Abstract
Hyperphosphataemia is a clinical consequence of the advanced stages of chronic kidney disease (CKD). Considerable evidence points to a role of hyperphosphataemia in the pathogenesis of CKD-associated cardiovascular (CV) complications, including vascular calcification, and with increased all-cause and CV mortality. These observations place management of hyperphosphataemia at the centre of CKD treatment. Although our increased understanding of the physiological role of FGF-23 may provide a long-term alternative biomarker of phosphate load and underlying disease progression, regular determination of serum phosphate is currently the most frequently used parameter to evaluate phosphate load in clinical practice. This review considers the challenges physicians and patients face in trying to control hyperphosphataemia. Amongst these are the limitations of dietary phosphate restriction, giving rise to the need for phosphate binder therapy to maintain serum phosphate control. Once the decision to use phosphate binders has been made, considerations include the relative efficacy, different potential side effects and pill burden associated with various phosphate binders. Although a number of phosphate binders are available, adherence poses a major obstacle to effective treatment. This emphasizes that further improvements to phosphate binder therapy can be made. Evaluation of novel agents and their potential role in the clinic should continue.
Keywords: chronic kidney disease, hyperphosphataemia, phosphate binder
Introduction
Currently, the global prevalence of chronic kidney disease (CKD) is estimated to be around 7% in people aged ≥30 years, with a higher prevalence (23–36%) in people aged ≥64 years [1]. CKD is a key determinant of poor health outcomes in patients with major noncommunicable diseases, contributing to their substantial worldwide burden [2]. Elevated serum phosphate levels (hyperphosphataemia) are an unavoidable clinical consequence of the advanced stages of CKD [3, 4]. Hyperphosphataemia is linked with a number of serious clinical complications, including vascular calcification [5] and left ventricular hypertrophy [6], as well as increased all-cause and cardiovascular (CV) mortality [7, 8]. Large observational studies have shown a graded association between levels of serum phosphate and all-cause mortality in patients undergoing dialysis [7–11]. Given these observations, one of the principal challenges in the management of patients in the advanced stages of CKD is control of hyperphosphataemia. This review focuses on selected questions surrounding the diagnosis and management of hyperphosphataemia in daily clinical practice.
What is the effect of hyperphosphataemia in patients?
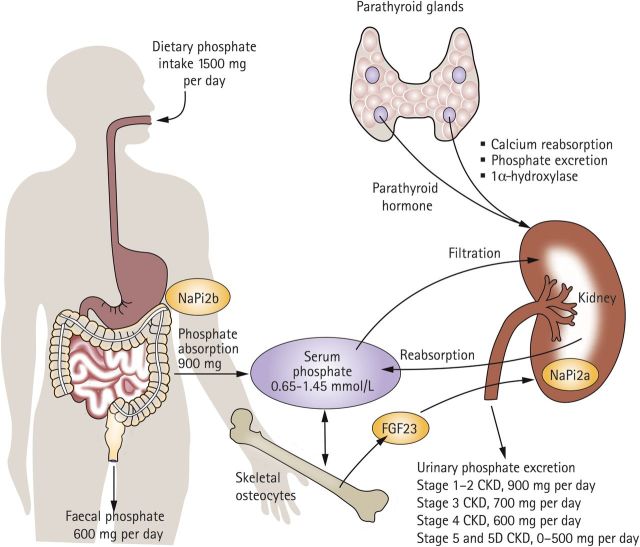
Although an in-depth description of the pathophysiology of hyperphosphataemia has been provided elsewhere [6], a brief overview is helpful to provide a basis for discussion of therapeutic approaches. In healthy individuals, phosphate homeostasis is maintained by regulation of dietary absorption by the gastrointestinal (GI) tract, bone turnover and mineralization, and renal excretion [12]. Following renal filtration, most of the serum phosphate is reabsorbed across the epithelium of the kidney proximal tubule (Figure 1) [13]. The sodium-dependent phosphate co-transporter proteins play a role in this process, mediating phosphate reabsorption from the filtrate across the renal proximal tubules (NaPi-2a and 2c) and phosphate absorption across the intestinal apical brush border (NaPi-2b; Figure 1) [13, 14]. In patients with impaired renal function, this homeostasis is disrupted as renal excretion of phosphate generally declines with increasing severity of CKD [12]. Initial compensatory mechanisms, including elevated secretion of parathyroid hormone (PTH) and fibroblast growth factor-23 (FGF-23) and temporarily elevated serum phosphate levels, reduce phosphate reabsorption and thus maintain serum phosphate levels in normal to near-normal range. However, hyperphosphataemia occurs almost inevitably in the later stages of CKD, when dietary intake of phosphate exceeds the rate of renal excretion (Figure 1) [12, 13].
Fig. 1.
Phosphate homeostasis is dysregulated in patients with late-stage CKD. Reprinted with permission from Macmillan Publishers Ltd [13].
Multiple putative mechanisms link elevated serum phosphate levels with increased CV morbidity and mortality. These include the direct mechanisms of vascular injury by means of vascular calcification, oxidative stress or endothelial dysfunction [12]. Indirect mechanisms associated with CV damage include chronically increased levels of FGF-23 [15], inhibition of calcitriol synthesis and increased levels of PTH [12]. Strictly speaking, the effect of high phosphate levels on the progression of secondary hyperparathyroidism is also in part a direct one, by prolonging the half-life of PTH mRNA in the parathyroid gland and favouring PTH secretion [16].
Of these pathogenetic mechanisms, research in patients with CKD has largely focused on the role of elevated phosphate levels in vascular calcification. Hyperphosphataemia drives vascular calcification by regulating gene expression in vascular smooth muscle cells, causing them to undergo an osteochondrogenic phenotype change [5]. Vascular calcifications are associated with CV morbidity and are an independent predictor of all-cause and CV mortality in patients with CKD [17, 18]. Even in patients with CKD who were not receiving dialysis (N = 181), those with a coronary artery calcification score of >100 AU (Agatston unit) were found to have a significantly higher risk of cardiac death or myocardial infarction than those with a score of ≤100 AU [hazard ratio (HR) for the former group: 4.11, confidence interval (CI): 1.77–9.57, P < 0.0006] [19]. Together, these observations support the link between vascular calcification, CV events and increased mortality. Given the high rate of CV mortality in patients with CKD, the necessity of screening patients for vascular calcification, determining which patients are at high risk of CV events and which steps to take to attenuate further progression, is a matter of ongoing debate [20]. Regardless of the outcome of this debate, evidence points to a role of hyperphosphataemia in the pathogenesis of CKD-associated CV complications, making it a focus of clinical management of the disease. However, of note in this context is that definitive data from prospective interventional studies comparing the efficacy of phosphate binder treatment with that of no phosphate binder treatment in patients with CKD stages 3–4 not receiving dialysis are scarce. Whereas one short-term study (N = 148) reported no beneficial effect from various phosphate binders on the progression of arterial calcification [21], a longer study (N = 90) demonstrated that non-calcium-based binders halted the progression of vascular calcification [22]. As yet, no prospective study has evaluated the long-term effects of phosphate binder treatment versus no phosphate binder treatment in dialysis patients.
Does evidence support assessment of FGF-23 to guide treatment decisions?
A cornerstone of clinical monitoring in patients with impaired renal function involves assessing and managing serum phosphate levels. The Kidney Disease: Improving Global Outcomes (KDIGO) and Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines highlight the importance of regular determination of serum phosphate and provide recommendations for phosphate levels (Table 1) [3, 23]. It is also acknowledged that serum phosphate fluctuates more than, for example, serum calcium; as such, trends in serum phosphate, rather than single values, should drive treatment choices [3, 4]. In this context, the more recent KDIGO guidelines no longer recommend target levels of the calcium × phosphate product [3], because this is indeed mostly driven by the serum phosphate concentrations and because serum calcium levels are not predictive of overall calcium balance.
Table 1.
Recommended serum calcium, albumin-corrected calcium, phosphate and PTH levels in stage 5 CKD [3,23]
| Organization (year) | Calcium | CAAlb | Phosphate | PTH |
|---|---|---|---|---|
| KDOQI (2003) | Not reported | Stage 3–4: within the normal range; stage 5: 2.10–2.37 mmol/L (8.4–9.5 mg/dL) | Stage 3–4: 0.87–1.49 mmol/L (2.7–4.6 mg/dL), Stage 5: 1.13–1.78 mmol/L (3.5–5.5 mg/dL) | 16.5–33.0 pmol/L (150–300 pg/mL) |
| KDIGO (2009) | Within the normal range | Not reported | Within the normal range; stage 5D: Toward the normal range | Stage 5D: 2–9× upper normal limit for the assay |
Serum phosphate does not increase until the estimated glomerular filtration rate falls below 0.5 mL/s/1.73m2 (30 mL/min/1.73m2), and as such it has been asserted that it may not be a sufficiently sensitive indicator of phosphate overload [6]. This has led to the investigation of possible alternative markers for future use in the clinical management of patients with CKD [24].
Emerging data highlight the potential of circulating FGF-23, a phosphatonin hormone that is released from osteocytes, most likely in response to phosphate overload [6], as a novel marker to identify patients with CKD who are at the highest risk of disease progression, CV disease and death [24–26]. In addition, it has been proposed that assessment of FGF-23 levels could help detect individuals who might benefit from early phosphate-lowering interventions before the onset of overt hyperphosphataemia [24–28]. Of particular interest is the proposal that elevated serum FGF-23 is independently associated with adverse outcomes in patients with CKD [6]. As such, it may be a biomarker of phosphate status, reflecting underlying disease progression.
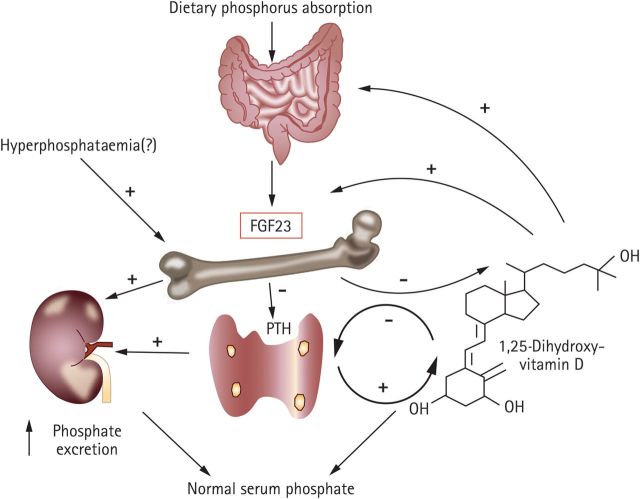
The primary physiological roles of FGF-23 are two-fold: it inhibits the reabsorption of renal phosphate, thereby increasing the rate of urinary phosphate excretion, and it suppresses the production of 1,25-dihydroxyvitamin D and increases its catabolism by the kidney, thereby protecting the body from excessive vitamin D exposure (Figure 2) [29, 30]. As such, FGF-23 plays a central adaptive role in phosphate and 1,25-dihydroxyvitamin D homeostasis in healthy individuals, but may equally be involved in the pathogenesis of CKD [31]. In patients with CKD, circulating concentrations of FGF-23 increase progressively with declining renal capacity for phosphate excretion [27, 31]. Findings from animal studies and genetic research suggest that elevated FGF-23 may reflect pathogenetic changes in bone and/or kidney health [32, 33]. Other studies have shown an independent association of FGF-23 with early pathogenetic mechanisms, such as increased left ventricular mass [34–36]. Taken together, these studies suggest that features such as left ventricular hypertrophy, which are commonly observed in patients with CKD, may be an adverse consequence of adaptive mechanisms that involve FGF-23, triggered in response to phosphate overload [6].
Fig. 2.
FGF-23 regulatory systems in phosphate metabolism. Reprinted with permission from Macmillan Publishers Ltd [30].
Although FGF-23 may have the potential to provide a better understanding of long-term phosphate status compared with the assessment of serum phosphate alone, the solution may not be entirely simple. The mechanism which regulates FGF-23 secretion from bone remains unclear, and the presence of modulators, or the down-regulation of co-factors such as klotho, may confound any signal from FGF-23 relating to underlying disease progression [33]. From a practical perspective, there is no validated standard assay for FGF-23 yet, and consequently, no reference range for interpretation of FGF-23 levels in clinical practice. Furthermore, in a recent study in a rat model of CKD, specific antagonism of FGF-23 increased mortality risk in the animals [37], calling into question the feasibility of developing therapies targeting FGF-23. In the meantime, assessment of FGF-23 may provide additional insights into the effect of different treatments on CKD progression to those provided by serum phosphate assessments. For example, an open-label randomized trial in 100 patients with stage 4 CKD showed that sevelamer was associated with a significant decrease in FGF-23 levels (P = 0.002) and increase in flow-mediated vasodilation (P < 0.001) from baseline compared with calcium acetate; both treatments were associated with a significant reduction in serum phosphate from baseline (P < 0.01), although this was more marked with the phosphate binder sevelamer [38]. Nonetheless, the measurement of serum phosphate and the fractional excretion of phosphate remain the primary tools for the physician, with regular determination of serum phosphate being the most frequently used parameter to evaluate phosphate load in clinical practice.
Can adequate phosphate control be achieved with dietary restriction?
Achieving recommended guideline serum phosphate levels can be challenging. In the Dialysis Outcomes and Practice Patterns Study (DOPPS) II, serum phosphate levels remained uncontrolled in 56% of patients with CKD receiving dialysis, with 9% and 47% of patients having serum phosphate levels <1.13 mmol/L (3.5 mg/dL) and >1.78 mmol/L (5.5 mg/dL), respectively [39]. While most of the phosphate burden is due to intestinal absorption, it remains to be considered that high or low turnover bone disease may also contribute to intractable hyperphosphataemia.
Therefore, multiple strategies can be implemented to control phosphate homeostasis in patients with CKD. These include dietary restriction and removal via dialysis or intensive (nocturnal or short daily) dialysis regimens. Pharmacologic interventions include reduction of intestinal phosphate absorption by administration of phosphate binders and suppression of PTH secretion and release by administration of calcimimetics, in order to normalize bone turnover in high turnover states. In addition, as overtreatment with active vitamin D analogues may favour both phosphate absorption and low bone turnover, dose reduction or cessation may have to be considered in individual patients.
Dietary restriction of phosphate is recommended in both the KDIGO and KDOQI guidelines [3, 23]. Support for this is provided by an observational study, which used questionnaires to assess dietary phosphorus and protein intake in 224 maintenance haemodialysis patients over 5 years of follow-up. The impact of phosphorus intake on patients was clear: higher levels of dietary phosphorus intake and a higher ratio of dietary phosphorus to protein were associated with an increased risk of death [40].
However, although dietary phosphate restriction can reduce serum phosphate levels, drawbacks to this approach include protein–energy wasting, which is itself an independent determinant of morbidity and mortality in dialysis patients [41]. Indeed, the risk associated with controlling serum phosphate by restricting dietary protein intake may outweigh the benefit of improved serum phosphate control [42]. This is supported by a post-hoc analysis of data from 1751 patients undergoing haemodialysis who were enrolled in the Haemodialysis Study, in which the prescribed recommendation for daily phosphate intake was recorded. The results showed that prescribed dietary phosphate restriction was not associated with a survival benefit [43].
A further complication of limiting dietary intake of phosphorus is that, whilst it may be relatively easy for patients to avoid foods which are naturally high in phosphate, it is more difficult to avoid consumption of processed food rich in phosphate-containing additives. These additives contain a form of phosphate that is more readily absorbed than that found in foods naturally high in phosphorus [44, 45]. The findings of the Chronic Renal Insufficiency Cohort study (N = 2879) showed higher serum phosphate levels in patients on the lowest income compared with those on the highest income, despite comparable phosphate intake. These observations were thought to reflect the different types of phosphates consumed in the different groups, with those on a low income consuming greater amounts of convenience food containing phosphate additives [46]. The widespread use of phosphate-containing additives has substantially increased our daily phosphate intake, and the frequent absence of phosphate levels on food labels makes it difficult to ascertain the phosphate content of food. These difficulties may even extend to the dieticians providing guidance to patients, as software programmes used to assess dietary composition have been shown to underestimate the phosphorus content of processed foods [47].
Poor knowledge of phosphate levels in food and phosphate management has been reported in haemodialysis patients, even in those patients taking phosphate binder medication [48]. In a randomized study, 145 dialysis patients received education on how to avoid foods containing phosphorus additives when purchasing food in shops or when eating out. These patients showed a 3-month decrease in serum phosphate levels of 0.19 mmol/L [0.6 mg/dL; 95% CI: –0.32 mmol/L to –0.032 mmol/L (–1.0 to –0.1 mg/dL)] greater than that observed in the control group (n = 134) which did not receive guidance [49]. This indicates that patient education can aid dietary phosphate restriction and contribute to clinically significant improvements in serum phosphate levels.
However, even with careful dietary modification, hidden sources of phosphate mean that dietary limitation of phosphate intake is difficult, and often remains an inadequate means of controlling hyperphosphataemia. Given these limitations, treatment with oral phosphate binders is an essential component of phosphate management for most patients undergoing dialysis. This may apply for those patients who are failing to achieve phosphate control through dietary restriction of phosphate intake, but also for those patients who are experiencing nutritional problems such as protein–energy wasting owing to following a strict dietary regimen.
What are the key considerations in phosphate binder treatment?
Data from three observational studies have shown a survival benefit associated with the early administration of phosphate binders [50–52]. In the DOPPS study mentioned above, which included 23 894 haemodialysis patients, 6283 deaths were observed during follow-up (median time at risk: 1.92 years). Patients receiving phosphate binder treatment had a 25% reduction in the risk of death compared with those who did not receive phosphate binders (HR: 0.75; 95% CI: 0.68–0.83); in models adjusted for nutritional factors, a 12% lower risk of death was reported (HR: 0.88; 95% CI: 0.80–0.97) [52]. A study of 8610 incident haemodialysis patients found that 1-year all-cause mortality of patients who received phosphate binders within 90 days of starting haemodialysis (n = 3555) was significantly lower than in those who did not (n = 5055; relative risk: 0.58; 95% CI: 0.52–0.66, P < 0.0001) [50]. Recently published data from 6321 patients on haemodialysis included in the COSMOS study also indicate that the use of phosphate binders, either alone or in combination regimens, was associated with a significantly lower risk of all-cause mortality [51].
With evidence suggesting that phosphate binder treatment should be considered a central component of the management of hyperphosphataemia, it is worth considering how to optimize this treatment approach. Ideally, a phosphate binder should effectively bind dietary phosphate regardless of pH, have minimal systemic absorption, few side effects, good palatability, a low pill burden and be available at a low cost [53].
In the 1970s, aluminium represented the mainstay of phosphate-binding therapy; this treatment was largely abandoned when cases of systemic aluminium toxicity arose [13]. However, it has since been established that systemic exposure to aluminium can also arise from high aluminium concentrations in haemodialysis water [54]. The next class of phosphate binders to be introduced, and still used extensively today, was the calcium-based binders, calcium carbonate or calcium acetate [13]. Calcium-based binders have been shown to be more effective in reducing serum phosphate levels than sevelamer hydrochloride (HCl) in dialysis patients in the randomized, double-blind CARE study (N = 100) [55] and in a retrospective chart review (N = 55) [56]. In addition, in a prospective 42-month study including 1347 haemodialysis patients, those prescribed sevelamer HCl had a higher mortality risk compared with those prescribed calcium carbonate (HR: 1.46; 95% CI: 1.1–1.9) [57]. However, after concerns about hypercalcaemia and the risk of vascular calcification [58, 59], the option of non-calcium-based agents was explored further.
One of these compounds is sevelamer, a non-calcium anion-exchange resin [13, 60]. Sevelamer was initially available as sevelamer HCl and most clinical studies have used this formulation. However, sevelamer HCl was associated with reduced serum bicarbonate concentration, prompting concerns about metabolic acidosis [61]. Subsequently, a different formulation, sevelamer carbonate, was developed [62, 63]. A Cochrane review and meta-analysis of studies including patients with CKD stages 3–5D according to KDOQI guidelines indicated that sevelamer significantly decreases end-of-treatment serum phosphate levels compared with placebo (based on one study only, including 36 patients), although comparisons of reduction in serum phosphate with calcium-based binders favoured the latter group [64]. In the key Dialysis Clinical Outcomes Revisited study (N = 2103), results of the primary analysis did not show any difference in overall mortality among patients on dialysis receiving sevelamer compared with those receiving a calcium-based binder [65]. A randomized, open-label study compared CV (primary endpoint), overall and non-CV mortality in incident dialysis patients who were treated with sevelamer (n = 232) with that in patients receiving calcium carbonate (n = 234). After a mean 28-month follow-up, CV mortality in the sevelamer group was ten times lower than that in the calcium carbonate group (P < 0.001). A significant reduction in all-cause mortality, though not in non-CV mortality, was also noted in the sevelamer group [66, 67]. Similar results were reported in an observational study in patients with stage 5D CKD, which found a significant reduction in all-cause and CV cumulative mortality in patients receiving sevelamer (n = 172) compared with a matched control group receiving calcium carbonate (n = 264) or no phosphate binder (n = 36) [68].
Investigations into the effects of sevelamer on vascular calcification have shown variable results, with some reporting that sevelamer attenuated progression of vascular calcification but others reporting no effect [22, 69–74]. A recent meta-analysis (N = 3271) suggested that the effect of sevelamer on vascular calcification in haemodialysis patients was not significant compared with that of calcium-based phosphate binders [75]. However, data from three clinical trials, two of which were included in the meta-analysis, have shown slower progression of coronary artery calcification in haemodialysis patients treated with sevelamer compared with those treated with a calcium-based binder [69, 72, 76]. Given the discrepancies between study results on this matter, additional large, prospective studies would be welcome to ascertain the effect of sevelamer on vascular calcification. Of note, it has also been reported that sevelamer is associated with pleiotropic effects in haemodialysis patients which may be beneficial for vascular protection, including a prolonged significant rise in serum levels of the calcification inhibitor fetuin A [77] and lowering of total and low-density lipoprotein cholesterol [71, 74, 77].
An increased risk of GI side effects has been reported with sevelamer compared with calcium-based binders [64], which may contribute to poor adherence. In addition, a major limitation of both sevelamer and calcium acetate in terms of their effect on treatment adherence is their high pill burden (6–12 tablets per day) [12].
Lanthanum carbonate, another non-calcium, metal-based phosphate-binding agent, has been shown to have similar efficacy to both calcium-based phosphate binders (78% of patients in the control arm were receiving calcium-based binders; N = 1359) [78] and sevelamer (N = 181) [79] in terms of reducing serum phosphate levels in haemodialysis patients. Similar to sevelamer, recent data suggest that lanthanum reduces FGF-23 levels in patients with stage 3 CKD [80]. Lanthanum carbonate is also associated with a lower pill burden than sevelamer or calcium-based binders [12]. In addition, in a Phase III, open-label study including 98 haemodialysis patients, more patients treated with lanthanum carbonate exhibited normalization of bone turnover than those treated with calcium carbonate, and fewer exhibited adynamic bone [81]. However, there are concerns about side effects associated with lanthanum carbonate, especially GI side effects [82, 83]. In addition, questions about the potential long-term accumulation of lanthanum in the liver have been raised, in part owing to the results from a rat model of chronic renal failure [84]. However, a post-hoc analysis of a subset of data from four Phase III trials of lanthanum, including data from some haemodialysis patients who were treated for up to 6 years, showed no detrimental changes in transaminase or bilirubin levels or significant increase in liver-associated adverse events compared with control groups [85].
Do patients take their phosphate binders?
One of the major potential drawbacks of treatment with currently available phosphate binders is that their effectiveness may be compromised by poor treatment adherence, possibly owing to side effects, high pill burden or a combination of these. Studies have shown that amongst haemodialysis patients, non-adherent patients are more likely to have elevated serum phosphate levels than their adherent counterparts [86, 87]. This is sobering, considering that the rates of non-adherence to phosphate binders are reported to range from 22 to 74%, with a mean in one systematic review of 51% [88]. The wide variation in reported rates of non-adherence can be attributed to differences in the way non-adherence was defined and assessed across studies [88].
Pill burden may be an important contributing factor to poor adherence in patients with CKD. A cross-sectional study conducted in 233 maintenance dialysis patients in the US showed that higher pill burden was associated with reduced adherence and lower health-related quality of life (HRQoL); almost two-thirds of patients (62%) were non-adherent [89]. Phosphate binders accounted for approximately one half of the daily pill burden, with the median daily pill number required for phosphate binder therapy being 9 (inter-quartile range: 6) [89]. Furthermore, this study showed that increasing the number of prescribed pills did not improve phosphate control [89].
These data suggest that the long-term maintenance of phosphate control using phosphate binders can be further improved for patients with CKD, in particular by considering ways of improving adherence, possibly by reducing pill burden.
Need for new therapies
A number of new therapeutic approaches to improve phosphate control in patients with CKD have been developed in recent years or are under clinical investigation; amongst these is intensive haemodialysis (more frequent or extended dialysis sessions). Analysis of data from the Frequent Haemodialysis Network Daily and Nocturnal Trials showed that daily and nocturnal dialysis regimens were associated with a reduction in mean serum phosphate of 0.15 mmol/L (0.46 mg/dL; 95% CI: 0.04–0.25 mmol/L [0.13–0.78 mg/dL]) and 0.40 mmol/L (1.24 mg/dL; 95% CI: 0.22–0.58 mmol/L [0.68–1.79 mg/dL]) compared with patients receiving conventional haemodialysis [90]. However, these intensive sessions may only be practicable for a relatively small proportion of patients [13].
Novel pharmacologic approaches may widen options for patients with CKD (Table 2). For example, inhibition of the sodium-dependent phosphate co-transporters is an additional opportunity for therapeutic intervention to control hyperphosphataemia. Illustrating this principle, administration of niacin or nicotinamide–which inhibits the sodium-dependent phosphate co-transporter NaPi-2b–concomitantly with phosphate binders was associated with a significant reduction in serum phosphate from 2.1 mmol/L (6.45 mg/dL) to 1.71 mmol/L (5.28 mg/dL; P = 0.002) during an 8-week study in 33 dialysis patients [91]. There have, however, been reports of patients experiencing GI side effects (diarrhoea) when receiving this combination [91, 92]. In addition, existing approaches can still be refined. There is a need for new phosphate binders that address the limitations of current treatments and enable patients to achieve and maintain adequate control of serum phosphate levels. Potentially, improved clinical outcomes can be achieved by improving the side effect profile, reducing pill burden, increasing treatment adherence, and allowing patients greater nutritional freedom.
Table 2.
| Compound | Class | Mechanism of action | Stage of clinical development | Company |
|---|---|---|---|---|
| Niacin/nicotinamide | Amide of vitamin B3 | Inhibits the sodium-dependent phosphate co-transporter | Phase III ongoing | N/A |
| Calcium acetate/magnesium carbonate | Combination phosphate binder | Calcium acetate and magnesium carbonate bind phosphate, forming non-absorbable complexes | Approved (EU only) | Fresenius Medical Care |
| PA21 | Iron-based phosphate binder | Iron(III)-oxyhydroxide binds phosphate by replacing hydroxide groups, forming non-absorbable complexes | Phase III ongoing | Vifor Pharma Ltd |
| Ferric citrate | Iron-based phosphate binder | Binds phosphate and forms non-absorbable complexes | Phase III ongoing | Numerous, including: Panion & BF Biotech Inc., Keryx Biopharmaceuticals, Torii Pharmaceutical Co., Ltd |
| Colestilan (MCI-196) | Non-calcium anion exchange resin | Binds phosphate and bile acid anions | Phase III ongoing | Mitsubishi Tanabe Pharma Corporation |
| HS219, a chitosan-loaded chewing gum | Natural polymer (dietary supplement) | Binds salivary phosphate | Phase II completed | KDL Inc. |
A combination calcium acetate/magnesium carbonate phosphate binder has shown a non-inferior reduction of serum phosphate levels compared with sevelamer in a 24-week randomized study in 255 haemodialysis patients, with no difference between groups in episodes of hypo- and hypercalcaemia [93]. In addition, a novel iron(III)-oxyhydroxide-based phosphate binder in clinical development may meet these criteria. Encouraging results have been reported in Phase I [94] and II studies [95]. In addition, the iron-based phosphate binder ferric citrate is also undergoing clinical evaluation [96]. The approach of binding salivary phosphate with a chitosan chewing gum during periods of fasting, to complement the use of phosphate binders, is also being investigated [97].
Conclusions
A strong evidence base places the diagnosis and management of hyperphosphataemia at the centre of patient care in CKD. Despite this, related diagnostic procedures and treatment decisions are far from straightforward and patients still suffer severe clinical complications and reduced QoL. Now that we have a greater understanding of the underlying pathophysiology of hyperphosphataemia, our focus can shift to identifying more reliable diagnostic markers of mineral- and bone-related disorders and more specific treatments to improve patients' clinical outcomes and their QoL. This requires us to assess the treatment of hyperphosphataemia in the context of the wider treatment that patients are receiving, consider pharmacologic interventions alongside available options for dialysis and dietary control, and continue to evaluate novel treatments and their potential place in the clinic.
Conflict of interest statement
None declared.
Acknowledgements
Funding. This review article was funded by Vifor Pharma Ltd. The authors would like to acknowledge medical writing support from Axon Communications in the preparation of this manuscript.
References
- 1.Zhang QL, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health. 2008;8:117. doi: 10.1186/1471-2458-8-117. doi:10.1186/1471-2458-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Couser WG, Remuzzi G, Mendis S, et al. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80:1258–1270. doi: 10.1038/ki.2011.368. doi:10.1038/ki.2011.368. [DOI] [PubMed] [Google Scholar]
- 3.Kidney Disease: Improving Global Outcomes. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD–MBD) Kidney Int Suppl. 2009;76:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 4.Kasiske BL, Zeier MG, Chapman JR, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int. 2010;77:299–311. doi: 10.1038/ki.2009.377. doi:10.1038/ki.2009.377. [DOI] [PubMed] [Google Scholar]
- 5.Giachelli CM. The emerging role of phosphate in vascular calcification. Kidney Int. 2009;75:890–897. doi: 10.1038/ki.2008.644. doi:10.1038/ki.2008.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Parra E, Tunon J, Egido J, et al. Phosphate: a stealthier killer than previously thought? Cardiovas Pathol. 2012;21:372–381. doi: 10.1016/j.carpath.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Floege J, Kim J, Ireland E, et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant. 2011;26:1948–1955. doi: 10.1093/ndt/gfq219. doi:10.1093/ndt/gfq219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;52:519–530. doi: 10.1053/j.ajkd.2008.03.020. doi:10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Block GA, Hulbert-Shearon TE, Levin NW, et al. Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. doi:10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 10.Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. JASN. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. doi:10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 11.Slinin Y, Foley RN, Collins AJ. Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: the USRDS waves 1, 3, and 4 study. JASN. 2005;16:1788–1793. doi: 10.1681/ASN.2004040275. doi:10.1681/ASN.2004040275. [DOI] [PubMed] [Google Scholar]
- 12.Tonelli M, Pannu N, Manns B. Oral phosphate binders in patients with kidney failure. N Engl J Med. 2010;362:1312–1324. doi: 10.1056/NEJMra0912522. doi:10.1056/NEJMra0912522. [DOI] [PubMed] [Google Scholar]
- 13.Hutchison AJ, Smith CP, Brenchley PE. Pharmacology, efficacy and safety of oral phosphate binders. Nat Rev Nephrol. 2011;7:578–589. doi: 10.1038/nrneph.2011.112. doi:10.1038/nrneph.2011.112. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto K, Haito-Sugino S, Kuwahara S, et al. Sodium-dependent phosphate cotransporters: lessons from gene knockout and mutation studies. J Pharm Sci. 2011;100:3719–3730. doi: 10.1002/jps.22614. doi:10.1002/jps.22614. [DOI] [PubMed] [Google Scholar]
- 15.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. doi:10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naveh-Many T, Bell O, Silver J, et al. Cis and trans acting factors in the regulation of parathyroid hormone (PTH) mRNA stability by calcium and phosphate. FEBS Lett. 2002;529:60–64. doi: 10.1016/s0014-5793(02)03259-3. doi:10.1016/S0014-5793(02)03259-3. [DOI] [PubMed] [Google Scholar]
- 17.Mizobuchi M, Towler D, Slatopolsky E. Vascular calcification: the killer of patients with chronic kidney disease. JASN. 2009;20:1453–1464. doi: 10.1681/ASN.2008070692. doi:10.1681/ASN.2008070692. [DOI] [PubMed] [Google Scholar]
- 18.Sigrist MK, Taal MW, Bungay P, et al. Progressive vascular calcification over 2 years is associated with arterial stiffening and increased mortality in patients with stages 4 and 5 chronic kidney disease. CJASN. 2007;2:1241–1248. doi: 10.2215/CJN.02190507. [DOI] [PubMed] [Google Scholar]
- 19.Russo D, Corrao S, Battaglia Y, et al. Progression of coronary artery calcification and cardiac events in patients with chronic renal disease not receiving dialysis. Kidney Int. 2011;80:112–118. doi: 10.1038/ki.2011.69. doi:10.1038/ki.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Block GA. Screening dialysis patients for vascular calcification. Semin Dial. 2010;23:271–276. doi: 10.1111/j.1525-139X.2010.00727.x. doi:10.1111/j.1525-139X.2010.00727.x. [DOI] [PubMed] [Google Scholar]
- 21.Block GA, Wheeler DC, Persky MS, et al. Effects of phosphate binders in moderate CKD. JASN. 2012;23:1407–1415. doi: 10.1681/ASN.2012030223. doi:10.1681/ASN.2012030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russo D, Miranda I, Ruocco C, et al. The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int. 2007;72:1255–1261. doi: 10.1038/sj.ki.5002518. doi:10.1038/sj.ki.5002518. [DOI] [PubMed] [Google Scholar]
- 23.KDOQI; National Kidney Foundation. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for anemia in chronic kidney disease. Am J Kidney Dis. 2006;47:S11–S145. doi: 10.1053/j.ajkd.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–1378. doi: 10.1038/ki.2011.47. doi:10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–2439. doi: 10.1001/jama.2011.826. doi:10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kendrick J, Cheung AK, Kaufman JS, et al. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. JASN. 2011;22:1913–1922. doi: 10.1681/ASN.2010121224. doi:10.1681/ASN.2010121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolf M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int. 2012;82:737–747. doi: 10.1038/ki.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliveira RB, Cancela AL, Graciolli FG, et al. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD–MBD therapy? CJASN. 2010;5:286–291. doi: 10.2215/CJN.05420709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin A, David V, Quarles LD. Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev. 2012;92:131–155. doi: 10.1152/physrev.00002.2011. doi:10.1152/physrev.00002.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isakova T, Gutiérrez OM, Wolf M. A blueprint for randomized trials targeting phosphorus metabolism in chronic kidney disease. Kidney Int. 2009;76:705–716. doi: 10.1038/ki.2009.246. doi:10.1038/ki.2009.246. [DOI] [PubMed] [Google Scholar]
- 31.Evenepoel P, Meijers B, Viaene L, et al. Fibroblast growth factor-23 in early chronic kidney disease: additional support in favor of a phosphate-centric paradigm for the pathogenesis of secondary hyperparathyroidism. CJASN. 2010;5:1268–1276. doi: 10.2215/CJN.08241109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bacchetta J, Salusky IB. Evaluation of hypophosphatemia: lessons from patients with genetic disorders. Am J Kidney Dis. 2012;59:152–159. doi: 10.1053/j.ajkd.2011.08.035. doi:10.1053/j.ajkd.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zoppellaro G, Faggin E, Puato M, et al. Fibroblast growth factor 23 and the bone-vascular axis: lessons learned from animal studies. Am J Kidney Dis. 2012;59:135–144. doi: 10.1053/j.ajkd.2011.07.027. doi:10.1053/j.ajkd.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 34.Kirkpantur A, Balci M, Gurbuz OA, et al. Serum fibroblast growth factor-23 (FGF-23) levels are independently associated with left ventricular mass and myocardial performance index in maintenance haemodialysis patients. Nephrol Dial Transplant. 2011;26:1346–1354. doi: 10.1093/ndt/gfq539. doi:10.1093/ndt/gfq539. [DOI] [PubMed] [Google Scholar]
- 35.Mirza MA, Larsson A, Melhus H, et al. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009;207:546–551. doi: 10.1016/j.atherosclerosis.2009.05.013. doi:10.1016/j.atherosclerosis.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Seiler S, Cremers B, Rebling NM, et al. The phosphatonin fibroblast growth factor 23 links calcium-phosphate metabolism with left-ventricular dysfunction and atrial fibrillation. Eur Heart J. 2011;32:2688–2696. doi: 10.1093/eurheartj/ehr215. doi:10.1093/eurheartj/ehr215. [DOI] [PubMed] [Google Scholar]
- 37.Shalhoub V, Shatzen EM, Ward SC, et al. FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Invest. 2012;122:2543–2553. doi: 10.1172/JCI61405. doi:10.1172/JCI61405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yilmaz MI, Sonmez A, Saglam M, et al. Comparison of calcium acetate and sevelamer on vascular function and fibroblast growth factor 23 in CKD patients: a randomized clinical trial. Am J Kidney Dis. 2012;59:177–185. doi: 10.1053/j.ajkd.2011.11.007. doi:10.1053/j.ajkd.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Port FK, Pisoni RL, Bommer J, et al. Improving outcomes for dialysis patients in the international dialysis outcomes and practice patterns study. CJASN. 2006;1:246–255. doi: 10.2215/CJN.01050905. [DOI] [PubMed] [Google Scholar]
- 40.Noori N, Kalantar-Zadeh K, Kovesdy CP, et al. Association of dietary phosphorus intake and phosphorus to protein ratio with mortality in hemodialysis patients. CJASN. 2010;5:683–692. doi: 10.2215/CJN.08601209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heng A-E, Cano NJM. Nutritional problems in adult patients with stage 5 chronic kidney disease on dialysis (both haemodialysis and peritoneal dialysis) NDT Plus. 2010;3:109–117. [Google Scholar]
- 42.Shinaberger CS, Greenland S, Kopple JD, et al. Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am J Clin Nutr. 2008;88:1511–1518. doi: 10.3945/ajcn.2008.26665. doi:10.3945/ajcn.2008.26665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lynch KE, Lynch R, Curhan GC, et al. Prescribed dietary phosphate restriction and survival among hemodialysis patients. CJASN. 2011;6:620–629. doi: 10.2215/CJN.04620510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ritz E, Hahn K, Ketteler M, et al. Phosphate additives in food—a health risk. Dtsch Arztebl Int. 2012;109:49–55. doi: 10.3238/arztebl.2012.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uribarri J. Phosphorus homeostasis in normal health and in chronic kidney disease patients with special emphasis on dietary phosphorus intake. Semin Dial. 2007;20:295–301. doi: 10.1111/j.1525-139X.2007.00309.x. doi:10.1111/j.1525-139X.2007.00309.x. [DOI] [PubMed] [Google Scholar]
- 46.Gutiérrez OM, Anderson C, Isakova T, et al. Low socioeconomic status associates with higher serum phosphate irrespective of race. JASN. 2010;21:1953–1960. doi: 10.1681/ASN.2010020221. doi:10.1681/ASN.2010020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uribarri J. Phosphorus additives in food and their effect in dialysis patients. CJASN. 2009;4:1290–1292. doi: 10.2215/CJN.03950609. [DOI] [PubMed] [Google Scholar]
- 48.Cupisti A, Ferretti V, D'Alessandro C, et al. Nutritional knowledge in hemodialysis patients and nurses: focus on phosphorus. J Ren Nutr. 2012;22:541–546. doi: 10.1053/j.jrn.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 49.Sullivan C, Sayre SS, Leon JB, et al. Effect of food additives on hyperphosphatemia among patients with end-stage renal disease: a randomized controlled trial. JAMA. 2009;301:629–635. doi: 10.1001/jama.2009.96. doi:10.1001/jama.2009.96. [DOI] [PubMed] [Google Scholar]
- 50.Isakova T, Gutiérrez OM, Chang Y, et al. Phosphorus binders and survival on hemodialysis. JASN. 2009;20:388–396. doi: 10.1681/ASN.2008060609. doi:10.1681/ASN.2008060609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cannata-Andia JB. The use of phosphate binding agents is associated with lower mortality: results from the COSMOS study. Paris, France: 49th ERA-EDTA Congress; 2012. May 24–27. [Google Scholar]
- 52.Lopes AA, Tong L, Thumma J, et al. Phosphate binder use and mortality among hemodialysis patients in the dialysis outcomes and practice patterns study (DOPPS): evaluation of possible confounding by nutritional status. Am J Kidney Dis. 2012;60:90–101. doi: 10.1053/j.ajkd.2011.12.025. doi:10.1053/j.ajkd.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barreto FC, de Oliveira RA, Oliveira RB, et al. Pharmacotherapy of chronic kidney disease and mineral bone disorder. Expert Opin Pharmacother. 2011;12:2627–2640. doi: 10.1517/14656566.2011.626768. doi:10.1517/14656566.2011.626768. [DOI] [PubMed] [Google Scholar]
- 54.Graf H, Stummvoll HK, Meisinger V. Dialysate aluminium concentration and aluminium transfer during haemodialysis. Lancet. 1982;319:46–47. doi: 10.1016/s0140-6736(82)92588-0. doi:10.1016/S0140-6736(82)92588-0. [DOI] [PubMed] [Google Scholar]
- 55.Qunibi WY, Hootkins RE, McDowell LL, et al. Treatment of hyperphosphatemia in hemodialysis patients: the calcium acetate renagel evaluation (CARE study) Kidney Int. 2004;65:1914–1926. doi: 10.1111/j.1523-1755.2004.00590.x. doi:10.1111/j.1523-1755.2004.00590.x. [DOI] [PubMed] [Google Scholar]
- 56.Brewster UC, Ciampi MA, Abu-Alfa AK, et al. Long-term comparison of sevelamer hydrochloride to calcium-containing phosphate binders. Nephrology. 2006;11:142–146. doi: 10.1111/j.1440-1797.2006.00544.x. doi:10.1111/j.1440-1797.2006.00544.x. [DOI] [PubMed] [Google Scholar]
- 57.Jean G, Lataillade D, Genet L, et al. Calcium carbonate, but not sevelamer, is associated with better outcomes in hemodialysis patients: results from the French ARNOS study. Hemodial Int. 2011;15:485–492. doi: 10.1111/j.1542-4758.2011.00575.x. doi:10.1111/j.1542-4758.2011.00575.x. [DOI] [PubMed] [Google Scholar]
- 58.Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. doi:10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 59.Moe SM, Chertow GM. The case against calcium-based phosphate binders. CJASN. 2006;1:697–703. doi: 10.2215/CJN.00560206. [DOI] [PubMed] [Google Scholar]
- 60.Wrong O, Harland C. Sevelamer. Nephrol Dial Transplant. 2008;23:2108. doi: 10.1093/ndt/gfn162. author reply 2101–2102 doi:10.1093/ndt/gfn162. [DOI] [PubMed] [Google Scholar]
- 61.De Santo NG, Frangiosa A, Anastasio P, et al. Sevelamer worsens metabolic acidosis in hemodialysis patients. J Nephrol. 2006;19:S108–S114. [PubMed] [Google Scholar]
- 62.Delmez J, Block G, Robertson J, et al. A randomized, double-blind, crossover design study of sevelamer hydrochloride and sevelamer carbonate in patients on hemodialysis. Clin Nephrol. 2007;68:386–391. doi: 10.5414/cnp68386. [DOI] [PubMed] [Google Scholar]
- 63.Pai AB, Shepler BM. Comparison of sevelamer hydrochloride and sevelamer carbonate: risk of metabolic acidosis and clinical implications. Pharmacotherapy. 2009;29:554–561. doi: 10.1592/phco.29.5.554. doi:10.1592/phco.29.5.554. [DOI] [PubMed] [Google Scholar]
- 64.Navaneethan SD, Palmer SC, Vecchio M, et al. Phosphate binders for preventing and treating bone disease in chronic kidney disease patients. Cochrane Database Syst Rev. 2011:CD006023. doi: 10.1002/14651858.CD006023.pub2. [DOI] [PubMed] [Google Scholar]
- 65.Suki WN, Zabaneh R, Cangiano JL, et al. Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int. 2007;72:1130–1137. doi: 10.1038/sj.ki.5002466. doi:10.1038/sj.ki.5002466. [DOI] [PubMed] [Google Scholar]
- 66.Molony D, Bellasi A, Bellizzi V, et al. Sevelamer Attenuates CV Mortality in Incident Hemodialysis Patients: Open Label, Randomized Clinical Trial of Efficacy and Safety (Independent Study) Paris, France: 49th ERA-EDTA Congress; 2012. 24–27 May 2012. [Google Scholar]
- 67.Di Iorio B, Bellasi A, Russo D, et al. Mortality in kidney disease patients treated with phosphate binders: a randomized study. CJASN. 2012;7:487–493. doi: 10.2215/CJN.03820411. [DOI] [PubMed] [Google Scholar]
- 68.Iimori S, Mori Y, Akita W, et al. Effects of sevelamer hydrochloride on mortality, lipid abnormality and arterial stiffness in hemodialyzed patients: a propensity-matched observational study. Clin Exp Nephrol. 2012;16:930–937. doi: 10.1007/s10157-012-0640-4. [DOI] [PubMed] [Google Scholar]
- 69.Block GA, Spiegel DM, Ehrlich J, et al. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005;68:1815–1824. doi: 10.1111/j.1523-1755.2005.00600.x. doi:10.1111/j.1523-1755.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 70.Raggi P, Bommer J, Chertow GM. Valvular calcification in hemodialysis patients randomized to calcium-based phosphorus binders or sevelamer. J Heart Valve Dis. 2004;13:134–141. [PubMed] [Google Scholar]
- 71.Braun J, Asmus HG, Holzer H, et al. Long-term comparison of a calcium-free phosphate binder and calcium carbonate—phosphorus metabolism and cardiovascular calcification. Clin Nephrol. 2004;62:104–115. doi: 10.5414/cnp62104. [DOI] [PubMed] [Google Scholar]
- 72.Chertow GM, Burke SK, Raggi P Treat to Goal Working Group. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245–252. doi: 10.1046/j.1523-1755.2002.00434.x. doi:10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 73.Qunibi W, Moustafa M, Muenz LR, et al. A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: the Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis. 2008;51:952–965. doi: 10.1053/j.ajkd.2008.02.298. doi:10.1053/j.ajkd.2008.02.298. [DOI] [PubMed] [Google Scholar]
- 74.Takei T, Otsubo S, Uchida K, et al. Effects of sevelamer on the progression of vascular calcification in patients on chronic haemodialysis. Nephron Clin Pract. 2008;108:c278–c283. doi: 10.1159/000127361. doi:10.1159/000127361. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Q, Li M, Lu Y, et al. Meta-analysis comparing sevelamer and calcium-based phosphate binders on cardiovascular calcification in hemodialysis patients. Nephron Clin Pract. 2010;115:c259–c267. doi: 10.1159/000313484. doi:10.1159/000313484. [DOI] [PubMed] [Google Scholar]
- 76.Kakuta T, Tanaka R, Hyodo T, et al. Effect of sevelamer and calcium-based phosphate binders on coronary artery calcification and accumulation of circulating advanced glycation end products in hemodialysis patients. Am J Kidney Dis. 2011;57:422–431. doi: 10.1053/j.ajkd.2010.10.055. doi:10.1053/j.ajkd.2010.10.055. [DOI] [PubMed] [Google Scholar]
- 77.Brandenburg VM, Schlieper G, Heussen N, et al. Serological cardiovascular and mortality risk predictors in dialysis patients receiving sevelamer: a prospective study. Nephrol Dial Transplant. 2010;25:2672–2679. doi: 10.1093/ndt/gfq053. doi:10.1093/ndt/gfq053. [DOI] [PubMed] [Google Scholar]
- 78.Finn WF SPD 405-307 Lanthanum Study Group. Lanthanum carbonate versus standard therapy for the treatment of hyperphosphatemia: safety and efficacy in chronic maintenance hemodialysis patients. Clinical Nephrol. 2006;65:191–202. doi: 10.5414/cnp65191. [DOI] [PubMed] [Google Scholar]
- 79.Sprague SM, Ross EA, Nath SD, et al. Lanthanum carbonate vs. sevelamer hydrochloride for the reduction of serum phosphorus in hemodialysis patients: a crossover study. Clinical Nephrol. 2009;72:252–258. doi: 10.5414/cnp72252. [DOI] [PubMed] [Google Scholar]
- 80.Gonzalez-Parra E, Gonzalez-Casaus ML, Galan A, et al. Lanthanum carbonate reduces FGF23 in chronic kidney disease stage 3 patients. Nephrol Dial Transplant. 2011;26:2567–2571. doi: 10.1093/ndt/gfr144. doi:10.1093/ndt/gfr144. [DOI] [PubMed] [Google Scholar]
- 81.D'Haese PC, Spasovski GB, Sikole A, et al. A multicenter study on the effects of lanthanum carbonate (Fosrenol) and calcium carbonate on renal bone disease in dialysis patients. Kidney Int Suppl. 2003;85:S73–S78. doi: 10.1046/j.1523-1755.63.s85.18.x. doi:10.1046/j.1523-1755.63.s85.18.x. [DOI] [PubMed] [Google Scholar]
- 82.Hutchison AJ, Maes B, Vanwalleghem J, et al. Efficacy, tolerability, and safety of lanthanum carbonate in hyperphosphatemia: a 6-month, randomized, comparative trial versus calcium carbonate. Nephron Clin Pract. 2005;100:c8–c19. doi: 10.1159/000084653. doi:10.1159/000084653. [DOI] [PubMed] [Google Scholar]
- 83.Joy MS, Kshirsagar A, Candiani C, Brooks T, Hudson JQ. Lanthanum carbonate. Ann Pharmacother. 2006;40:234–240. doi: 10.1345/aph.1G224. doi:10.1345/aph.1G224. [DOI] [PubMed] [Google Scholar]
- 84.Lacour B, Lucas A, Auchère D, et al. Chronic renal failure is associated with increased tissue deposition of lanthanum after 28-day oral administration. Kidney Int. 2005;67:1062–1069. doi: 10.1111/j.1523-1755.2005.00171.x. doi:10.1111/j.1523-1755.2005.00171.x. [DOI] [PubMed] [Google Scholar]
- 85.Hutchison AJ, Barnett ME, Krause R, et al. Lanthanum Carbonate Study Group. Lanthanum carbonate treatment, for up to 6 years, is not associated with adverse effects on the liver in patients with chronic kidney disease stage 5 receiving hemodialysis. Clinical Nephrol. 2009;71:286–295. [PubMed] [Google Scholar]
- 86.Arenas MD, Malek T, Álvarez-Ude F, et al. Phosphorus binders: preferences of patients on haemodialysis and its impact on treatment compliance and phosphorus control. Nefrologia. 2010;30:522–530. doi: 10.3265/Nefrologia.pre2010.may.10275. [DOI] [PubMed] [Google Scholar]
- 87.Arenas MD, Malek T, Gil MT, et al. Challenge of phosphorus control in hemodialysis patients: a problem of adherence? J Nephrol. 2010;23:525–534. [PubMed] [Google Scholar]
- 88.Karamanidou C, Clatworthy J, Weinman J, et al. A systematic review of the prevalence and determinants of nonadherence to phosphate binding medication in patients with end-stage renal disease. BMC Nephrol. 2008;9:2. doi: 10.1186/1471-2369-9-2. doi:10.1186/1471-2369-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chiu YW, Teitelbaum I, Misra M, et al. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. CJASN. 2009;4:1089–1096. doi: 10.2215/CJN.00290109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Daugirdas JT, Chertow GM, Larive B, et al. Effects of frequent hemodialysis on measures of CKD mineral and bone disorder. JASN. 2012;23:727–738. doi: 10.1681/ASN.2011070688. doi:10.1681/ASN.2011070688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cheng SC, Young DO, Huang Y, et al. A randomized, double-blind, placebo-controlled trial of niacinamide for reduction of phosphorus in hemodialysis patients. CJASN. 2008;3:1131–1138. doi: 10.2215/CJN.04211007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Delanaye P, Weekers L, Krzesinski JM. Diarrhea induced by high doses of nicotinamide in dialysis patients. Kidney Int. 2006;69:1914. doi: 10.1038/sj.ki.5000381. doi:10.1038/sj.ki.5000381. [DOI] [PubMed] [Google Scholar]
- 93.de Francisco AL, Leidig M, Covic AC, et al. Evaluation of calcium acetate/magnesium carbonate as a phosphate binder compared with sevelamer hydrochloride in haemodialysis patients: a controlled randomized study (CALMAG study) assessing efficacy and tolerability. Nephrol Dial Transplant. 2010;25:3707–3717. doi: 10.1093/ndt/gfq292. doi:10.1093/ndt/gfq292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Geisser P, Philipp E. PA21: a novel phosphate binder for the treatment of hyperphosphatemia in chronic kidney disease. Clinical Nephrol. 2010;74:4–11. doi: 10.5414/cnp74004. [DOI] [PubMed] [Google Scholar]
- 95.Wüthrich RP, Chonchol M, Covic A, et al. Randomized clinical trial of the iron-based phosphate binder PA21 in hemodialysis patients. Clin J Am Soc Nephrol. 2012 doi: 10.2215/CJN.08230811. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Umanath K, Sika M, Niecestro R, et al. Rationale and study design of a three-period, 58-week trial of ferric citrate as a phosphate binder in patients with ESRD on dialysis. Hemodial Int. 2012 doi: 10.1111/j.1542-4758.2012.00711.x. doi:10.1111/j.1542-4758.2012.00711.x. [DOI] [PubMed] [Google Scholar]
- 97.Savica V, Calò LA, Monardo P, et al. Salivary phosphate-binding chewing gum reduces hyperphosphatemia in dialysis patients. JASN. 2009;20:639–644. doi: 10.1681/ASN.2008020130. doi:10.1681/ASN.2008020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Locatelli F, Dimkovic N, Pontoriero G, et al. Effect of MCI-196 on serum phosphate and cholesterol levels in haemodialysis patients with hyperphosphataemia: a double-blind, randomized, placebo-controlled study. Nephrol Dial Transplant. 2010;25:574–581. doi: 10.1093/ndt/gfp445. doi:10.1093/ndt/gfp445. [DOI] [PubMed] [Google Scholar]