient received a 6-month course of cyclophosphamide and daily steroids which were tapered off after another 2 months. Renal manifestations and prognosis showed significant heterogeneity in reported cases, ranging from hematuria to acute kidney injury and irreversible chronic kidney disease requiring hemodialysis. Three patients in the series became dialysis dependent [1–3]. While four patients died [1, 7], others survived with therapy.
Though the precise pathogenesis of pulmonary hemorrhage in IgA nephropathy is unknown, three putative mechanisms have been suggested. Firstly, pulmonary hemorrhage may be a non-specific alveolar capillary response to injury triggered by the IgA immune response to associated (synpharyngitic) upper respiratory tract infection. Consistent with this suggestion is the report of a patient with IgA nephropathy on renal replacement therapy who developed recurrent bloody peritoneal dialysate during respiratory tract infection [10]. Secondly, pulmonary hemorrhage in IgA nephropathy may be due to alveolar capillary damage due to anti-glomerular basement membrane antibody (anti-GBM) attack. Interestingly, Fervenza et al. recently described a patient who developed anti-GBM disease due to IGA antibodies in a patient with Goodpasture's syndrome [11]. However, no such circulating antibodies have consistently been shown, and IgA deposition in lung biopsy on immunofluorescence is an inconsistent finding [1]. Thirdly, it has also been suggested that pulmonary hemorrhage occurring with IgA is an immune-complex mediated capillaritis [3]. According to this hypothesis, the pulmonary hemorrhage represents a pulmonary manifestation of immune complex activity. Consistent with this notion, some patients with IgAN have shown IgA and C3 depositions in sites distant from the kidney such as skin [12].
When our patient presented with pulmonary hemorrhage and hematuria, other causes of pulmonary renal syndrome were diligently excluded and pulmonary hemorrhage was confirmed with bronchoscopy and bronchial lavage fluid cytology showing hemorrhagic fluid with pigment laden macrophages. In other reported cases, pulmonary capilliritis [2, 3, 5] characterized by the presence of patchy areas of red blood cells and focal fibrin within airspaces and multiple small foci of organizing pneumonia pattern (Masson bodies) were observed. It is interesting to note that none of the patients that received lung biopsy showed deposition of IgA on the alveolar walls. Pulmonary histology in fatal cases involving Henoch–Schönlein purpura has shown a leucocytoclastic vasculitis and IgA deposition along alveolar septa in areas of hemorrhage [13, 14]. Lung biopsy was not done in our case and thus the exact lung pathology could not be confirmed. The patient's renal biopsy was typical for IgA nephropathy. The absence of rash, lack of joint and abdominal involvement make Henoch–Schönlein vasculitis highly unlikely.
Other lung diseases associated with IgA nephropathy include idiopathic pulmonary hemosiderosis (IPH) [15, 16], IgA immune complex-mediated pneumonitis [17], pulmonary tuberculosis [18] and bronchiolitis obliterans [19]. Though pulmonary hemorrhage can be a rare manifestation of IgA nephropathy, if it is not recognized, it can run a rapidly fatal course. Prompt recognition and appropriate immunosuppressive therapy leads to a good outcome [20]. Our case highlights the fact that IgA nephropathy should be considered in the differential diagnosis of pulmonary hemorrhage. This presentation may also be a reflection of Floege's suggestion that IgA nephropathy is a dynamic disease and the defects driving this disease may reside outside the kidney [21].
Conflict of interest statement. None declared.
References
- 1.Medcalf JF, Brownjohn AM, Turney JH. Pulmonary haemorrhage in association with IgA nephropathy. Nephrol Dial Transplant. 1996;11:1148–1149. [PubMed] [Google Scholar]
- 2.Srivaths P, Dishop MK, Elidemir O, et al. IgA nephropathy presenting with renal failure and pulmonary hemorrhage. Pediatr Nephrol. 2010;25:535–538. doi: 10.1007/s00467-009-1382-8. [DOI] [PubMed] [Google Scholar]
- 3.Anantham D, Chan KP, Chuah KL, et al. Pulmonary capillaritis in IgA nephropathy. South Med J. 2007;100:605–607. doi: 10.1097/SMJ.0b013e31804853dc. [DOI] [PubMed] [Google Scholar]
- 4.Afessa B, Cowart RG, Koenig SM. Alveolar hemorrhage in IgA nephropathy treated with plasmapheresis. South Med J. 1997;90:237–239. doi: 10.1097/00007611-199702000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Lai FM, Li EK, Suen MW, et al. Pulmonary hemorrhage. A fatal manifestation in IgA nephropathy. Arch Pathol Lab Med. 1994;118:542–546. [PubMed] [Google Scholar]
- 6.Endo Y, Minato H, Taki R, et al. Myeloperoxidase-antineutrophil cytoplasmic antibody-negative microscopic polyangiitis with pulmonary haemorrhage and IgA nephropathy. Case Rep Dermatol. 2011;3:22–27. doi: 10.1159/000324422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreepala C, Changsirikulchai S, Chalermsanyakorn P. Pulmonary hemorrhage with acute renal injury in a patient with IgA nephropathy. J Med Assoc Thai. 2009;92(Suppl 3):S80–S84. [PubMed] [Google Scholar]
- 8.Fung M, Churchill D, Alexopoulou I, et al. IgA nephropathy and pulmonary hemorrhage in an adult. Am J Nephrol. 2001;21:318–322. doi: 10.1159/000046268. [DOI] [PubMed] [Google Scholar]
- 9.Travis WD, Colby TV, Lombard C, et al. A clinicopathologic study of 34 cases of diffuse pulmonary hemorrhage with lung biopsy confirmation. Am J Surg Pathol. 1990;14:1112–1125. doi: 10.1097/00000478-199012000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Rambausek M, Ritz E, Waldherr R. Recurrent bloody dialysate during upper respiratory tract infection in mesangial IgA glomerulonephritis. Nephron. 1987;46:213. doi: 10.1159/000184346. [DOI] [PubMed] [Google Scholar]
- 11.Borza DB, Chedid MF, Colon S, et al. Recurrent Goodpasture's disease secondary to a monoclonal IgA1-kappa antibody autoreactive with the alpha1/alpha2 chains of type IV collagen. Am J Kidney Dis. 2005;45:397–406. doi: 10.1053/j.ajkd.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 12.Faille-Kuyper EH, Kater L, Kuijten RH, et al. Occurrence of vascular IgA deposits in clinically normal skin of patients with renal disease. Kidney Int. 1976;9:424–429. doi: 10.1038/ki.1976.52. [DOI] [PubMed] [Google Scholar]
- 13.Kathuria S, Cheifec G. Fatal pulmonary Henoch–Schonlein syndrome. Chest. 1982;82:654–656. doi: 10.1378/chest.82.5.654. [DOI] [PubMed] [Google Scholar]
- 14.Payton CD, Allison ME, Boulton-Jones JM. Henoch Schonlein purpura presenting with pulmonary haemorrhage. Scott Med J. 1987;32:26–27. doi: 10.1177/003693308703200113. [DOI] [PubMed] [Google Scholar]
- 15.Kjellman B, Elinder G, Garwicz S, et al. Idiopathic pulmonary haemosiderosis in Swedish children. Acta Paediatr Scand. 1984;73:584–588. doi: 10.1111/j.1651-2227.1984.tb09978.x. [DOI] [PubMed] [Google Scholar]
- 16.Ohga S, Takahashi K, Miyazaki S, et al. Idiopathic pulmonary haemosiderosis in Japan: 39 possible cases from a survey questionnaire. Eur J Pediatr. 1995;154:994–995. doi: 10.1007/BF01958645. [DOI] [PubMed] [Google Scholar]
- 17.Harland RW, Becker CG, Brandes JC, et al. Immunoglobulin A (IgA) immune complex pneumonitis in a patient with IgA nephropathy. Ann Intern Med. 1992;116:220–222. doi: 10.7326/0003-4819-116-3-220. [DOI] [PubMed] [Google Scholar]
- 18.Cohen AJ, Rosenstein ED. IgA nephropathy associated with disseminated tuberculosis. Arch Intern Med. 1985;145:554–556. [PubMed] [Google Scholar]
- 19.Hernandez JI, Gomez-Roman J, Rodrigo E, et al. Bronchiolitis obliterans and IgA nephropathy. A new cause of pulmonary-renal syndrome. Am J Respir Crit Care Med. 1997;156:665–668. doi: 10.1164/ajrccm.156.2.9610043. [DOI] [PubMed] [Google Scholar]
- 20.Floege J, Eitner F. Current therapy for IgA nephropathy. J Am Soc Nephrol. 2012;22:1785–1794. doi: 10.1681/ASN.2011030221. [DOI] [PubMed] [Google Scholar]
- 21.Floege J. The pathogenesis of IgA nephropathy: what is new and how does it change therapeutic approaches? Am J Kid Dis. 2011;58:992–1004. doi: 10.1053/j.ajkd.2011.05.033. [DOI] [PubMed] [Google Scholar]