Abstract
Systemic mastocytosis (SM) is characterized by infiltration of neoplastic mast cells in one or more organ systems. SM in association with plasma cell dyscrasia is very rare. We report a first case of indolent SM (ISM) associated with light chain deposition disease (LCDD) in a kidney biopsy from a 59-year-old female presenting with skin rash, elevated serum creatinine, hematuria and mild proteinuria. Subsequent workup demonstrated IgG kappa monoclonal protein in serum and urine. A bone marrow biopsy revealed neoplastic mast cells involving bone marrow without evidence of clonal myeloid or lymphoid proliferation. Kidney biopsy demonstrated modest mesangial expansion detected by light microscopy and unequivocal evidence of monoclonal kappa light chain deposition within glomerular capillaries, tubular basement membranes and vascular walls detected by immunofluorescence and/or electron microscopy, along with equivocal evidence of light chain cast nephropathy. Despite treatment with bortezomib and dexamethasone, her renal function was progressively declined over the next 6 months. This case is a reminder that SM can coincide with LCDD, which requires clinical suspicion and multimodality workup on a kidney biopsy including immunofluorescence and electron microscopy to reach the correct diagnosis.
Keywords: systemic mastocytosis, light chain deposition disease
Introduction
Mastocytosis is a rare disease characterized by neoplastic proliferation of mast cells with a spectrum of clinical and morphological manifestation. Mastocytosis can be limited to skin [cutaneous mastocytosis (CM)] or it can also involve bone marrow and/or other extracutaneous organs [systemic mastocytosis (SM)] with or without skin involvement [1]. SM with concurrent plasma cell dyscrasia is very rare. So far, only a handful of cases of SM with plasma cell myeloma have been reported, which comprises the very rare subsets of reported SM with associated clonal hematological non-mast cell lineage disease (SM-AHNMD) [2–4]. However, there has been no report of SM associated with paraprotein-related kidney disease.
Here, we report a first case of light chain deposition disease (LCDD) diagnosed on a kidney biopsy in a patient with indolent systemic mastocytosis (ISM) and serum and urine monoclonal kappa light chains but without evidence of other neoplasms of myeloid or lymphoid lineage.
Case report
Clinical history and initial laboratory data
A 59-year-old white female presented with an incidentally discovered increased serum creatinine of 2.7 mg/dL (236 μmol/L) from a baseline of 0.8 mg/dL (71 μmol/L) measured 5 years ago. Physical examination showed mild hypertension (145/70 mmHg). Cardiac and pulmonary examinations were unremarkable and no splenomegaly, hepatomegaly or peripheral lymphadenopathy were noted. Skin examination revealed a diffuse reddish-brown macular rash on her upper torso and legs, which had been unchanged for years. Urinalysis showed 3+ blood, no albumin, 2 white blood cells/high power field (HPF) and 1 red blood cell/HPF. Subsequent workup demonstrated a positive serum protein electrophoresis with 1.4 g/dL of a monoclonal protein identified as IgG kappa on immunofixation. The kappa/lambda-free light chain ratio was elevated at 48.97 (normal 0.26–1.65). Urine protein electrophoresis and immunofixation confirmed monoclonal-free kappa light chain quantified at 333 mg/24 h (30% of total proteins measured at 1092 mg/24 h). Past medical history was significant for SM diagnosed 7 years earlier by recurrent urticaria and skin rash, and a skin biopsy showed increased mast cells. A bone marrow biopsy at the time of diagnosis showed multiple paratrabecular and perivascular mast cell aggregates comprising 30–50% of the marrow space, as well as many fusiform mast cells. Both CD2- and CD25-positive mast cells (detected by immunohistochemistry) and the D816V kit mutation (detected by polymerase chain reaction on a paraffin block) were present, confirming SM diagnosis. Serum tryptase was elevated at 97.2 ng/mL (normal <1 ng/mL). She was classified as having ISM. Her symptoms were stable and managed with fexofenadine, cromolyn sulfate, aspirin and diphenhydramine over the next few years. A repeat bone marrow biopsy confirmed the diagnosis of SM, but there was no evidence of a myeloid/myelomonocytic neoplasm, plasma cell neoplasm or other lymphoproliferative disorders. She then underwent a kidney biopsy to determine the cause of renal insufficiency.
Kidney biopsy findings
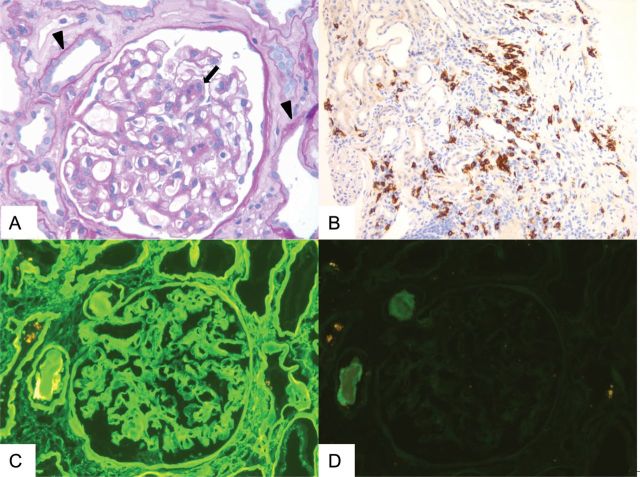
Light microscopic examination showed glomeruli with mild segmental mesangial expansion due to mildly increased cellularity and matrix but without overt mesangial nodules (Figure 1A). Glomeruli were otherwise unremarkable. There was also widespread acute tubular injury, characterized by flattening of tubular epithelial cells and loss of brush borders (Figure 1A). Rare intratubular casts showed fractured and/or metachromatic appearance. In addition, a single intratubular cast was surrounded by epithelioid cells, suspicious of syncytial cell reaction. The interstitium showed patchy focally dense mononuclear inflammatory cell infiltrates in the background of at least mild interstitial fibrosis and tubular atrophy. Congo-red stain was negative for amyloid deposition. Immunoperoxidase stain for CD117 (c-kit), a marker for mast cells [5], showed scattered-positive cells, some in small clusters , notably with <15 cells, in close proximity to areas with dense mononuclear inflammatory cell infiltrates (Figure 1B).
Fig. 1.
Evaluation of renal biopsy by light and immunofluorescence microscopy and immunohistochemistry. (A) Modest segmental mesangial expansion of a glomerulus due to increased cellularity and matrix (allow) and acute tubular injury characterized by flattening of tubular epithelial cells and loss of brush borders (arrow head). (Periodic acid-Schiff stain 40×). (B) Immunoperoxidase stain for CD117/c-kit highlighting the scattered mast cells with focal small aggregates (20×). (C and D) Bright linear staining of glomerular basement membranes, Bowman's capsule and tubular basement membranes for kappa light chains (C) but not lambda light chains (D) detected by immunofluorescence microscopy (40×).
Immunofluorescence microscopy demonstrated bright (3–4+; on a scale of 0–4) linear staining of glomeruli, Bowman's capsules and tubular basement membranes for kappa light chain (Figure 1C). The walls of some arteries and arterioles similarly showed diffuse bright staining for kappa light chains in an extracellular matrix pattern. There was no significant staining of glomeruli, vessels, tubular basement membranes or interstitium for lambda light chains (Figure 1D) or other immunoreactants. Some tubular casts with fractured appearance were brightly positive for kappa light chains but not for lambda light chains.
On ultrastructural examination, there were diffuse finely granular, ‘powdery’, electron dense deposits with linear arrangement within the glomerular basement membranes (predominantly localized in intramembranous and focally towards the subendothelial aspect of the glomerular basement membranes) and tubular basement membranes (Figure 2). We did not observe any obvious evidence of light chain proximal tubulopathy by light, immunofluorescence or electron microscopy.
Fig. 2.
Electron microscopic findings. Finely granular (‘powdery’) electron dense deposits with linear distribution along the glomerular capillary walls (arrow head) (magnification × 15 200).
Based on the characteristic linear staining of glomerular and tubular basement membranes and vascular walls for kappa light chain without lambda light chain detected by immunofluorescence microscopy and ‘powdery’ electron dense deposits along the glomerular and tubular basement membranes detected by electron microscopy, a diagnosis of monoclonal kappa LCDD was established. The presence of rare atypical intratubular casts, which brightly stained for kappa light chain, but not for lambda light chain by immunofluorescence microscopy, together with the presence of widespread acute tubular injury and rare possible syncytial cell reaction was interpreted highly suggestive of a concomitant light chain cast nephropathy (LCCN).
Clinical follow-up
After her kidney biopsy results returned, the patient was started with bortezomib and dexamethasone. Urinary protein electrophoresis showed an initial decrease in M-spike from 333 mg/24 h (30% of total proteins) pre-treatment to 107 mg/24 h (6% of total proteins) after the first cycle of above chemotherapy and 81 mg/24 h (4.6% of total proteins) after the second cycle. Kappa/lambda free light chain ratios also decreased from 49 pre-treatment to 28 and 10 after the first and second cycles, respectively. However, serum M-spike increased after the third cycle to 379 mg/24 h (9% of total proteins) and serum creatinine, which had been 2.3 mg/mL (203 μmol/L) before her first cycle of treatment continued to rise to 4.6 mg/dL (407 μmol/L).
Discussion
SM-associated kidney diseases have been rarely reported in the literature, including a case of membranous nephropathy [6] and two cases of mesangial proliferative glomerulopathy [7, 8]. Of note, one of these patients with mesangial proliferative glomerulopathy showed serum and urine IgG-kappa monoclonal paraprotein, raising the possibility that the observed lesions represented a paraprotein-related process [8]. To our knowledge, the present case is the first report of SM associated with biopsy-proven paraprotein-related kidney disease. LCDD is characterized by the deposition of monoclonal kappa or lambda light chains with structural abnormalities [9] in various organs [10–14]. The disease is frequently associated with plasma cell myeloma or other lymphoproliferative diseases. One of the unique features of LCDD in this biopsy is the absence of typical nodular glomerulosclerosis, which closely resembles Kimmelstiel–Wilson nodules of diabetic nephropathy, suggesting that the disease is in the early stage. The present biopsy also demonstrated some features suggestive of concurrent LCCN. It is well recognized that LCDD can be associated with other paraprotein-related kidney disease, such as LCCN or AL-type amyloidosis [15, 16]. In the present case, congo-red stain was negative, excluding the latter possibility.
Immunoperoxidase stain for mast cells (CD117/c-kit) showed scattered, focally aggregated mast cells in renal parenchyma. However, due to the extremely limited literature on kidney involvement in SM and expected mast cell density in nonspecific inflammatory infiltrates in areas of tubulointerstitial fibrosis [17, 18], it is difficult to unequivocally interpret this finding as renal involvement by SM. The demonstration of c-KIT D816V mutation or aberrant expression of CD2 and/or CD25 on mast cells, which could serve as evidence of neoplastic mast cell infiltration, was not investigated in the present kidney biopsy.
Although there was no evidence of detectable plasma cell or other lymphoid malignancies in our case, the presence of LCDD in the renal biopsy along with paraprotein detected in the serum and urine suggested the presence of an undetected monoclonal lymphoplasmacytic population in this patient. In ∼40% of LCDD, a definitive diagnosis of multiple myeloma cannot be established [10, 15]. However, dysproteinemia can be documented in most patients with LCDD or light chain amyloidosis [10], suggestive of an underlying plasma cell dyscrasia. Despite a low tumor burden in such patients, target organ injury may develop if untreated.
SM in association with lymphoproliferative disorders is well documented and it comprises the very small subsets of SM-AHNMD [2–4, 19]. However, the pathophysiologic link between SM and lymphoproliferative disorders remains unclear. In cases of SM associated with lymphoproliferative disorders, including plasma cell neoplasm, D816V C-KIT mutation was detected in the neoplastic mast cells but not in the neoplastic lymphoid population, suggesting that SM and coexisting lymphoid neoplasm were clonally separate [19–21]. Interestingly, there are several lines of in vitro evidence demonstrating the ability of neoplastic mast cells to support the growth of associated neoplastic lymphoplasmacytic population [22, 23]. Together with a chronological sequence of LCDD in our case, which developed 7 years after the diagnosis of SM, we favor that LCDD was related to, and perhaps triggered by SM in this case, although we cannot rule out that these were two independent concurrent processes.
Conflict of interest statement
None declared.
References
- 1.Horny HP, Metcalfe DD, Bennett JM, et al. WHO classification of tumors of the haematopoietic and lymphoid tissues. Lyon, France: LARC Press; 2008. Mastocytosis; pp. 54–63. [Google Scholar]
- 2.Motwani P, Kocoglu M, Lorsbach RB. Systemic mastocytosis in association with plasma cell dyscrasias: report of a case and review of the literature. Leuk Res. 2009;33:856–859. doi: 10.1016/j.leukres.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Filanovsky K, Lev S, Haran M, et al. Systemic mastocytosis associated with smoldering multiple myeloma: an unexpected diagnosis in a patient with a rash. Leuk Lymphoma. 2010;51:1152–1154. doi: 10.3109/10428191003743452. [DOI] [PubMed] [Google Scholar]
- 4.Horny HP, Sotlar K, Sperr WR, et al. Systemic mastocytosis with associated clonal haematological non-mast cell lineage diseases: a histopathological challenge. J Clin Pathol. 2004;57:604–608. doi: 10.1136/jcp.2003.014860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arber DA, Tamayo R, Weiss LM. Paraffin section detection of the c-kit gene product (CD117) in human tissues: value in the diagnosis of mast cell disorders. Hum Pathol. 1998;29:498–504. doi: 10.1016/s0046-8177(98)90066-1. [DOI] [PubMed] [Google Scholar]
- 6.Lal SM, Brooks CS, Luger AM, et al. Systemic mastocytosis associated with membranous nephropathy and peripheral neuropathy. Am J Kidney Dis. 1988;12:538–543. doi: 10.1016/s0272-6386(88)80108-2. [DOI] [PubMed] [Google Scholar]
- 7.Poliantseva LR, Klepikov PV, Varshavskii VA. Nephrotic syndrome in a patient with systemic mastocytosis. Klin Med (Mosk) 1983;61:115–119. [PubMed] [Google Scholar]
- 8.Diamantidis MD, Myrou AD, Kaiafa GD, et al. Aggressive systemic mastocytosis associated with mesangioproliferative glomerulonephritis. Acta Haematol. 2011;125:153–159. doi: 10.1159/000322286. [DOI] [PubMed] [Google Scholar]
- 9.Decourt C, Cogne M, Rocca A. Structural peculiarities of a truncated V kappa III immunoglobulin light chain in myeloma with light chain deposition disease. Clin Exp Immunol. 1996;106:357–361. doi: 10.1046/j.1365-2249.1996.d01-841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasr SH, Valeri AM, Cornell LD, et al. Renal monoclonal immunoglobulin deposition disease: a report of 64 patients from a single institution. Clin J Am Soc Nephrol. 2012;7:231–239. doi: 10.2215/CJN.08640811. [DOI] [PubMed] [Google Scholar]
- 11.Pozzi C, D'Amico M, Fogazzi GB, et al. Light chain deposition disease with renal involvement: clinical characteristics and prognostic factors. Am J Kidney Dis. 2003;42:1154–1163. doi: 10.1053/j.ajkd.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 12.Toor AA, Ramdane BA, Joseph J, et al. Cardiac nonamyloidotic immunoglobulin deposition disease. Mod Pathol. 2006;19:233–237. doi: 10.1038/modpathol.3800524. [DOI] [PubMed] [Google Scholar]
- 13.Girelli CM, Lodi G, Rocca F. Kappa light chain deposition disease of the liver. Eur J Gastroenterol Hepatol. 1998;10:429–430. doi: 10.1097/00042737-199805000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Bhargava P, Rushin JM, Rusnock EJ, et al. Pulmonary light chain deposition disease: report of five cases and review of the literature. Am J Surg Pathol. 2007;31:267–276. doi: 10.1097/01.pas.0000213358.18380.d2. [DOI] [PubMed] [Google Scholar]
- 15.Lin J, Markowitz GS, Valeri AM, et al. Renal monoclonal immunoglobulin deposition disease: the disease spectrum. J Am Soc Nephrol. 2001;12:1482–1492. doi: 10.1681/ASN.V1271482. [DOI] [PubMed] [Google Scholar]
- 16.Lorenz EC, Sethi S, Poshusta TL, et al. Renal failure due to combined cast nephropathy, amyloidosis and light-chain deposition disease. Nephrol Dial Transplant. 2010;25:1340–1343. doi: 10.1093/ndt/gfp735. [DOI] [PubMed] [Google Scholar]
- 17.Moore AE, Johnston WH, Hever A, et al. Systemic mastocytosis presenting with acute oliguric renal failure: report of a case and review of the literature. Int Urol Nephrol. 2010;44:639–642. doi: 10.1007/s11255-010-9878-5. [DOI] [PubMed] [Google Scholar]
- 18.Hiromura K, Kurosawa M, Yano S, et al. Tubulointerstitial mast cell infiltration in glomerulonephritis. Am J Kidney Dis. 1998;32:593–599. doi: 10.1016/s0272-6386(98)70022-8. [DOI] [PubMed] [Google Scholar]
- 19.Horny HP, Sotlar K, Stellmacher F, et al. An unusual case of systemic mastocytosis associated with chronic lymphocytic leukaemia (SM-CLL) J Clin Pathol. 2006;59:264–268. doi: 10.1136/jcp.2005.026989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim Y, Weiss LM, Chen YY, et al. Distinct clonal origins of systemic mastocytosis and associated B-cell lymphoma. Leuk Res. 2007;31:1749–1754. doi: 10.1016/j.leukres.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Sotlar K, Colak S, Bache A, et al. Variable presence of KITD816V in clonal haematological non-mast cell lineage diseases associated with systemic mastocytosis (SM-AHNMD) J Pathol. 2010;220:586–595. doi: 10.1002/path.2677. [DOI] [PubMed] [Google Scholar]
- 22.Tournilhac O, Santos DD, Xu L, et al. Mast cells in Waldenstrom's macroglobulinemia support lymphoplasmacytic cell growth through CD154/CD40 signaling. Ann Oncol. 2006;17:1275–1282. doi: 10.1093/annonc/mdl109. [DOI] [PubMed] [Google Scholar]
- 23.Ho AW, Hatjiharissi E, Ciccarelli BT, et al. CD27-CD70 interactions in the pathogenesis of Waldenstrom macroglobulinemia. Blood. 2008;112:4683–4689. doi: 10.1182/blood-2007-04-084525. [DOI] [PMC free article] [PubMed] [Google Scholar]