Abstract
We describe a case of filariasis presenting with severe chyluria and nephrotic-range proteinuria. There were no obvious findings of glomerulonephritis in the renal biopsy. Technetium-99m-human serum albumin (Tc-99m-HSA) lymphoscintigraphy revealed the presence of communications between lymphatic channels and the urinary tract. Ezetimibe (10 mg/day) was administered during hospitalization. Chyluria was decreased within a few days following the administration of ezetimibe. Moreover, a remission was obtained from nephrotic-range proteinuria. Tc-99m-HSA lymphoscintigraphy showed a reduction of lymph flow to the urinary tract three months later. In our patient, therapeutic intervention by ezetimibe may have resulted in a reduction of chylous lymph absorption from the intestine and the prevention of mucosal rupture into the renal pelvis and calyx via reduced intralymphatic pressure. Ezetimibe may be an effective and safe treatment for this indication, and should be considered when filarial patients present with chyluria and massive proteinuria before employing invasive surgical procedures.
Keywords: chyluria, ezetimibe, filariasis, proteinuria
Background
Chyluria is the presence of a milky white lymphatic fluid that is rich in protein and dietary lipids in the form of chylomicrons in urine. The most important cause of chyluria is impaired circulation of lymph flow by thoracic duct obstruction which arises from a variety of causes, including filariasis, trauma, surgical procedure, tumor and aneurysm. Chyluria occurs in 5.5% of filarial-affected patients [1]. Filariasis is endemic in the tropical parts of Africa, India and southern Asia. It is commonly caused by the parasitic filarial nematode Wuchereria bancrofti (W. bancrofti).
A strict low-fat diet is recommended in the conservative management of chyluria for the purpose of reducing intralymphatic pressure via decreasing chylous lymph absorption from the intestine. However, it is uncertain whether inhibition of cholesterol absorption with ezetimibe reduces intralymphatic pressure in filarial patients.
We report a case of filariasis presenting with severe chyluria and nephrotic-range proteinuria in which inhibition of cholesterol absorption with ezetimibe has been remarkably effective.
Case report
A 75-year-old woman was admitted to our department with proteinuria and bilateral lower-extremity edema. She lived in the prefecture of Nagasaki, a filarial endemic region, from birth to 74 years of age. The patient first presented with chyluria in her 40s and had been diagnosed by lymphangiography with chyluria due to filariasis in 1980. Since then, she had suffered from intermittent chyluria and leg edema. She developed lower-extremity edema three months before the current admission, and her primary-care doctor referred her to our department for treatment on September 2010.
On examination, she looked well and her vital signs were stable; blood pressure was normal (125/73 mmHg) and pulse rate was 76 beats/min. The body temperature was normal (36.6°C). The chest examination showed no abnormalities. The abdomen was soft and slightly enlarged. The liver and the spleen were not palpable. No fluid wave suggestive of ascites was perceived on palpation. Both legs showed prominent pitting edema.
Laboratory examinations revealed a white blood cell count of 4.36 × 103/μL (4.36 × 109/L). Albumin level had decreased to 2.8 g/dL (28 g/L) and total cholesterol level was 266 mg/dL (6.88 mmol/L), with triglycerides at 229 mg/dL (2.59 mmol/L). Urinalysis showed the presence of protein (3+) and occult blood (3+) using a dipstick and massive proteinuria (8.9 g/24 h). There was no evidence of urinary stone or intra-abdominal space-occupying lesion or ascites fluid on the computed tomography scan of abdomen. Technetium-99m-human serum albumin (Tc-99m-HSA) lymphoscintigraphy revealed the presence of communications between the lymphatic system and the urinary tract (Figure 1A).
Fig. 1.
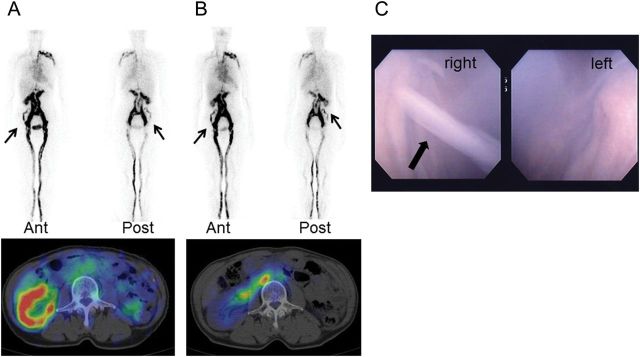
(A) Technetium-99m-human serum albumin (Tc-99m-HSA) lymphoscintigraphy (pre-administration of ezetimibe) shows the presence of communications between the lymphatic system and the urinary tract (arrow). Ant, anterior view image; post, posterior view image. (B) Tc-99m-HSA lymphoscintigraphy (three months post-administration of ezetimibe) shows a reduction of lymph flow to the urinary tract (arrow). (C) Cystoscopy reveals the presence of chyluria flowing out from the right ureteral orifice (arrow).
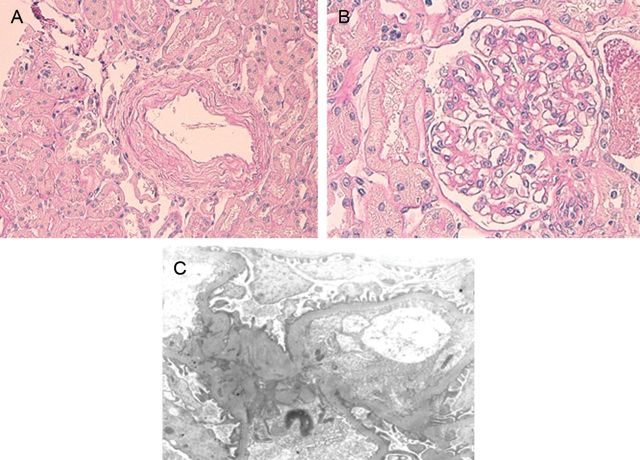
The diagnosis of chyluria was confirmed by the ether test, which showed complete clearing of urinary opacity by the addition of ether to her milky urine. There were no obvious findings of glomerulonephritis in the renal biopsy (Figure 2). On the basis of the clinical course, the laboratory findings and imaging studies, filariasis was considered to be involved in the pathogenesis of chyluria and nephrotic-range proteinuria as well in this case.
Fig. 2.
(A, B) Light microscopy of the patient's renal biopsy showing a slight degree of tubulointerstitial atrophy, fibrosis and intimal thickening of arteries. Glomerular abnormalities are not observed (PAS staining, ×400). (C) Electron microscopy of this patient's renal biopsy. Effacement of the foot processes is not found.
Because this patient showed moderate dyslipidemia, ezetimibe (10 mg/day) was administered on the 22nd hospital day. Chyluria disappeared within a few days following administration of ezetimibe. Moreover, the urinary protein excretion decreased markedly to ∼0.2 g/24 h and hematuria also disappeared. As the urinary protein decreased, the edema in the lower extremities disappeared and the hypoproteinemia improved (albumin level, 4.2 g/dL or 42 g/L) during outpatient follow-up. Total cholesterol level had decreased to 210 mg/dL (5.43 mmol/L), with triglycerides at 69 mg/dL (0.779 mmol/L) following remission. Tc-99m-HSA lymphoscintigraphy showed a reduction of lymph flow to the urinary tract three months later (Figure 1B).
Discussion
Our patient with filariasis showed remarkable improvement in both nephrotic-range proteinuria and severe chyluria after treatment with ezetimibe. The renal biopsy in our patient showed benign nephrosclerosis, which did not explain such a massive amount of proteinuria. Many reports have previously documented the incidence of glomerulonephritis associated with acute filarial infection, which is called ‘filarial nephropathy’ [2–4]. Using light microscopic examination, these reports have described various types of renal injuries such as membranous, mesangiocapillary and chronic sclerosing glomerulonephritis [4–7]. Using immunofluorescence microscopy, granular deposits of IgG and C3 have also been reported [5, 7]. In our patient, light and immunofluorescent microscopic studies showed no evidence of glomerulonephritis. Thus, mechanisms other than renal lesions, such as retrograde lymphatic hypertension and the rupture of lymph vessels into the urinary tract, may have played an important role in the development of proteinuria and chyluria.
The histological changes of lymphatics in filariasis result from inflammatory damage to the lymphatics caused by adult worms. Adult worms migrate to lymphatics and stimulate inflammatory processes which cause lymphatic obstruction and prevent intestinal lymph outflow. As a result, the elevated intralymphatic pressure triggers the rupture of vulnerable sites in the mucous membranes of the renal pelvis and calyx, which causes a leakage of chyle into the urinary tract [8]. In our case, Tc-99m-HSA lymphoscintigraphy also showed communications between the lymphatic system and the urinary tract.
Inhibition of cholesterol absorption with ezetimibe may be remarkably effective in the treatment of massive proteinuria and chyluria in filariasis, as occurred in our patient. Ezetimibe is the first of a new class of drugs that inhibits dietary and biliary cholesterol absorption by blocking at the brush border of the intestine. The molecular target for ezetimibe was recently identified to be the Niemann-Pick C1-like 1 (NPC1L1), the intestinal cholesterol transport protein at the small intestine, especially expressed in the brush border [9]. In our patient, therapeutic intervention with ezetimibe may have resulted in a reduction of chylous lymph absorption from the intestine and the prevention of mucosal rupture in the renal pelvis and calyx via reduced intralymphatic pressure. This fact may suggest an important role of intralymphatic pressure in the pathogenesis of chyluria and nephrotic-range proteinuria in filariasis.
There is no definitive treatment for filarial chyluria. In mild cases, rest and a strict low-fat diet with high protein and fluid intake are recommended as conservative management. Surgical treatment is indicated in the patient who presents with refractory severe chyluria with large amounts of proteinuria, hypoproteinemia, progressive weight loss and an immunodeficiency state from hypogammaglobulinemia [10]. From the viewpoint of invasiveness and the difficulties of a second surgery, the risks of surgical treatment should not be considered less serious. There are some reports in the literature that spontaneous remission can be seen in up to 50% of patients with chyluria [11, 12]; in our patient, any other factors contributing to the continuation or remission of chylous urine were not apparent, so we cannot completely exclude the possibility that her improvement was a spontaneous remission unrelated to ezetimibe. However, the average duration of chyluria in a series of cases with spontaneous remission was 44.3 months [11]. The recurrence of chyluria after discontinuation of ezetimibe and a remission following re-administration would have more convincingly clarified the contribution of this agent to the remission of the chyluria. However, the patient expressed a strong wish to continue on ezetimibe due to concerns about the relapse of chyluria and has therefore continued to receive ezetimibe and a fat-restricted diet through our department. In the six months since discharge, there has been no relapse of chyluria.
Here, we describe a case of filariasis with remarkable improvement in both severe chyluria and nephrotic-range proteinuria on treatment with ezetimibe. Treatment with ezetimibe may have the potential to become an effective and safe treatment for the management of chyluria, and should be considered when filarial patients present with chyluria and massive proteinuria before resorting to invasive surgical procedures.
Conflict of interest Statement
None declared.
References
- 1.Udonsi JK. The status of human filariasis in relation to clinical signs in endemic areas of the Niger Delta. Ann Trop Med Parasitol. 1986;80:425–432. doi: 10.1080/00034983.1986.11812043. [DOI] [PubMed] [Google Scholar]
- 2.Derouiche A, El Atat R, Mechri M, et al. Post-kidney transplantation lymphocele due to a lymphatic filariasis. Transplant Proc. 2010;42:2808–2812. doi: 10.1016/j.transproceed.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 3.Gulati S, Gupta N, Singh NP, et al. Chyluria with proteinuria or filarial nephropathy? An enigma. Parasitol Int. 2007;56:251–254. doi: 10.1016/j.parint.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Pillay VK, Kirch E, Kurtzman NA. Glomerulopathy associated with filarial loiasis. JAMA. 1973;225:179. doi: 10.1001/jama.1973.03220290057028. [DOI] [PubMed] [Google Scholar]
- 5.Yap HK, Woo KT, Yeo PP, Chiang GS, Singh M, Lim CH. The nephrotic syndrome associated with filariasis. Ann Acad Med Singapore. 1982;11:60–63. [PubMed] [Google Scholar]
- 6.Ngu JL, Chatelanat F, Leke R, Ndumbe P, Youmbissi J. Nephropathy in Cameroon: evidence for filarial derived immune-complex pathogenesis in some cases. Clin Nephrol. 1985;24:128–134. [PubMed] [Google Scholar]
- 7.Date A, Gunasekaran V, Kirubakaran MG, Shastry JC. Acute eosinophilic glomerulonephritis with Bancroftian filariasis. Postgrad Med J. 1979;55:905–907. doi: 10.1136/pgmj.55.650.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunkwall J, Simonsen O, Bergqvist D, Jonsson K, Bergentz SE. Chyluria treated with renal autotransplantation: a case report. J Urol. 1990;143:793–796. doi: 10.1016/s0022-5347(17)40098-x. [DOI] [PubMed] [Google Scholar]
- 9.Betters JL, Yu L. NPC1L1 and cholesterol transport. FEBS Lett. 2010;584:2740–2747. doi: 10.1016/j.febslet.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okamoto K, Nagayoshi H, Matsuyama M, Nagano T, Nagata K. Chyluria in Japan—report of the present status. Nihon Hinyokika Gakkai Zasshi. 1976;67:677–688. [PubMed] [Google Scholar]
- 11.Ohyama C, Saita H, Miyasato N. Spontaneous remission of chyluria. J Urol. 1979;121:316–317. doi: 10.1016/s0022-5347(17)56767-1. [DOI] [PubMed] [Google Scholar]
- 12.Diamond E, Schapira HE. Chyluria—a review of the literature. Urology. 1985;26:427–431. doi: 10.1016/0090-4295(85)90147-5. [DOI] [PubMed] [Google Scholar]