Abstract
Background
Autosomal dominant polycystic kidney disease (ADPKD) is an inherited disorder that results in the growth of cysts in the kidneys and other organs. Multisystemic involvement is common including affection of the central nervous system with cerebral aneurysms and arachnoid cysts.
Methods
This is a prospective cohort study to investigate the prevalence and growth rate of arachnoid cysts in ADPKD patients. Participants enrolled in the SUISSE ADPKD cohort were offered cranial imaging for the detection of intracranial alterations. In the case of identified arachnoid cysts, patients were suggested to undergo follow-up imaging to assess the growth rate of the cysts. Volume of arachnoid cysts at the baseline and at follow-up visits was assessed by manual segmentation on a dedicated workstation.
Results
A total of 109 ADPKD patients agreed to undergo cranial imaging. In 14 (12.8%) patients (9 males and 5 females), 18 singular arachnoid cysts were identified. The baseline volumes of individual cysts ranged from 1.8 to 337.6 cm3. During a mean follow-up period of 24 months, the volume changes of 12 individual arachnoid cysts of nine patients ranged from −3.1 to 3.7 cm3. Cystic lesions were mostly localized in the middle fossa. All affected patients were clinically asymptomatic.
Conclusions
We found a higher prevalence of arachnoid cysts in ADPKD patients with more advanced disease. There was a large variability in size and growth. These arachnoid cysts were clinically silent and their growth pattern was subtle and unpredictable, in contrast to the much more foreseeable growth of the renal cysts.
Keywords: arachnoid cyst, autosomal dominant polycystic kidney disease, extrarenal manifestation, intracranial alteration
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited renal disorder characterized by the arising of innumerable cysts in the kidneys most likely causing end-stage renal disease in adulthood [1, 2].
Displaying a multisystemic feature, ADPKD often includes extrarenal organ involvement such as cystic lesions in the liver, pancreas, spleen and lung as well as presenting with hypertension, chronic back pain, cardiac valve defects, abdominal hernias, haematuria, urinary tract infection, kidney stones, dysregulated phosphate homeostasis, impaired quality of life and intracranial alterations [2–6].
Classical intracranial manifestations are cerebral aneurysms and arachnoid cysts which are considered to be the result of a misguided cell differentiation [2, 7–9]. Structurally, arachnoid cysts consist of one or multiple layers of arachnoid mesothelium, which normally covers the cerebral fluid absorbing arachnoid villi and granulations [10, 11]. When failing to develop a connection to the dural sinus, the mesothelium is thought to form a cavity containing a cerebrospinal fluid-like [12] liquid. Previously demonstrated Na+K+ATPase activity and increased expression of NKCC1 in the cyst lining cells indicate a secretory mechanism towards the lumen, which subsequently leads to cyst enlargement [10, 11, 13, 14].
The prevalence of arachnoid cysts is described with 0.5–1% in the general population [15, 16] amounting to 5.2–8.1% in patients with ADPKD [13, 17, 18]. Clinical features mostly have been reported as benign, while symptomatic cysts can be associated with mental retardation, seizures, cerebral haemorrhage and focal neurological deficits [19–21].
However, the natural course of arachnoid cysts in patients with ADPKD has not been systematically examined up to now. Here, we report on the prevalence and the volume change of arachnoid cysts in a well-characterized cohort of patients with ADPKD.
Materials and methods
Patients and study procedure
From 2006 to 2012, 194 ADPKD patients were enrolled in the SUISSE ADPKD cohort at the University Hospital of Zürich. The SUISSE ADPKD cohort is a prospective longitudinal ongoing study; patients are eligible for enrolment if they have ADPKD according to the Ravine criteria, aged 18–50 years with an estimated glomerular filtration rate (eGFR) of >60 mL/min/1.73 m2 [22]. At enrolment, all patients were offered cranial imaging for the screening of cerebral aneurysms and arachnoid cysts, which is part of standard care at our centre. In the case of identified arachnoid cysts, patients were suggested to undergo follow-up imaging within 1–2 years to assess the growth rate of the cysts.
Clinical and laboratory parameters including serum creatinine, blood pressure, antihypertensive medication, neurological symptoms and patient and family history were recorded at the baseline and follow-up visit. The GFR was estimated according to the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation. Total kidney volume (TKV) was determined as described previously [22].
The study was conducted according to the guidelines for Good Clinical Practice and the Declaration of Helsinki Principles. Study approval was obtained from the local ethics committee, and all patients gave written, informed consent.
Imaging studies
Cranial magnetic resonance imaging (MRI) without gadolinium contrast was performed using eight-channel head coil (SENSE). Protocols for cranial MRI were as follows: coronal T1 [TR (ms)/TE (ms) 580/10, slice thickness 4 mm, flip angle 73°, NEX 3.00, FOV 24 × 18, matrix size 320 × 192]. Axial T2 FRFSE [TR (ms)/TE (ms) 6720/87.1, slice thickness 4 mm, flip angle 90°, NEX: 2.00, FOV 24 × 24, matrix size 384 × 256]. Time-of-flight MRA sequence [TR (ms)/TE (ms) 23/2.4, slice thickness 4 mm, flip angle 20°, NEX: 1.00, FOV 24 × 18, matrix size 320 × 224] was applied to investigate possible intracranial aneurysms. Arachnoid cyst volumes were calculated on T2-weighted images by manually tracing the outlines on every slice and subsequently summing up the volumes of each section. The procedure was conducted twice by the same observer in random order with an interval of at least one week. The intraobserver variability calculated by using Spearman's ρ correlation coefficient amounted to 0.995.
Statistical analysis
For comparison of baseline characteristics of affected and non-affected ADPKD patients, the Mann–Whitney U-test, the Pearson's χ2 test and the Fisher's exact test were applied. The correlation between volume changes and follow-up time was evaluated using Spearman's rank correlation.
Results
We offered cranial imaging to 194 ADPKD cohort patients and 109 (56%) participants agreed to undergo the procedure. Study subjects with neurocranial imaging were no different from patients who refused the procedure with regards to age, gender and eGFR.
A total of 18 separate arachnoid cysts of variable size were detected in 14 patients (9 males and 5 females), corresponding to a prevalence of 12.8% (Table 1).
Table 1.
Patient baseline characteristicsa
| Characteristic | Arachnoidcysts,n = 14 | Controls,n = 95 | Significance(two-sided),P |
|---|---|---|---|
| Age (years) | 35 (17) | 31 (12) | 0.037b |
| Gender [no. (%)] | |||
| Female | 5 (36) | 43 (45) | 0.502c |
| Male | 9 (64) | 52 (55) | |
| Creatinine (μmol/L) | 100 (28) | 86 (31) | 0.022b |
| eGRF (CKD EPI) (mL/min/1.73 m2) | 81 (33) | 97 (36) | 0.037b |
| Blood pressure (mmHg) | |||
| Systolic | 138 (34) | 134 (17) | 0.526b |
| Diastolic | 90 (14) | 85 (13) | 0.267b |
| Antihypertensive treatment [no. (%)] | 12 (86) | 44 (46) | 0.008c |
| ACEi/ARB | 12 (86) | 40 (42) | |
| Kidney volume (cm3) | 1112 (779) | 820 (807) | 0.120b |
| Presence of liver cysts [no. (%)] | 10 (71) | 67 (71) | 0.756d |
| Intracranial aneurysms [no. (%)] | 2 (14) | 4 (4) | 0.170d |
| Family history for intracranial aneurysms [no. (%)] | 3 (21) | 19 (20) | 1.000d |
aBaseline values were recorded at time point of cranial imaging. Data are expressed as either median (IQR) or number of patients (%).
bMann–Whitney's U-test.
cPearson's χ2 test.
dFisher's exact test.
Eleven of the 18 cystic lesions were situated in the middle cranial fossa with 7 in posterior localization, one cyst classified as posterior was situated in the cerebellopontine angle. The arachnoid cysts were evenly distributed between the right and the left hemicranium: eight lesions were right-sided, eight left-sided and two paramedian or bilateral. In T2-weighted images, the cyst wall was thin and delicate and the arachnoid cyst content appeared homogenous, suggesting that no recent intracystic bleeding or infection occurred.
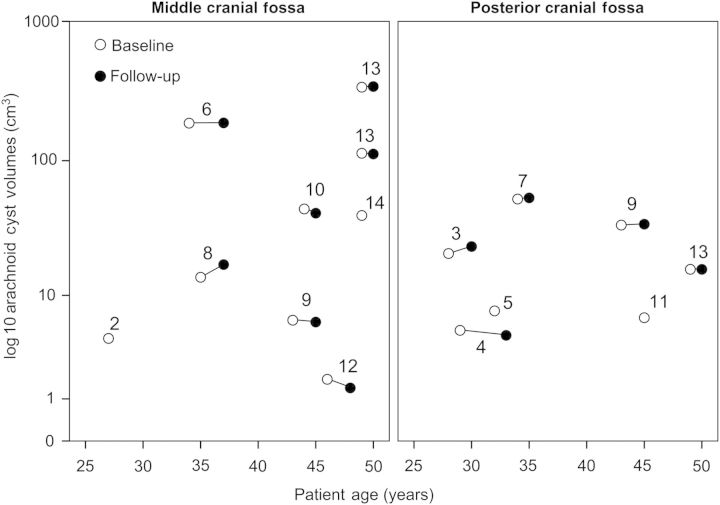
The individual arachnoid cyst volumes were highly variable, ranging from 1.8 to 337.6 cm3 (Figure 1). Two male patients showed extreme dimensions of lesions with cystic expansion over the entire hemicranium (Figure 2). Nine participants with 12 separate arachnoid cysts underwent follow-up cranial imaging at a median interval of 543 days [inter-quartile range (IQR) 398–1092 days]. Absolute and annualized volume changes of individual cysts ranged from 3.7 to −3.1 cm3 and from 4.4 to −2.8 cm3, respectively (Table 2). The volume changes of arachnoid cysts did not correlate with the follow-up interval (rs = 0.033, P = 0.932), whereas TKV changes were moderately correlated with follow-up time (rs = 0.559, P = 0.059) (Figure 3a and b).
Fig. 1.
Volumes of individual arachnoid cysts at the baseline and follow-up visits. Patient nos 2, 5, 11 and 14 did not have follow-up imaging. Patient no. 1 was excluded from volumetric analysis due to technical reasons.
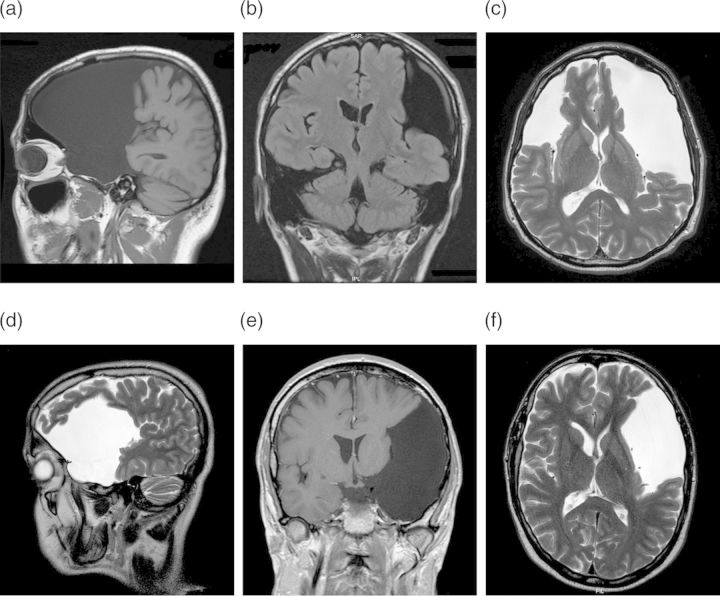
Fig. 2.
T1- and T2-weighted MRI demonstrating extreme dimensions of arachnoid cysts. MRI in Patient no. 13 (a–c) shows three cysts left and right in the middle and posterior fossa; MRI in Patient no. 6 (d–f) displays a large arachnoid cyst encompassing the left middle fossa.
Table 2.
Characteristics of singular arachnoid cystsa
| Patient no. | Age atbaseline(years) | Gender | Cranialfossa | Cyst localization | Cyst side | Baselinevolume(cm3) | Follow-upvolume(cm3) | Calculatedannualgrowth (cm3) | Follow-uptime(months) | Aneurysm |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 22 | F | Middle | Temporopolar | Left | N/A | N/A | |||
| 1 | 22 | F | Middle | Temporopolar | Right | N/A | N/A | |||
| 2 | 27 | F | Middle | Temporopolar | Left | 4.4 | ||||
| 3 | 28 | F | Posterior | Cerebellar | Left | 20.9 | 23.6 | 1.2 | 26 | |
| 4 | 29 | M | Posterior | Cerebellopontine | Right | 5.2 | 4.7 | −0.1 | 48 | |
| 5 | 32 | M | Posterior | Retrocerebellar | Paramedian | 7.5 | ||||
| 6 | 34 | M | Middle | Frontotemporal | Left | 186.3 | 187.1 | 0.3 | 38 | ACoA |
| 7 | 34 | M | Posterior | Retrocerebellar | Left | 52.7 | 53.7 | 0.9 | 14 | |
| 8 | 35 | M | Middle | Temporopolar | Left | 13.8 | 17.3 | 1.5 | 27 | |
| 9 | 43 | F | Middle | Temporopolar | Left | 6.3 | 6.1 | −0.2 | 14 | |
| 9 | 43 | F | Posterior | Retrocerebellar | Bilateral | 34.0 | 34.5 | 0.4 | 14 | |
| 10 | 44 | F | Middle | Temporopolar | Right | 44.6 | 41.5 | −2.8 | 12 | |
| 11 | 45 | M | Posterior | Retrocerebellar | Right | 6.6 | ACoA | |||
| 12 | 46 | M | Middle | Temporopolar | Right | 1.8 | 1.4 | −0.2 | 20 | |
| 13 | 49 | M | Posterior | Retrocerebellar | Right | 15.9 | 15.9 | 0.0 | 9 | |
| 13 | 49 | M | Middle | Frontotemporal | Right | 113.1 | 111.7 | −1.6 | 9 | |
| 13 | 49 | M | Middle | Frontotemporal | Left | 337.6 | 341.3 | 4.4 | 9 | |
| 14 | 49 | M | Middle | Temporal | Right | 40.0 |
aF, female; M, male; N/A, not available; ACoA, anterior communicating artery.
Fig. 3.
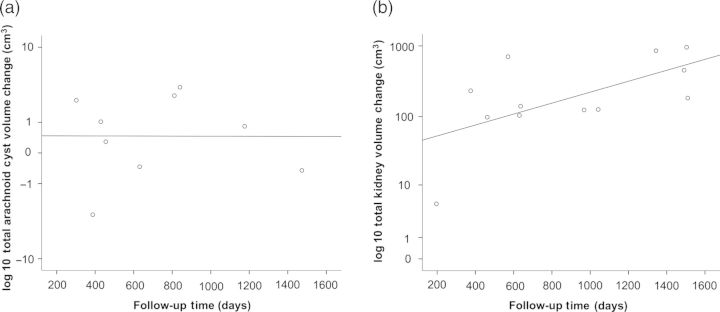
(a) Total volume change of arachnoid cysts. Arachnoid cyst volume data of Patient no. 1 were not available due to technical reasons (b). Change of corresponding TKVs.
A detailed review of the medical history did not reveal neurological signs and symptoms in patients with arachnoid cysts. In the two patients with extreme dimensions of arachnoid cysts (nos 6 and 13), a detailed neurological assessment revealed normal cranial nerve status, motor, sensory and global cognitive function.
Baseline characteristics of the 14 patients with arachnoid cysts and the 95 controls are displayed in Table 1. Patients with arachnoid cysts were older (35 versus 31 years), were predominantly male (64 versus 55%), had a lower eGFR (81 versus 97 mL/min/1.73 m2) and were often hypertensive (86 versus 46%), and their TKV was larger (1112 versus 820 cm3) than patients without arachnoid cysts. The family history for arachnoid cysts was negative in all 14 individuals.
Discussion
Here, we report that arachnoid cysts occur in a substantial number of patients with ADPKD when these lesions are sought for by neurocranial MR or computed tomography (CT) imaging procedures. In our study, we detected arachnoid cysts in 12.8% of all examined Suisse ADPKD patients. Aside from several case reports [19–21, 23–27], there are three analyses of ADPKD cohorts reporting a prevalence of arachnoid cysts which ranges from 5.2 to 8.1% (Table 3) [13, 17, 18].
Table 3.
Forty-two ADPKD patients affected by arachnoid cysts reported in the literature
| Study |
||||
|---|---|---|---|---|
| Torres et al. [13] | Schievink et al. [18] | Romao et al. [17] | Suisse ADPKD cohort study | |
| Setting, methods and participants | ||||
| Type of study | Prospective. Regular ADPKD patients seen in the author's office, asymptomatic for cranial haemorrhage |
Retrospective. Consecutive group of neurologically asymptomatic ADPKD patients screened for intracranial aneurysms at the author's institution |
Prospective. Neurologically asymptomatic ADPKD patients referred for magnetic resonance imaging as a subgroup of a retrospectively investigated cohort |
Prospective. Neurologically asymptomatic participants of Suisse ADPKD cohort screened for intracranial alterations |
| Aim of the study | To determine the impact of imaging techniques in the presymptomatic diagnosis of aneurysms and intracranial pathology | To investigate the prevalence of intracranial cysts among ADPKD patients screened for aneurysms | To determine the prevalence of renal and extrarenal manifestations and to investigate the occurrence and characteristics of events related to ADPKD in the central nervous system | To determine the prevalence, localization, growth behaviour and associated clinical symptoms of AC in ADPKD patients of our cohort |
| Total no. of examined ADPKD patients | 96 | 247 | 42 | 109 |
| Age of cohort, mean ± SD | 41 ± 12 years | 44 (range 13–72) years | 35.6 ± 14.3 years | Median 34.5 years (IQR 16 years) |
| Gender distribution of cohort (male:female) | 43:53 (44.8% male) | 96:151 (38.8% male) | 18:24 (42.9% male) | 61:48 (56% male) |
| Serum creatinine of cohort | <132–264 μmol/L (<1.5 to ≥3 mg/dL) | Not available | 122 ± 70 μmol/L (1.39 ± 0.79 mg/dL) | Median 99 μmol/L (IQR 18 μmol/L) [median 1.12 mg/dL (IQR 0.21 mg/dL)] |
| No. of patients with polycystic liver | Not available | 136 (55.1%) | Not available | 76 (69.7%) |
| No. of patients with aneurysm | 11 indeterminate (11.5%) | 15 (10.8%) | 3 (7.1%) | 6 (5.5%) |
| Imaging technique | HRCT, MRI, angiography | MRI, CT, MRA | MRA | MRI |
| Control group | 192 age- and gender-matched non-ADPKD patients who previously underwent cerebral angiography, to study the significance of concomitant intracranial pathologies | 247 age and gender matched non-ADPKD patients who had undergone cranial imaging | None, comparison to literature | 95 ADPKD patients who underwent cranial imaging, not affected with AC |
| Results for arachnoid cysts (AC) | ||||
| No. of patients (prevalence) | 5 (5.2%) | 20 (8.1%) | 3 (7.1%) | 14 (12.8%) |
| No. of patients with multiple cysts | Not available | 2 (10%) | Not available | 2 (14.3%) |
| Age | Mean 42.6 years | Mean 45 years (range 17–56) | Mean 49 years (range 39–58) | Median 35 years |
| Gender distribution (male:female) | 3:2 (60% male) | 5:15 (25% male) | 1:2 (33% male) | 9:5 (64.3% male) |
| Serum creatinine | Not available | Not available | Range 97–176 μmol/L (range 1.1–2.0 mg/dL) | Median 100 μmol/L (IQR 28 μmol/L) [median 1.13 mg/dL (IQR 0.32 mg/dL)] |
| Localization of AC | 2 anterior cranial fossa 2 middle cranial fossa 1 posterior cranial fossa |
4 anterior cranial fossa 8 middle cranial fossa 4 posterior cranial fossa |
1 middle cranial fossa 2 posterior cranial fossa |
11 middle cranial fossa 7 posterior cranial fossa |
| Range of size of AC | 1.5 cm × 1 cm to 5 cm × 3 cm | Not available | Not available | 1.8–337.6 cm3 |
| Follow-up imaging | None | 3 patients after 12, 16 and 51 months, respectively | Not available | 9 patients, median follow-up time 24 months |
| No. of patients with polycystic liver | Not available | 17 (85%) | 3 (100%) | 10 (71.4%) |
| No. of patients with concomitant aneurysms | 0 | 1 (7.1%) | 0 | 2 (14.3%) |
Torres et al. [13] performed MRI and high resolution computed tomography (HRCT) on 96 ADPKD patients aged (mean ± SD) 41 ± 12 years in order to determine prospectively the impact of cranial imaging in the diagnosis of intracranial pathology. Serum creatinine ranged from <132 to 264 μmol/L (<1.5 to ≥3.0 mg/dL) and 44.8% of all participants were male. The authors found arachnoid cysts in five patients (60% male), none of them showing neurological symptoms. The mean age at time point of imaging was 42.6 years. Arachnoid cysts were located equally in the anterior (2), middle (2) and posterior (1) cranial fossa. No concomitant aneurysm was reported.
A major study was conducted by Schievink et al. [18] to investigate the prevalence of intracranial cysts in ADPKD. The study cohort consisted of 247 participants (38.8% male) aged 13–72 years (mean 44 years). Serum creatinine was not described. Arachnoid cysts were detected in 20 asymptomatic patients (25% males) and 2 individuals had multiple cysts. The mean age was 45 years (range 17–56 years). The middle fossa was the predominant site of occurrence (eight cases), followed by anterior and posterior fossae (four cases, respectively). One patient was affected with a concomitant aneurysm.
Romao et al. [17] generally searched for intracranial alterations in 42 neurologically asymptomatic ADPKD patients (42.9% males). The mean age of the investigated cohort was lower than in the other two studies (35.6 ± 14.3 years). The mean serum creatinine was 122 μmol/L (1.39 mg/dL) and three patients were already on dialysis. The authors describe arachnoid cysts in three patients (one male and two females) aged 39–58 years (mean 49 years) with a serum creatinine ranging from 97 to 176 μmol/L (1.1 to 2.0 mg/dL). Cystic lesions were found in the middle and posterior cranial fossa. All of the three cases were affected with concomitant polycystic liver disease, but none had an intracranial aneurysm.
In our study, we found a higher prevalence of arachnoid cysts than others, a fact that needs to be addressed. Since genotyping is not described in either study, the nature of the mutation in these patients, and hence the course of the disease, is subject to hypothesis. The mutation of PKD1 is responsible for ∼85% of ADPKD cases, whereas only 15% of all patients possess a mutation in PKD2 [28]. The latter are believed to have a slower rate of disease progression. Our cohort mostly consists of patients who previously were enrolled in the Suisse ADPKD Study. Selected by kidney volume increase not less than 2% in 6 months, our patients are considered PKD1 rather than PKD2. We hypothesize that the higher frequency of arachnoid cysts corresponds to our selection of clinically more manifest cases of ADPKD, namely patients with PKD1 mutation.
The distribution of gender in our report matches data in the general population showing a male dominance, although one study described female overrepresentation in patients with ADPKD.
The main site of cystic lesions was the middle (61%), followed by the posterior cranial fossa (39%). This is not different from the distribution of arachnoid cysts in the general population where the localization of arachnoid cysts is reported to be predominant in the middle cranial cavity, more precisely the Sylvian fissure [29].
Cyst size was variable ranging from very small cysts to bizarrely shaped large cysts encompassing major parts of the hemicranium. The volume changes of the cysts that we measured over a mean interval of 2 years were subtle and inconsistent, not aligning with the steady and more substantial growth of the kidney cysts compressing the adjacent renal parenchyma, provoking tissue fibrosis and a loss of renal function.
The absence of clinical symptoms even in patients with very large cysts contrasts with the cystic pathology in the kidney, suggesting a different behaviour of the arachnoid cysts. Thus, the occurrence of arachnoid cysts can be regarded as benign, manifesting mostly as an incidental finding on MR or CT images, and does not need to be monitored further unless there are neurological symptoms, but the latter are rare.
Although arachnoid cysts and cerebral aneurysms have a higher prevalence in patients with ADPKD, their coexistence has only rarely been published [18, 30]. In our series of 14 patients with arachnoid cysts, we found two patients with a coexisting cerebral aneurysm.
In conclusion, we found a higher prevalence of arachnoid cysts in clinically more preceded ADPKD patients. These arachnoid cysts are clinically silent and their growth pattern was subtle and unpredictable, in contrast to the much more foreseeable growth of the renal cysts.
Conflict of interest statement
None declared.
Acknowledgements
Funding for this study was supported by the Swiss National Science Foundation (No. 310000-118166). GE Healthcare Switzerland provided an Advantage Workstation for volumetric analysis of magnetic resonance imaging data.
References
- 1.Gabow PA. Autosomal dominant polycystic kidney disease. N Engl J Med. 1993;329:332–342. doi: 10.1056/NEJM199307293290508. [DOI] [PubMed] [Google Scholar]
- 2.Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369:1287–1301. doi: 10.1016/S0140-6736(07)60601-1. [DOI] [PubMed] [Google Scholar]
- 3.Guay-Woodford LM. Renal cystic diseases: diverse phenotypes converge on the cilium/centrosome complex. Pediatr Nephrol. 2006;21:1369–1376. doi: 10.1007/s00467-006-0164-9. [DOI] [PubMed] [Google Scholar]
- 4.Pei Y. Diagnostic approach in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2006;1:1108–1114. doi: 10.2215/CJN.02190606. [DOI] [PubMed] [Google Scholar]
- 5.Rizk D, Chapman A. Treatment of autosomal dominant polycystic kidney disease (ADPKD): the new horizon for children with ADPKD. Pediatr Nephrol. 2008;23:1029–1036. doi: 10.1007/s00467-007-0706-9. [DOI] [PubMed] [Google Scholar]
- 6.Rizk D, Jurkovitz C, Veledar E, et al. Quality of life in autosomal dominant polycystic kidney disease patients not yet on dialysis. Clin J Am Soc Nephrol. 2009;4:560–566. doi: 10.2215/CJN.02410508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bannister CM, Russell SA, Rimmer S, et al. Fetal arachnoid cysts: their site, progress, prognosis and differential diagnosis. Eur J Pediatr Surg. 1999;9(Suppl 1):27–28. doi: 10.1055/s-2008-1072308. [DOI] [PubMed] [Google Scholar]
- 8.Robinson RG. Congenital cysts of the brain: arachnoid malformations. Prog Neurol Surg. 1971;4:133–174. [Google Scholar]
- 9.Vega-Sosa A, de Obieta-Cruz E, Hernandez-Rojas MA. Intracranial arachnoid cyst. Cir Cir. 2010;78:551–556. [PubMed] [Google Scholar]
- 10.Go KG, Houthoff HJ, Blaauw EH, et al. Arachnoid cysts of the sylvian fissure. Evidence of fluid secretion. J Neurosurg. 1984;60:803–813. doi: 10.3171/jns.1984.60.4.0803. [DOI] [PubMed] [Google Scholar]
- 11.Go KG, Houthoff HJ, Hartsuiker J, et al. Fluid secretion in arachnoid cysts as a clue to cerebrospinal fluid absorption at the arachnoid granulation. J Neurosurg. 1986;65:642–648. doi: 10.3171/jns.1986.65.5.0642. [DOI] [PubMed] [Google Scholar]
- 12.Berle M, Wester KG, Ulvik RJ, et al. Arachnoid cysts do not contain cerebrospinal fluid: a comparative chemical analysis of arachnoid cyst fluid and cerebrospinal fluid in adults. Cerebrospinal Fluid Res. 2010;7:8. doi: 10.1186/1743-8454-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres VE, Wiebers DO, Forbes GS. Cranial computed tomography and magnetic resonance imaging in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1990;1:84–90. [PubMed] [Google Scholar]
- 14.Helland CA, Aarhus M, Knappskog P, et al. Increased NKCC1 expression in arachnoid cysts supports secretory basis for cyst formation. Exp Neurol. 2010;224:424–428. doi: 10.1016/j.expneurol.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Helland CA, Lund-Johansen M, Wester K. Location, sidedness, and sex distribution of intracranial arachnoid cysts in a population-based sample. J Neurosurg. 2010;113:934–939. doi: 10.3171/2009.11.JNS081663. [DOI] [PubMed] [Google Scholar]
- 16.Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357:1821–1828. doi: 10.1056/NEJMoa070972. [DOI] [PubMed] [Google Scholar]
- 17.Romao EA, Moyses Neto M, Teixeira SR, et al. Renal and extrarenal manifestations of autosomal dominant polycystic kidney disease. Braz J Med Biol Res. 2006;39:533–538. doi: 10.1590/s0100-879x2006000400014. [DOI] [PubMed] [Google Scholar]
- 18.Schievink WI, Huston J, 3rd, Torres VE, et al. Intracranial cysts in autosomal dominant polycystic kidney disease. J Neurosurg. 1995;83:1004–1007. doi: 10.3171/jns.1995.83.6.1004. [DOI] [PubMed] [Google Scholar]
- 19.Alehan FK, Gurakan B, Agildere M. Familial arachnoid cysts in association with autosomal dominant polycystic kidney disease. Pediatrics. 2002;110(1 Pt 1):e13. doi: 10.1542/peds.110.1.e13. [DOI] [PubMed] [Google Scholar]
- 20.Allen A, Wiegmann TB, MacDougall ML. Arachnoid cyst in a patient with autosomal-dominant polycystic kidney disease. Am J Kidney Dis. 1986;8:128–130. doi: 10.1016/s0272-6386(86)80125-1. [DOI] [PubMed] [Google Scholar]
- 21.Leung GK, Fan YW. Chronic subdural haematoma and arachnoid cyst in autosomal dominant polycystic kidney disease (ADPKD) J Clin Neurosci. 2005;12:817–819. doi: 10.1016/j.jocn.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 22.Kistler AD, Poster D, Krauer F, et al. Increases in kidney volume in autosomal dominant polycystic kidney disease can be detected within 6 months. Kidney Int. 2009;75:235–241. doi: 10.1038/ki.2008.558. [DOI] [PubMed] [Google Scholar]
- 23.Herman TE, Siegel MJ. Autosomal dominant polycystic disease with associated arachnoid cysts and subdural cystic hygroma requiring shunting. J Perinatol. 2010;30:566–568. doi: 10.1038/jp.2010.67. [DOI] [PubMed] [Google Scholar]
- 24.Howe G, Liddell J, Hunn A. Adult polycystic kidney disease and arachnoid cyst formation: Case report. J Clin Neurosci. 1995;2:269–270. doi: 10.1016/s0967-5868(95)80014-x. [DOI] [PubMed] [Google Scholar]
- 25.Peces R, Peces C, Fernandez EJ, et al. Arachnoid cysts in autosomal dominant polycystic kidney. Nefrologia. 2006;26:510–512. [PubMed] [Google Scholar]
- 26.Pirson Y. Extrarenal manifestations of autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 2010;17:173–180. doi: 10.1053/j.ackd.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Wijdicks EF, Torres VE, Schievink WI. Chronic subdural hematoma in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2000;35:40–43. doi: 10.1016/S0272-6386(00)70299-X. [DOI] [PubMed] [Google Scholar]
- 28.Igarashi P, Somlo S. Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2002;13:2384–2398. doi: 10.1097/01.asn.0000028643.17901.42. [DOI] [PubMed] [Google Scholar]
- 29.Rengachary SS, Watanabe I. Ultrastructure and pathogenesis of intracranial arachnoid cysts. J Neuropathol Exp Neurol. 1981;40:61–83. [PubMed] [Google Scholar]
- 30.de Oliveira JG, Giudicissi-Filho M, Rassi-Neto A, et al. Intracranial aneurysm and arachnoid cyst: a rare association between two cerebral malformations. Br J Neurosurg. 2007;21:406–410. doi: 10.1080/02688690701466313. [DOI] [PubMed] [Google Scholar]