Abstract
Background
Dengue is a growing public health problem in Pakistan and acute kidney injury (AKI) is one of the least studied complications of dengue virus infection (DVI). The aim of this study was to determine the frequency, severity and predictors of AKI in patients with DVI and to study the impact of AKI on the length of hospital stay and mortality.
Methods
We retrospectively reviewed medical records of patients aged ≥14 years hospitalized with a primary diagnosis of DVI at Aga Khan University Hospital Karachi between January 2008 and December 2010. Binary logistic regression models were constructed to identify factors associated with the development of AKI and to study the impact of AKI on hospital stays of more than 3 days.
Results
Out of 532 patients, AKI was present in 13.3% (71/532). Approximately two-thirds (64.8%) of these patients had mild AKI and a third (35.2%) had moderate to severe AKI. Independent predictors for AKI were male gender [odds ratio (OD) 4.43; 95% CI 1.92–10.23], presence of dengue hemorrhagic and dengue shock syndrome (DSS, OD 2.14; 95% CI 1.06–4.32), neurological involvement (OD 12.08; 95% CI 2.82–51.77) and prolonged activated partial thromboplastin time (aPTT, OD 1.81; 95% CI 1.003–3.26). AKI was associated with a length of stay ≥3 days when compared with those who did not have AKI (OD 2.98; 95% CI 1.66–5.34). Eight patients (11.3%) with AKI died whereas there were no mortalities in patients without AKI (P < 0.001). Only 5 patients (7%) had persistent kidney dysfunction at discharge.
Conclusions
AKI in DVI is associated with neurological involvement, prolongation of aPTT, greater length of hospital stay and increased mortality.
Keywords: acute kidney injury, dengue, length of hospital stay, mortality, predictors for AKI
Introduction
Dengue is one of the commonest mosquito-borne infections caused by the flavi virus. Owing to rising trade activities and tourism across the world, the virus has been transported from the endemic region to various other parts of the world [1, 2]. As a result, compared with nine reporting countries in 1950, currently the geographic distribution includes >100 countries. Each year 50 million people are affected by dengue and around 2.5 billion people are at risk [3]. Dengue was first documented in 1982 in Pakistan [4]. Pakistan has had multiple epidemics ever since the first episode in 1994 [5]. The virus is now endemic in all provinces of the country [6]. Dengue can affect various organs of the body including liver, hematological system, respiratory system and brain. Acute kidney injury (AKI) is one of the least studied complications of dengue.
The majority of previous studies used variable definition of AKI in dengue virus infection (DVI), and included only patients with dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS). The Acute Kidney Injury Network (AKIN) definition of AKI can potentially pick up from mild to severe AKI [7] and hence is used in our study. We included all cases with primary diagnosis of DVI, irrespective of severity of the DVI using the World Health Organization (WHO) 1997 definition.
There are only a few case reports [8–15] in the literature and very few studies done on AKI in DVI [16–18]. Due to the frequent epidemics and endemic nature of infection in Pakistan, this retrospective study was planned to gather more information about AKI in dengue. We report the largest case series from South Asia (Pakistan) on AKI in DVI. Our aim was to study the frequency, severity and predictors of AKI. In addition, the impact of AKI on the length of stay and mortality was also studied.
Materials and methods
Setting and study population
This was a retrospective case series study conducted at Aga Khan University Hospital Karachi, Pakistan. Aga Khan University Hospital is a tertiary care health facility located in the largest city of Pakistan, Karachi, which has a population of around 15 million. It has 15 inpatient units with a total capacity of 563 beds. Data were collected after receiving approval from the ethics review committee of the institution. Study subjects included inpatients admitted with a primary diagnosis of DVI. They were identified from a central computerized record for a period of 3 years from January 2008 to December 2010 with the help of the Department of Health Information Management System. Records of the cases were retrieved through codes using International Classification of Diseases 9th Revision Clinical Modification (ICD-9 CM) 061 for DVI and ICD-9 CM 065.4 for DHF and DSS. Study subjects included patients aged >14 years admitted with the primary diagnosis of DVI and confirmed dengue IgM antibodies, irrespective of severity of DVI. Patients with chronic kidney disease, those with no laboratory evidence of DVI and malaria were excluded. Patients were divided into two cohorts (those with and those without AKI) in order to determine independent predictors of AKI. Similarly, two cohorts for length of stay inside the hospital were also made in order to study impact of AKI on the length of hospital stay. Data on demographics, clinical features, laboratory data, length of stay, recovery of renal functions and mortality were noted on a pro forma. We used the AKIN definition for classification of AKI Stages [7]. Stage 1 AKI was defined as an increase in serum creatinine >26.5 µmole/L (≥0.3 mg/dL) or 1.5 to 2-fold increase from baseline. Stage 2 AKI was defined as an increase in serum creatinine of >2–3-fold from baseline. Stage 3 AKI was defined as an increase in serum creatinine of >3-fold from baseline or an absolute serum creatinine of >354 µmol/L (≥4 mg/dL). The WHO 1997 classification was used to classify DVI into dengue fever (DF), DHF and DSS [19].
The length of stay was divided into ≤3 days and >3 days. Mild hepatitis was defined as alanine aminotransferase (ALT) 0.75–5.01 µkat/L (45–300 IU/L) and severe hepatitis was defined as ALT > 5.01 µkat/L (>300 IU/L).
Data analysis
Descriptive statistics were used to summarize baseline values and demographic data. Quantitative and qualitative data were expressed as mean, median, interquartile range and standard deviation (SD) and number of observations with percentage (%), respectively. To evaluate the association between outcomes (AKI and without AKI group, length of hospital stay) and each of the factors, 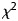 -test or Fisher's exact tests of independence were used to compare proportions where appropriate, and the Student's t-test was used to analyze continuous data. Odds ratios (OR) and their 95% confidence intervals (CI) were estimated using binary logistic regression, with AKI and length of hospital stay (≤3 versus >3 days) as outcomes. Multivariable models were constructed, including variables that showed an effect in the prediction of AKI and length of hospital stay >3 days in the univariate analysis. All P-values were based on two-sided tests and significance was set at a P-value <0.05. The analyses were performed using SPSS version 19.
-test or Fisher's exact tests of independence were used to compare proportions where appropriate, and the Student's t-test was used to analyze continuous data. Odds ratios (OR) and their 95% confidence intervals (CI) were estimated using binary logistic regression, with AKI and length of hospital stay (≤3 versus >3 days) as outcomes. Multivariable models were constructed, including variables that showed an effect in the prediction of AKI and length of hospital stay >3 days in the univariate analysis. All P-values were based on two-sided tests and significance was set at a P-value <0.05. The analyses were performed using SPSS version 19.
Results
General characteristics of the study population
Out of 532 patients, two-thirds (70.9%) were male. The mean age was 35.2 ± 14.7 years (range 15–85 years). DF was found in the majority of patients (84.4%). DHF was present only in 76 (14.3%) patients, followed by DSS in only 7 (1.3%) patients. Seventy-one (13.3%) patients developed AKI. Approximately two-thirds (64.8%) of the subjects had AKIN Stage 1 AKI. AKIN Stage 2 AKI was present in 13 (18.3%) patients and Stage 3 AKI was present in 12 (16.9%) patients. Among other manifestations, 329 (61.8%) had mild hepatitis with ALT 0.75–5.01 µkat/L (45–300 IU/L) and 47 patients (8.8%) had severe hepatitis with ALT > 5.01 µkat/L (>300 IU/L). Prolongation of prothrombin time was found in 64 (12%), while 225 (42.3%) patients had prolonged aPTT. Fourteen (2.6%) patients had respiratory failure and 11 (2.1%) patients had neurological involvement. Among those with AKI, eight patients (11.3%) died, whereas there was no mortality in patients who did not have AKI. Among survivors at the time of discharge from hospital, 58 patients (81.7%) had complete recovery of the kidney function and only 5 (7%) continued to have some degree of renal dysfunction (Table 1).
Table 1.
General characteristics of the study population (n = 532)
| Variables | Mean ± SD | Median/range/percentages |
|---|---|---|
| Age (years) | 35.29 ± 14.70 | 32 (15–85) |
| Gender | ||
| Male | 377 | 70.9% |
| Female | 155 | 29.1% |
| Length of hospital stay | 3.46 ± 3.45 | 3 (1–33 days) |
| Peak creatinine (µmol/L) | 98.124 ± 83.98 | 79.56 (17.68–875.16) |
| Peak creatinine in AKI (µmol/L) | 229.84 ± 174.15 | 149.6 (114.92–875.16) |
| Admission creatinine (µmol/L) | 90.16 ± 61.88 | 79.56 (17.68–857.48) |
| Admission hematocrit (Proportion of 1.0) | 0.4102 ± 0.063 | 0.415 (0.199–0.58) |
| Peak hematocrit (Proportion of 1.0) | 0.422 ± 0.0563 | 0.424 (0.264–0.58) |
| Platelets | 38.65 ± 42.14 | 23 (2–427) |
| Alanine aminotransferase (µkat/L) | 2.73 ± 5.86 | 1.27 (0.050–60.55) |
| Prothrombin time (s) | 12.82 ± 8.70 | 11.10 (3–120) |
| aPTT (s) | 36.02 ± 15.12 | 33.35 (11.2–120) |
| Central nervous system involvement | 11 | 2.1% |
| Vasopressin | 5 | 0.9% |
| Respiratory failure | 14 | 2.6% |
| WHO classification | ||
| DF | 449 | 84.4% |
| DHF | 76 | 14.3% |
| DSS | 7 | 1.3% |
| Length of stay in hospital (days) | ||
| ≤3 | 386 | 72.6% |
| >3 | 146 | 27.4% |
| Alanine aminotransferase | ||
| Normal | 156 | 29.3% |
| Mild hepatitis 0.75–5.01 µkat/L | 329 | 61.8% |
| Severe hepatitis >5.01 µkat/L | 47 | 8.8% |
| Prothrombin time (s) | ||
| ≤15 | 468 | 88% |
| >15 | 64 | 12% |
| aPTT (s) | ||
| ≤35 | 307 | 57.7% |
| >35 | 225 | 42.3% |
| Platelets (per µL) | ||
| <50 000 | 406 | 76.3% |
| 50 000–100 000 | 93 | 17.5% |
| 100 000–150 000 | 23 | 4.3% |
| >150 000 | 10 | 1.9% |
Independent predictor for AKI
On binary logistic regression male gender (OD 4.43; 95% CI 1.92–10.23), presence of DHF and DSS (OD 2.14; 95% CI 1.06–4.32), neurological involvement (OD 12.08; 95% CI 2.82–51.77) and prolongation of aPTT (OD 1.81; 95% CI 1.003–3.26) were found to be independent predictors for the development of AKI (Tables 2 and 3).
Table 2.
Frequency, severity and outcome of AKI at discharge
| Total number of patients | 532 |
|---|---|
| AKI | 71 (13.3%) |
| AKIN-1 AKI | 46 (64.8%) |
| AKIN-2 AKI | 13 (18.3%) |
| AKIN-3 AKI | 12 (16.9%) |
| Outcome at discharge | Fully recovered = 58 (81.7%) Persistence kidney dysfunction = 5 (7%) Mortality = 8 (11.3%) |
Table 3.
Predictors of AKI in individuals aged ≥14 years hospitalized for DVI (n = 532)
| Variables | Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|
| AKI | No AKI | P-value | OD [95% CI] | P-value | |
| Age (years) | 43 ± 18.39 | 34.12 ± 13.70 | <0.001 | 1.02 (1.007–1.04) | 0.006 |
| Gender | |||||
| Male | 62 (87.3) | 315 (68.3) | 0.001 | 4.43 (1.92–10.23) | <0.001 |
| Female | 9 (12.7) | 146 (31.7) | |||
| CNS | 7 (9.9) | 4 (0.9) | <0.001 | 12.08 (2.82–51.77) | 0.001 |
| Respiratory failure | 9 (12.7) | 5 (1.1) | <0.001 | ||
| DF | 52 (73.2) | 397 (86.1) | <0.001 | ||
| DHF | 12 (16.9) | 64 (13.9) | 2.14 (1.06–4.32) | 0.03 | |
| DSS | 7 (9.9) | 0 | |||
| Normal | 20 (28.2) | 136 (29.5) | 0.01 | ||
| Mild hepatitis 0.75–5.01 µkat/L | 38 (53.5) | 291 (63.1) | |||
| Severe hepatitis >5.01 µkat/L | 13 (18.3) | 34 (7.4) | |||
| Length of hospital stay (days) | |||||
| ≤3 | 29 (40.8) | 357 (77.4) | <0.001 | ||
| >3 | 42 (59.2) | 104 (22.6) | 3.07 (1.68–5.62) | <0.001 | |
| Prothrombin time (s) | |||||
| ≤15 | 54 (76.1) | 414 (89.8) | 0.001 | ||
| >15 | 17 (23.9) | 47 (10.2) | |||
| aPTT | 1.81 (1.003–3.26) | 0.04 | |||
| <35 | 26 (36.6) | 281 (61) | <0.001 | ||
| >35 | 45 (63.4) | 180 (39) | |||
| Platelets (per µL) | |||||
| <50 000 | 61 (85.9) | 345 (74.8) | 0.12 | ||
| 50000–100 000 | 7 (9.9) | 86 (18.7) | |||
| 100000–150 000 | 1 (1.4) | 22 (4.8) | |||
| >150000 | 2 (2.8) | 8 (1.7) | |||
Independent predictor for the length of hospital stay
Three hundred and eighty-six patients (72.6%) had length of hospital stay <3 days, while the rest (27.4%) had length of stay >3 days. We found that AKI was an independent predictor for increased length of hospital stay (OD 2.98; 95% CI 1.66–5.34) (Table 4).
Table 4.
Impact of AKI on the length of hospital stay and mortality
| Length of stay in hospital | |||
| AKI ≤ 3 days | AKI > 3 days | Univariate analysis | Multivariate analysis |
| 29 (7.5%) | 42 (28.8%) | P < 0.001 | OD [95%CI 2.98 (1.66–5.34)] P ≤ 0.001 |
| Mortality | |||
| AKI | No AKI | Univariate analysis | Multivariate analysis |
| 8 (11.3%) | 0 (0%) | P < 0.001 | |
Discussion
Tropical acute febrile illnesses are common causes of AKI in developing countries. DF along with other tropical infections like malaria, scrub typhus, enteric fever, leptospirosis and hantavirus have been reported to cause AKI [20]. AKI is a complication of DVI which has not been studied much. There are multiple proposed mechanisms for etiopathogenesis of renal impairment in DVI. Dengue causes capillary leakage and loss of fluid from the intravascular compartment leading to shock [9, 10] which may lead to decreased kidney perfusion and acute tubular necrosis. Possible etiological factors for AKI in DF include hypotension with either hemolysis or rhabdomyolysis and shock as reported in various case reports [8, 11–14]. On the other hand, unexplained AKI has also been reported in the literature [15]. Interestingly, dengue may cause glomerular injury in addition to the above-mentioned mechanisms as reported in one study [21]. The presence of viral antigen in tubular epithelial cells has been demonstrated [22]. Two experimental studies also provide evidence supporting possible glomerular injury in DVI [23, 24].
The incidence of AKI found in our study is higher than reported in other studies. Laoprasopwattana et al. have reported an incidence of 0.9% [16] in Thai children. Whereas Lee et al. described an incidence of 3.3% [17]. This difference could be because of different selection criteria used for defining AKI. However, it is interesting to note that both of the above-mentioned studies defined AKI as a rapid elevation of serum creatinine >2 mg/dL. They might have under-recognized AKIN Stage 1 AKI, which accounted for 64.8% of the patients in our study. A more recent study by Mehra et al. reported an incidence of 10.8% using the AKIN definition and their findings are comparable with ours [25] (Table 5).
Table 5.
Summary of case series on AKI in DVI
| Author | Year | Country | Study design/size | Main theme | Outcome |
|---|---|---|---|---|---|
| Laoprasopwattana et al. [16] | 2010 | Thailand | Case series/2893 cases | Outcome of DHF-caused AKI in Thai Children | Twenty-five patients (0.9%) developed AKI. Patients with DHF induced AKI were matched with those without AKI. AKI with DHF has a mortality of 64% and was associated with DHF-Grade IV (odds ratio 16.9; 95% CI) and obesity (odds ratio 6.3; 95% CI). Respiratory and liver failure along with major bleeding was found in those with AKI |
| Lee et al. [17] | 2009 | Taiwan | Case series/304 cases | Clinical characteristics, risk factors and outcomes in adults experiencing DHF complicated with acute renal failure. | 10 out of 304 patients with DSS had AKI. The rest were taken as control. DSS was independently associated with AKI and mortality was high in those with AKI. |
| Wiwanitkit et al. [18] | 2005 | Thailand | Case series | Acute renal failure in the fatal cases ofDHF, a summary in Thai death cases | AKI was found in 33.3% of cases of fatal DHF in contrast to 0.3% of all cases of DHF |
| Mehra et al. [25] | 2012 | India | Case series/233 cases | AKI in DF using AKIN criteria: incidence and risk factors | Twenty-four patients (10.8%). developed AKI and all-cause mortality was 9% |
We found coagulopathy and derangement of both intrinsic and extrinsic pathways in our case series. However, AKI was significantly associated with prolongation of aPTT. Thus, hypotension in combination with coagulation derangement might be the possible etiology for the development of AKI. Male gender was over represented in our case series and had a higher risk of developing AKI. Increased mobility of male population in our society might be putting them at higher risk of mosquito bite or it might be just because they have better access to health care in our part of the world.
Dengue is associated with significant morbidity and mortality as well as an enormous economic burden [26, 27]. The mean length of hospital stay in patients with dengue has been reported to be 3–4 days in various studies. Khan et al. [28] from Saudi Arabia reported length of stay of 4 days. Similarly, a study from Singapore reported the mean stay as 3 days [29]. Parkash et al. [30] reported a mean hospital stay of 4 days in patients with associated hepatitis. The mean length of stay in our study was 3.46 days, which is comparable with internationally reported data. Moreover, we found that AKI was associated with a longer hospital stay and hence is an independent predictor for length of hospitalization. We did not come across any published literature looking at the impact of AKI on hospital stay in patients with DVI.
Various studies from Pakistan have reported mortality of 2.6–2.7% in the general population infected with DVI [30, 31]. International data report a variable mortality ranging from 0 to 3.7% [32–35]. However, impact of AKI on mortality in dengue is less well studied. We found a significantly increased rate of mortality (11.3%) in patients with AKI, and interestingly no mortality in patients without AKI. Therefore, the presence of AKI in DVI predicts increased morbidity and mortality.
This study has several limitations. The study is retrospective in nature and is of limited clinical use as the study focused on inpatients, therefore excluding patients who visited outpatient clinics and other hospitals. Also, the study was limited to a single center. Moreover, histopathology reports in clinically indicated cases were not available to elucidate etiopathogenesis of AKI. Patients were only followed up until discharge and there was a lack of long-term follow-up. Prospective studies are needed with renal biopsy in clinically indicated cases along with a long-term follow-up to know more about the etiopathogenesis and outcome of AKI in DVI.
Conclusions
AKI in DVI causes significant morbidity and mortality. The presence of AKI in patients with DVI should be vigilantly monitored preferably in a special care unit. The presence of AKI should alert clinicians for admission and early initiation of supportive treatment under close monitoring in order to avoid morbidity and mortality associated with this complication.
Authors' contributions
M.A.K. planned and wrote the final manuscript. S.S., M.A.C., B.M. and Z.K. carried out data collection and entry into SPSS. J.T. and S.Y. reviewed the first draft of the manuscript. S.A.H. supervised and reviewed the study protocol and gave important suggestions throughout the study and in writing the manuscript.
Acknowledgements
The authors acknowledge Mr. Ghulam Rasool for typing the manuscript.
Conflict of interest statement. None declared.
References
- 1.O'Brien D, Tobin S, Brown GV, et al. Fever in returned travelers: review of hospital admissions for a 3-year period. Clin Infect Dis. 2001;33:603–609. doi: 10.1086/322602. [DOI] [PubMed] [Google Scholar]
- 2.MacLean J, Lalonde R, Ward B. Fever from the tropics. Travel Med Advisor. 1994;5:27.1–27.14. [Google Scholar]
- 3.Muto R. Summary of dengue situation in WHO Western Pacific Region. Dengue Bull. 1998;22:12–19. [Google Scholar]
- 4.Hayes CG, Baqar S, Ahmed T, et al. West Nile virus in Pakistan. 1. Sero-epidemiological studies in Punjab Province. Trans R Soc Trop Med Hyg. 1982;76:431–436. doi: 10.1016/0035-9203(82)90130-4. [DOI] [PubMed] [Google Scholar]
- 5.Chan YC, Salahuddin NI, Khan J, et al. Dengue haemorrhagic fever outbreak in Karachi, Pakistan, 1994. Trans R Soc Trop Med Hyg. 1995;89:619–620. doi: 10.1016/0035-9203(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 6.Ahsan T. Dengue fever: a regular epidemic? J Pak Med Assoc. 2008;58:1–2. [PubMed] [Google Scholar]
- 7.Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karakus A, Banga N, Voorn GP, et al. Dengue shock syndrome and rhabdomyolysis. Neth J Med. 2007;65:78–81. [PubMed] [Google Scholar]
- 9.George R, Liam CK, Chua CT, et al. Unusual clinical manifestations of dengue virus infection. Southeast Asian J Trop Med Public Health. 1988;19:585–590. [PubMed] [Google Scholar]
- 10.Chacko B, John GT, Jacob CK, et al. Dengue shock syndrome in a renal transplant recipient. Transplantation. 2004;77:634–635. doi: 10.1097/01.tp.0000114608.44000.d7. [DOI] [PubMed] [Google Scholar]
- 11.Garcia JH, Rocha TD, Viana CF, et al. Dengue shock syndrome in a liver transplant recipient. Transplantation. 2006;82:850–851. doi: 10.1097/01.tp.0000235151.60237.fe. [DOI] [PubMed] [Google Scholar]
- 12.Gunasekera HH, Adikaram AV, Herath CA, et al. Myoglobinuric acute renal failure following dengue viral infection. Ceylon Med J. 2000;45:181. [PubMed] [Google Scholar]
- 13.Radakovic-Fijan S, Graninger W, Muller C, et al. Dengue hemorrhagic fever in a British travel guide. J Am Acad Dermatol. 2002;46:430–433. doi: 10.1067/mjd.2002.111904. [DOI] [PubMed] [Google Scholar]
- 14.Wiersinga WJ, Scheepstra CG, Kasanardjo JS, et al. Dengue fever-induced hemolytic uremic syndrome. Clin Infect Dis. 2006;43:800–801. doi: 10.1086/507111. [DOI] [PubMed] [Google Scholar]
- 15.Lima EQ, Gorayeb FS, Zanon JR, et al. Dengue haemorrhagic fever-induced acute kidney injury without hypotension, haemolysis or rhabdomyolysis. Nephrol Dial Transplant. 2007;22:3322–3326. doi: 10.1093/ndt/gfm431. [DOI] [PubMed] [Google Scholar]
- 16.Laoprasopwattana K, Pruekprasert P, Dissaneewate P, et al. Outcome of dengue hemorrhagic fever-caused acute kidney injury in Thai children. J Pediatr. 2010;157:303–309. doi: 10.1016/j.jpeds.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Lee IK, Liu JW, Yang KD. Clinical characteristics, risk factors, and outcomes in adults experiencing dengue hemorrhagic fever complicated with acute renal failure. Am J Trop Med Hyg. 2009;80:651–655. [PubMed] [Google Scholar]
- 18.Wiwanitkit V. Acute renal failure in the fatal cases of dengue hemorrhagic fever, a summary in Thai death cases. Ren Fail. 2005;27:647. doi: 10.1080/08860220500200916. [DOI] [PubMed] [Google Scholar]
- 19.Dengue hemorrhagic fever: diagnosis, treatment, prevention and control. Geneva: World Health Organization; 1997. [Google Scholar]
- 20.Basu G, Chrispal A, Boorugu H, et al. Acute kidney injury in tropical acute febrile illness in a tertiary care centre–RIFLE criteria validation. Nephrol Dial Transplant. 2011;26:524–531. doi: 10.1093/ndt/gfq477. [DOI] [PubMed] [Google Scholar]
- 21.Boonpucknavig V, Bhamarapravati N, Boonpucknavig S, et al. Glomerular changes in dengue hemorrhagic fever. Arch Pathol Lab Med. 1976;100:206–212. [PubMed] [Google Scholar]
- 22.Jessie K, Fong MY, Devi S, et al. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J Infect Dis. 2004;189:1411–1418. doi: 10.1086/383043. [DOI] [PubMed] [Google Scholar]
- 23.Boonpucknavig S, Vuttiviroj O, Boonpucknavig V. Infection of young adult mice with dengue virus type 2. Trans R Soc Trop Med Hyg. 1981;75:647–653. doi: 10.1016/0035-9203(81)90142-5. [DOI] [PubMed] [Google Scholar]
- 24.Barreto DF, Takiya CM, Paes MV, et al. Histopathological aspects of Dengue-2 virus infected mice tissues and complementary virus isolation. J Submicrosc Cytol Pathol. 2004;36:121–130. [PubMed] [Google Scholar]
- 25.Mehra N, Patel A, Abraham G, et al. Acute kidney injury in dengue fever using acute kidney injury network criteria: incidence and risk factors. Tropical doctor. 2012;42:160–162. doi: 10.1258/td.2012.120023. [DOI] [PubMed] [Google Scholar]
- 26.Meltzer MI, Rigau-Perez JG, Clark GG, et al. Using disability-adjusted life years to assess the economic impact of dengue in Puerto Rico: 1984–1994. Am J Trop Med Hyg. 1998;59:265–271. doi: 10.4269/ajtmh.1998.59.265. [DOI] [PubMed] [Google Scholar]
- 27.Okanurak K, Sornmani S, Indaratna K. The cost of dengue hemorrhagic fever in Thailand. Southeast Asian J Trop Med Public Health. 1997;28:711–717. [PubMed] [Google Scholar]
- 28.Khan NA, Azhar EI, El-Fiky S, et al. Clinical profile and outcome of hospitalized patients during first outbreak of dengue in Makkah, Saudi Arabia. Acta Trop. 2008;105:39–44. doi: 10.1016/j.actatropica.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Lye D, Chan M, Lee V, et al. Do young adults with uncomplicated dengue fever need hospitalisation? A retrospective analysis of clinical and laboratory features. Singapore Med J. 2008;49:476–479. [Original article] [PubMed] [Google Scholar]
- 30.Parkash O, Almas A, Jafri SM, et al. Severity of acute hepatitis and its outcome in patients with dengue fever in a tertiary care hospital Karachi, Pakistan (South Asia) BMC Gastroenterol. 2010;10:43. doi: 10.1186/1471-230X-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wasay M, Channa R, Jumani M, et al. Changing patterns and outcome of Dengue infection; report from a tertiary care hospital in Pakistan. J Pak Med Assoc. 2008;58:488–489. [PubMed] [Google Scholar]
- 32.Seet R, Ooi EE, Wong HB, et al. An outbreak of primary dengue infection among migrant Chinese workers in Singapore characterized by prominent gastrointestinal symptoms and a high proportion of symptomatic cases. J Clin Virol. 2005;33:336–340. doi: 10.1016/j.jcv.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez D, Castro OE, Kouri G, et al. Classical dengue hemorrhagic fever resulting from two dengue infections spaced 20 years or more apart: Havana, dengue 3 epidemic, 2001–2002. Int J Infect Dis. 2005;9:280–285. doi: 10.1016/j.ijid.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Wichmann O, Hongsiriwon S, Bowonwatanuwong C, et al. Risk factors and clinical features associated with severe dengue infection in adults and children during the 2001 epidemic in Chonburi, Thailand. Trop Med Int Health. 2004;9:1022–1029. doi: 10.1111/j.1365-3156.2004.01295.x. [DOI] [PubMed] [Google Scholar]
- 35.Harris E, Videa E, Perez L, et al. Clinical, epidemiologic, and virologic features of dengue in the 1998 epidemic in Nicaragua. Am J Trop Med Hyg. 2000;63:5–11. doi: 10.4269/ajtmh.2000.63.5. [DOI] [PubMed] [Google Scholar]


