Abstract
Cardiovascular (CV) disease is the leading cause of morbidity and mortality in chronic kidney disease (CKD) patients. Although about half of the deaths are due to CV causes, only a minority are directly linked to myocardial infarction and it is estimated that cardiac arrest or cardiac arrhythmias account for about a quarter of all deaths registered in dialysis patients. Thus, simple non-invasive tools such as electrocardiogram (ECG) may detect those patients at increased risk for arrhythmias. The QT interval on the standard 12-lead ECG is the time from ventricular depolarization (Q wave onset) to cardiac repolarization completion (end of the T wave) and represents a marker of cardiac repolarization defects. Numerous studies suggest a direct association between QT abnormalities and poor prognosis in the general population, CKD patients and dialysis patients. Of note, multivariable adjustments for different traditional and CKD-specific risk factors for CV events attenuate but do not cancel these associations. We herein review the clinical significance of simple non-invasive tools such as the QT tract on ECG for detecting those patients at increased risk of CV event and possibly for treatment individualization.
Keywords: chronic kidney disease, dialysis, morbidity, mortality, QT interval
Introduction
Cardiovascular diseases (CVD), including ventricular arrhythmias and sudden cardiac death, are the leading causes of morbidity and mortality among subjects with chronic kidney disease (CKD) accounting for about half of the deaths [1, 2]. Although atherosclerosis is accelerated in CKD, only a minority of cardiovascular (CV) deaths are due to ischaemic heart disease, whereas the incidence of cardiac arrhythmias and sudden death (SD) is significantly increased when compared with the general population [1, 2]. Several traditional and dialysis-specific factors have been studied to explain this extraordinary CV vulnerability to SD. Cardiac arrhythmias may be triggered by electrolyte imbalances, especially in the hearts of CKD patients who are susceptible to atherosclerosis, myocardiocytic fibrosis, calcification and myocardial ischaemia [3].
Simple, non-invasive biomarkers of CVD such as QT tract prolongation on a standard 12-lead electrocardiogram (ECG) have been associated with subclinical atherosclerosis as well as structural and electrophysiological changes that may predispose to cardiac arrhythmias [4]. We herein summarize the clinical significance and usefulness of QT tract abnormality detection in CKD patients receiving and not receiving dialysis.
Corrected QT interval and QT interval dispersion: definition
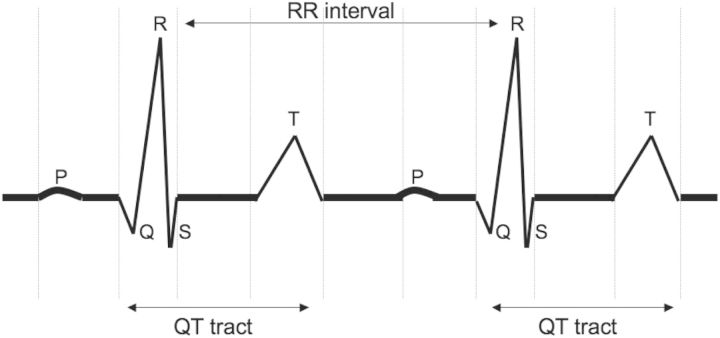
The QT interval on the standard 12-lead ECG represents the time from ventricular depolarization (Q wave onset) to cardiac repolarization completion (end of the T wave). The QT interval is conventionally assessed at a paper speed and gain of 25 mm/s and 10 mm/mV, respectively. Two different approaches for QT interval assessment are available: the threshold method which defines the end of the T wave as the intersection of the terminal limb of the T wave with the isoelectric baseline and the tangent method which defines the end of the T wave as the intersection of a line drawn from the peak of the T wave to the steepest point of the descending limb of the T wave (tangent) and the isoelectric baseline [5]. However, data suggest that all reported methods for QT assessment are comparable with QT assessed via the tangent method shorter by up to 10ms [5].
Since the QT interval depends on the heart rate, several methods are available to yield a corrected measure (QTc: corrected QT). The most commonly used and generally adopted in automatic or semiautomatic electrocardiographs is the Bazzett formula [6]. According to this formula, QTc is the ratio of the QT interval and the square root of the RR interval (Figure 1), which represents the cardiac cycle duration: QTc = QT/(RR)1/2. Although this formula is the most commonly used, it tends to over- and under-adjust at high and low heart rates, respectively. Thus, other methods to account for the heart rate have been developed. Fridericia [7], for instance, proposed to adjust the QT interval for the cubic root of the RR interval: QTc = QT/(RR)1/3. Nevertheless, it is now widely accepted that linear regression represents the most accurate way to account for the heart rate. Accordingly, as proposed by Sagie et al. [8], QTc = QT + 0.154(1 − RR). Regardless of the equation used, a normal corrected QT should vary between 300 and 440 ms (450 ms for women). A simple practical rule suggests that a normal QTc should be less than half of the RR interval.
Fig. 1.
QT and RR interval on ECG.
QT dispersion (QTd) is defined as the difference between the longest and the shortest QT interval measured on a standard 12-lead ECG. Numerous studies documented that a QTd interval shorter than 30 ms, between 30 and 60 ms and longer than 60 ms is associated with low-, intermediate- and high-risk of cardiac arrhythmias, respectively [9–11].
Risk factors for cardiac depolarization disorders in the general population and CKD patients
Several genetic or environmental factors may result in a prolonged QT interval (Table 1). Genetic defects of the myocardial sodium and potassium channels may be responsible for the intrinsic cardiac repolarization disorders that characterize the congenital long-QT syndrome [12]. Numerous drugs (e.g., antiarrhythmic, antihistaminic, psychotropic and antibiotic medications) and electrolyte disturbances (e.g., hypocalcaemia, hypokalaemia and hypomagnesaemia) may prolong the QT interval. Of note, in the absence of other abnormalities of the cardiac repolarization, this prolongation may be reversed with the discontinuation of any of the environmental factors [12] (Table 1).
Table 1.
Factors associated with QT tract abnormalities in the general population and CKD patients
| Genetic factors |
| Defects of the myocardial sodium and potassium channels |
| Defects of the myocardial potassium channels |
| Environmental factors |
Electrolytes shift during HD:
|
| Use of calcium-containing phosphate binders |
| Excessive Mg intake |
Drugs
|
| Comorbidities |
| Extensive vascular calcification (coronary artery calcification) |
| History of ischaemic heart disease |
| LVH |
| Central nervous system injury |
Traditional CV risk factors and various medical conditions such as central nervous system injury, as well as pre-existing CV heart diseases, have also been associated with QT tract abnormalities. Indeed, the QT tract may be prolonged in patients with elevated blood pressure, chronic heart failure, mitral valve prolapse and ischaemic heart disease [4, 13] (Table 1).
A recent study shows a robust association between the QT interval and the non-traditional CV risk factors such as mineral metabolism abnormalities [14]. In a pooled analysis of 7312 men and women from NHANES III and 14 825 men and women from the ARIC study, the QT tract was directly and indirectly associated with serum phosphorus and calcium levels, respectively [14].
The transmembrane electrolyte shifts that occur during each haemodialysis (HD) session may explain the association between dialysis and QT interval abnormalities. Indeed, different ionic countercurrents such as calcium (Ca) and potassium (K) currents control the different phases of the ventricular depolarization and repolarization [15]. A reduction in both K and Ca levels in the plasma induces a significant prolongation of the duration of the action potential and, thus, of the QT interval on ECG [15]. At each dialysis session, both K and Ca undergo a rapid shift. While potassium tends to increase during the inter-dialytic time, it is usually rapidly lowered during each dialysis session. Contrarily, the calcium concentration is normally increased at the end of dialysis. The degree and the speed of these shifts depend on the concentration gradient between the plasma and the dialysis bath of these solutes as well as the convection/diffusion rate and they may trigger ventricular depolarization/repolarization abnormalities [16, 17]. Overall, it seems that the greater the potassium, calcium and bicarbonate gradient between blood and dialysate, the longer is the QT prolongation at dialysis session completion [15–27].
Other factors may impact the QT tract in CKD patients. In a previous study [28], we showed a strong and independent association between coronary artery calcification (CAC) detected via cardiac computed tomography (CT) and QT interval in a small cohort of 32 patients on maintenance dialysis (age: 69 ± 16 years, time on dialysis 32 ± 27 months) and 12 patients with CKD 4 (age 66 ± 17 years, uraemia duration 38 ± 16 months) [28]. In spite of an overall good mineral control in the study cohort (serum phosphate levels: 3.6 ± 1.3 mg/dL and 4.3 ± 1.6 mg/dL; CaXP product: 46 ± 17 and 49 ± 16 mg2/dL2, in CKD 4 and HD patients, respectively), a CAC score of ≥400 was highly prevalent in both the groups. When the study cohort was stratified according to the presence of CAC score >400, we found that patients with an increased CAC had on average a significant 11% longer corrected QTd (P < 0.0001). Indeed, QTd showed a linear correlation with CAC scores in both CKD 4 (r = 0.91, P < 0.0001) and HD patients (r = 0.899, P < 0.0001) [28]. These data suggest that cardiac calcification is potentially linked to dysrhythmias in end-stage renal disease (ESRD) [28].
We further tested this hypothesis in a subsequent observational, prospective study of 132 incident HD patients [29]. At uni- and multivariable analyses, CAC progression was associated with a significant increase in both QTd and pulse wave velocity (PWV). Every 20 unit increase in the CAC score corresponded to a significant 23% (95% CI 1.12–1.27; P < 0.001) and 32% (95% CI 1.09–1.37; P < 0.01) increased risk of experiencing a 1 m/s increase in PWV and 1ms in QTd, respectively [29]. Notably, QT and other markers of cardiovascular disease (CVD) deterioration were associated with the use of calcium-containing phosphate binders [29].
Recently, Claes et al. [30] confirmed the link between vascular calcification and QT tract abnormalities in 193 patients (118 men, 52 years old) with CKD stage 5D. A prolonged corrected QT interval was detected in 26% of the study cohort and was directly associated with the extent of aortic calcifications (P = 0.0004) independent of age, gender, CVD history, electrolytes and parameters of mineral metabolism [30].
Though the mechanisms underlying the positive association between QT duration and arterial calcification remain largely unknown, we designed a prospective randomized controlled trial to test in a large cohort of incident dialysis patients whether attenuation of vascular calcification progression also reduces QT tract prolongation and mortality (INDEPENDENT study; registered on ClinicalTrials.Gov, NCT00710788) [31].
Epidemiology of cardiac depolarization disorders in the general population and CKD patients
QT interval prolongation (QTc interval ≥440ms) represents a common ECG finding both among subjects from the general population and CKD patients.
In a recent systemic review of the medical literature, among the 36 031 individuals from the general population included in the studies considered, a total of 2677 (8.7%) subjects had a QTc interval longer than 440ms [12].
Data from the CV Health Study (CHS) suggest that subjects with CKD not yet receiving dialysis also exhibit longer corrected QT intervals compared with peers without CKD (estimated glomerular filtration rate, eGFR, >60 mL/min1.73 m2) [32]. In this large community-based study of 3238 adults older than 65 years and free from CVD, the unadjusted QT prolongation index [QTI: represents the percentile prolongation of the QT interval with respect to the median value of a large North American sample: QTI % = QT/656 × (heart rate + 100)] was significantly longer among participants with CKD compared with the remainder of the study cohort [32]. However, these differences were explained by the different prevalence of traditional CV risk factors as well as CV medication use [32].
In 1999, two independent research groups [33, 34] reported on a significant QT tract prolongation and dispersion in 34 and 50 patients on maintenance dialysis. Morris et al. [33] showed that QTd was significantly higher in HD patients (63.1±20.6 ms) compared with controls (36.0 ±13.7 ms; P < 0.001) and rose after HD to levels comparable with those seen in patients with coronary artery disease. Lorincz et al. [34] confirmed the impact of HD on the QT interval reporting on a significant increase of QTc (from 482 ±42 to 519 ±33 ms; P < 0.01) and QTd (from 62 ±18 to 95 ±17 ms; P < 0.001) after dialysis [34]. A few other studies that have investigated the impact of dialysis on the QT interval yielded similar results [3, 4, 35–37]. Some studies concluded that dialysis is associated with QTd prolongation [4, 35], others have demonstrated an association between dialysis and QTc prolongation but not QTd [36, 37] and finally one study documented that both QTc and QTd are both increased after dialysis [3].
Cardiac depolarization disorders and outcomes in the general population and CKD patients
Considering that QT represents a measure of the cardiac repolarization time, an abnormally prolonged QT interval reflects a cardiac repolarization defect and hence a greater vulnerability to left ventricular arrhythmias (i.e. torsade de pointes and ventricular fibrillation) and sudden cardiac death [9–12].
Numerous epidemiological studies investigated the association between QT tract and CV outcomes in the general population. While the majority of these studies suggest that QT may serve as a predictor of CV events and sudden cardiac death, a few studies failed to demonstrate that a prolonged QT tract portends a poor prognosis (Table 2).
Table 2.
Association of QT tract elongation and outcome in the general and CKD populationa
| Study | Study population | Main results |
|---|---|---|
|
General population | ||
| Schouten et al. [41] |
N = 3091 men and women Age: 40–65 years |
15-year follow-up |
| Moderate QTc (420–440 ms) elongation predicted all-cause (adjusted RR 1.5 and 1.7, in men and women, respectively) mortality. | ||
| Moderate QTc (420–440 ms) elongation predicted CV and IHD mortality in men (RR 1.6 and 1.8, respectively), but not in women. | ||
| Extensive QTc (>440 ms) elongation predicted all-cause (adjusted RR 1.7 and 1.6, in men and women, respectively) mortality. | ||
| Extensive QTc (>440 ms) elongation predicted CV and IHD mortality in men (RR 1.8 and 2.1, respectively), but not in women. | ||
| RR = relative risk fully adjusted for confounders | ||
| Dekker et al. [42] | 877 men Age: first group of middle-age (∼50 years) and a second group of older (∼70 years) men |
15 years of follow-up |
| QTc increases with age and is associated with traditional CV risk factors. | ||
| Middle-aged men: rate ratios for coronary heart death and SD were more than double in the intermediate QTc (385–420 ms) and more than triple in the long-QTc (>420 ms) categories. | ||
| In the elderly, a similar association was documented for the long (>440 ms) but not for the intermediate QTc. Age-adjusted rate ratios for myocardial infarction incidence and coronary artery disease mortality were 2.8 and 5.0, respectively. | ||
| Adjustment for age, systolic blood pressure, body mass index, total serum cholesterol and smoking only slightly lowered the observed rate ratios. | ||
| de Bruyne et al. [43] | 2358 men and 3454 women Age: >55 years (mean 63.9 ± 9 years) |
3–6.5 years (mean 4 years) follow-up |
| QTc dispersion (>60 ms) associated with a 2-fold increased risk of cardiac death (HR: 2.5; 95% CI 1.6–4.0) and sudden cardiac death (HR: 1.9; 95% CI 1.0–3.7) and a 40% increased risk of total mortality (HR: 1.4; 95% CI 1.2–1.8). | ||
| Additional adjustment for potential confounders, including history of myocardial infarction, hypertension and overall QTc, did not materially change the risk estimates. | ||
| Elming et al. [44] | 1797 men and 1658 women Age: 30–60 years (mean 44 ± 11 years) |
13 years of follow-up |
| Prolonged QTc (>430 ms) associated with CV death and fatal and non-fatal cardiac morbidity [relative risk ratios: 2.9 (1.1–7.8) and 2.7 (1.4–5.5), respectively] | ||
| Prolonged QTd (>80 ms) associated with CV death and fatal and non-fatal cardiac morbidity [relative risk ratios: 4.4 (1.0–19.1) and 2.2 (1.1–4.0), respectively] | ||
| Relative risk ratios adjusted for age, gender, myocardial infarct, angina pectoris, diabetes mellitus, arterial hypertension, smoking habits, serum cholesterol level and heart rate | ||
| Algra et al. [45] | 6693 men and women (53% men) Middle-aged (median age ∼60 years) |
2 years of follow-up |
| In patients without evidence of cardiac dysfunction (history of symptoms of pump failure or an ejection fraction <40%), QTc (>440 ms) was associated with a 2.3 times higher risk of SD (RR 2.3, 95% CI 1.4, 3.9). | ||
| In patients with evidence of cardiac dysfunction, the RR was 1.0 (95% CI 0.5, 1.9). | ||
| Adjustment for age, gender, history of myocardial infarction, heart rate and the use of drugs did not alter these relative risks. | ||
| Social Insurance Institution's Coronary Heart Disease study [13]. | 5598 and 5119 men and women Age: 30–59 years |
23-year follow-up |
| Nomogram-corrected QT (QTn) interval prolongation associated with blood pressure and signs of CVD | ||
| In men with heart disease, over 10% prolongation of QTnc predicted SD (RR: 2.17; 95% CI 0.67–7–45); total CV mortality (RR: 2.12; 95% CI 1–25–3.59); all-cause mortality (RR: 1–92; 95% CI 1.23–3.00). | ||
| In men without heart disease, no association detected with the exception of healthy men with a low heart rate in which QTnc predicted SD (RR: 2–75; 95% CI 1–00–7.40). | ||
| Over 10% shortened QTnc predicted CV death in men with heart disease who smoked (RR: 3.72; 95% CI 1.45–9.54) | ||
| Non-smoking men with short QTnc had low mortality risks irrespective of possible signs of CVD. | ||
| The trends in mortality risks were similar but weaker for women. | ||
| Rotterdam study [46]. | 3105 men and 4878 women Age: >55 years (mean 69.2 ± 9.0 years) |
6.7 (2.3) years follow-up |
| Prolonged QTc interval (>450 ms in men; >470 ms in women) was associated with a 2.5-fold increased risk of sudden cardiac death (HR: 2.5; 95% CI 1.3–4.7), | ||
| In patients with an age below the median of 68 years, the corresponding relative risk was 8.0 (95% CI 2.1–31.3). | ||
| Association independent of age, gender, body mass index, hypertension, cholesterol/high-density lipoprotein ratio, diabetes mellitus, myocardial infarction, heart failure and heart rate | ||
| Framingham Heart Study [47] | 5125 men and women 30–62 years of age |
30 years follow-up |
| No significant differences in the risk of total mortality, sudden cardiac death or death due to coronary artery disease according to QTc. | ||
| A similar lack of significant association between QTc and these three outcomes was observed among all persons studied and in the two sexes | ||
| Point estimates adjusted for age, gender, cigarette smoking, serum total cholesterol, systolic systemic blood pressure and Framingham relative weight. | ||
| Chronic kidney disease patients | ||
| CHS [32] | 3238 participants with and without CKD (defined as eGFR < 60 mL/min) Age >65 years 18.5% (N = 600) individuals with CKD |
9.2 years of median follow-up |
| Participants with CKD had longer PR and corrected QT intervals compared with those without CKD; however, traditional CV risk factors and CV medication use explained these differences. | ||
| Each 10 ms increase in the QRS interval was associated with a 15% greater risk of incident heart failure (95% CI 1.04–1.27), a 13% greater risk of CHD (95% CI 1.04–1.24) and a 17% greater risk of mortality (95% CI 1.09, 1.25) among CKD participants. | ||
| Each 5% increase in QTI was associated with a 42% (95% CI 1.23–1.65), 22% (95% CI 1.07–1.40) and 10% (95% CI 0.98–1.22) greater risk of HF, CHD and mortality, respectively. | ||
| Associations seemed stronger for participants with CKD; however, no significant interactions were detected. | ||
| QTI: QT prolongation index, which represents the percentile prolongation of the QT interval with respect to the median value of a large North American sample QTI % = (QT/656) X(heart rate + 100). | ||
| Hage et al. [39] | 280 patients on maintenance HD. | 40 ± 28 months of follow-up |
| 39% of patients exhibited a prolonged QTc (460 ms) | ||
| Age: 53 ± 9 years 40 ± 28 months of follow-up |
Female gender, decreasing LVEF, and decreasing severity of CAD on angiography were independent predictors of prolonged QTc | |
| Patients with a prolonged QTc had 1-, 3- and 5-year death rates of 12, 36, and 47%, respectively, versus 8, 24 and 36% for those with a normal QTc (log-rank P = 0.03). | ||
| QTc was associated with the risk of death (HR: 1.008, 95% CI 1.001–1.014, P = 0.016 per 1 ms increase). | ||
| Association independent of age, gender, diabetes mellitus, myocardial infarction, presence and severity of CAD on angiography, LVH, LVEF and multiple other variables, | ||
| Beaubien et al. [40] | 147 patients on maintenance HD (N = 49) or peritoneal (N = 98) dialysis Age: 58.5 ± 16.7 years |
5–9 years of follow-up |
| A prolonged QTdc (>74 ms) was detected in 46.9 and 52% of HD and peritoneal dialysis patients, respectively. | ||
| QTdc was associated with the presence of diabetes mellitus, mean QT interval, corrected calcium levels. | ||
| QTdc was an independent predictor of total (RR = 1.53) and CV mortality (RR = 1.57). A trend towards arrhythmia-related mortality was also noted. | ||
aRR, relative risk; CVD, cardiovascular disease; IHD, ischaemic heart disease; CV, cardiovascular; HR, hazard ratio; SD, sudden death; CKD, chronic kidney disease; HF, heart failure; LVEF, left ventricular ejection fraction; CAD, coronary artery diease; LVH, left ventricular hypertrophy; HD, haemodialysis; CHS, cardiovascular health study.
However, the great heterogeneity among study cohorts and designs, the different historical period in which the studies were carried out and the potential for residual confounding may account for the reported inconsistencies in different subgroups of individuals from the general population (Table 2). Overall, it seems that a prolonged QTc (i.e. >440 ms) or Qtcd (>70 ms) interval is strongly associated with the risk of all-cause mortality, all cause of CV death and SD. Though a tight association with traditional CV risk factors and coexisting CVD is almost always reported, these electrocardiographic abnormalities may identify those individuals at higher risk for any CV event. Further studies should elaborate on the impact of gender, age and underlying medical conditions on QT tract prolongation (Table 2). Indeed, it seems that the prognostic significance of QT is attenuated in women, elderly and among subjects with a known CV condition.
CVD is accelerated in CKD patients and accounts for much of the observed mortality in this population. Although about half of the deaths are due to CV causes, only a minority are directly linked to myocardial infarction and it is estimated that cardiac arrest or cardiac arrhythmias accounts for about a quarter of all deaths registered in dialysis patients [38].
As reported in the general population, QT tract abnormalities portend a poor prognosis in chronic kidney disease (CKD) and dialysis patients. Data from the CHS [32] showed that among the 3238 participants with and without CKD, each 5% increase in the QT interval was associated with a significant 42% (95% CI 1.23–1.65), 22% (95% CI 1.07–1.40) and 10% (95% CI 0.98–1.22) greater risk of heart rate failure, coronary artery disease and mortality, respectively [32]. Though no significant interactions were detected, these associations seemed to be stronger for participants with CKD versus without CKD (defined as eGFR < 60 mL/min).
In a retrospective study of 280 patients on maintenance dialysis evaluated for kidney transplantation, Hage et al. [39] reported on independent and additive prognostic information gained from the QTc evaluation. A prolonged QTc (>460 ms) was described in 47% of the study cohort and was associated with the risk of death independently of age, gender, diabetes mellitus, myocardial infarction, presence and severity of coronary artery disease on angiography, LVH and left ventricular ejection fraction (LVEF) [39].
Finally, a study by Beaubien et al. [40] showed that a prolonged QTd (>74 ms) predicted the risk of serious ventricular arrhythmias or sudden cardiac death (SD) in a retrospective cohort of adult ESRD patients starting peritoneal dialysis or HD [40].
Though data on CKD patients are still limited and mainly based on the retrospective studies, it seems that QT interval abnormalities identify a subset of patients with a high CV vulnerability as described in the general population.
Conclusion
QT interval prolongation is a common finding among CKD patients. Furthermore, data suggest that a prolonged QT interval represents a marker of cardiac repolarization defects and is linked to the risk of cardiac arrhythmias and sudden cardiac death. Though, the pathophysiology of QT abnormalities is still largely obscure, data suggest that traditional and non-traditional CV risk factors are linked to QT interval abnormalities. Indeed, calcium and potassium channel remodelling of the heart due to concomitant CVD, such as ischaemic heart disease, extensive CAC, hypertension, left ventricular hypertrophy (LVH), heart failure and exposure to proarrhythmic drugs, may contribute to the QT interval prolongation in CKD. Furthermore, a large body of evidence supports the notion that the rapid transmembrane electrolyte shifts occurring during dialysis treatment also hamper the cardiac repolarization and increase the QT interval.
Considering that about a quarter of cardiac deaths are due to cardiac arrhythmias in CKD patients and the excessive proarrhythmic drugs to which CKD patients are exposed [23], QT tract evaluation appears to be a simple in-office, non-invasive tool for CV assessment that provides additive prognostic information of use for treatment individualization in CKD patients.
Conflict of interest statement
None declared.
References
- 1.Gruppo Emodialisi E Patologie Cardiovascolari. Multicentre, cross-sectional study of ventricular arrhythmias in chronically haemodialysed patients. Lancet. 1988;332:305–309. [PubMed] [Google Scholar]
- 2.Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease; 2004. Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. US Renal Data System (USRDS) 2007 . Available from: http://www.usrds.org/2007/pdf/00_intro_07.pdf. (2 January 2013, date last accessed) [Google Scholar]
- 3.Howse M, Sastry S, Bell GM. Changes in the corrected QT interval and corrected QT dispersion during haemodialysis. Postgrad Med J. 2002;78:273–275. doi: 10.1136/pmj.78.919.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu VC, Lin LY, Wu KD. QT interval dispersion in dialysis patients. Nephrology (Carlton) 2005;10:109–112. doi: 10.1111/j.1440-1797.2005.00391.x. [DOI] [PubMed] [Google Scholar]
- 5.Panicker GK, Karnad DR, Natekar M, et al. Intra- and interreader variability in QT interval measurement by tangent and threshold methods in a central electrocardiogram laboratory. J Electrocardiol. 2009;42:348–352. doi: 10.1016/j.jelectrocard.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Bazett HC. An analysis of the time-relations of electrocardiograms. Heart. 1920;7:353–370. [Google Scholar]
- 7.Fridericia LS. The duration of systole in the electrocardiogram of normal subjects and of patients with heart disease. Acta Medica Scandinavica. 1920;53:469–486. [Google Scholar]
- 8.Sagie A, Larson MG, Goldberg RJ, et al. An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study) Am J Cardiol. 1992;70:797–801. doi: 10.1016/0002-9149(92)90562-d. [DOI] [PubMed] [Google Scholar]
- 9.Day CP, McComb JM, Campbell RW. QT dispersion: an indication of arrhythmia risk in patients with long QT intervals. Br Heart J. 1990;63:342–344. doi: 10.1136/hrt.63.6.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen S, Rasmussen V, Torp-Pedersen C, et al. QT intervals and QT dispersion determined from a 12-lead 24-hour Holter recording in patients with coronary artery disease and patients with heart failure. Ann Noninvasive Electrocardiol. 2008;13:22–30. doi: 10.1111/j.1542-474X.2007.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zabel M, Lichtlen PR, Haverich A, et al. Comparison of ECG variables of dispersion of ventricular repolarization with direct myocardial repolarization measurements in the human heart. J Cardiovasc Electrophysiol. 1998;9:1279–1284. doi: 10.1111/j.1540-8167.1998.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 12.Montanez A, Ruskin JN, Hebert PR, et al. Prolonged QTc interval and risks of total and cardiovascular mortality and sudden death in the general population: a review and qualitative overview of the prospective cohort studies. Arch Intern Med. 2004;164:943–948. doi: 10.1001/archinte.164.9.943. [DOI] [PubMed] [Google Scholar]
- 13.Karjalainen J, Reunanen A, Ristola P, et al. QT interval as a cardiac risk factor in a middle aged population. Heart. 1997;77:543–548. doi: 10.1136/hrt.77.6.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Post WS, Dalal D, et al. Serum 25-hydroxyvitamin D, calcium, phosphorus, and electrocardiographic QT interval duration: findings from NHANES III and ARIC. J Clin Endocrinol Metab. 2011;96:1873–1882. doi: 10.1210/jc.2010-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genovesi S, Dossi C, Vigano MR, et al. Electrolyte concentration during haemodialysis and QT interval prolongation in uraemic patients. Europace. 2008;10:771–777. doi: 10.1093/europace/eun028. [DOI] [PubMed] [Google Scholar]
- 16.Genovesi S, Rivera R, Fabbrini P, et al. Dynamic QT interval analysis in uraemic patients receiving chronic haemodialysis. J Hypertens. 2003;21:1921–1926. doi: 10.1097/00004872-200310000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Nappi SE, Virtanen VK, Saha HH, et al. QTc dispersion increases during hemodialysis with low-calcium dialysate. Kidney Int. 2000;57:2117–2122. doi: 10.1046/j.1523-1755.2000.00062.x. [DOI] [PubMed] [Google Scholar]
- 18.Buemi M, Aloisi E, Coppolino G, et al. The effect of two different protocols of potassium haemodiafiltration on QT dispersion. Nephrol Dial Transplant. 2005;20:1148–1154. doi: 10.1093/ndt/gfh770. [DOI] [PubMed] [Google Scholar]
- 19.Severi S, Ciandrini A, Grandi E, et al. Cardiac response to hemodialysis with different cardiovascular tolerance: heart rate variability and QT interval analysis. Hemodial Int. Int Symp Home Hemodial. 2006;10:287–293. doi: 10.1111/j.1542-4758.2006.00110.x. [DOI] [PubMed] [Google Scholar]
- 20.Severi S, Grandi E, Pes C, et al. Calcium and potassium changes during haemodialysis alter ventricular repolarization duration: in vivo and in silico analysis. Nephrol Dial Transplant. 2008;23:1378–1386. doi: 10.1093/ndt/gfm765. [DOI] [PubMed] [Google Scholar]
- 21.Severi S, Vecchietti S, Cavalcanti S, et al. Electrocardiographic changes during hemodiafiltration with different potassium removal rates. Blood Purif. 2003;21:381–388. doi: 10.1159/000073440. [DOI] [PubMed] [Google Scholar]
- 22.Di Iorio B, Torraca S, Piscopo C, et al. Dialysate bath and QTc interval in patients on chronic maintenance hemodialysis: pilot study of single dialysis effects. J Nephrol. 2012;25:653–660. doi: 10.5301/jn.5000036. [DOI] [PubMed] [Google Scholar]
- 23.Gussak I, Gussak HM. Sudden cardiac death in nephrology: focus on acquired long QT syndrome. Nephrol Dial Transplant. 2007;22:12–14. doi: 10.1093/ndt/gfl587. [DOI] [PubMed] [Google Scholar]
- 24.Santoro A, Mancini E, Gaggi R, et al. Electrophysiological response to dialysis: the role of dialysate potassium content and profiling. Contrib Nephrol. 2005;149:295–305. doi: 10.1159/000085691. [DOI] [PubMed] [Google Scholar]
- 25.Drighil A, Madias JE, Benjelloun M, et al. Changes in the QT intervals, QT dispersion, and amplitude of T waves after hemodialysis. Ann Noninvasive Electrocardiol. 2007;12:137–144. doi: 10.1111/j.1542-474X.2007.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki R, Tsumura K, Inoue T, et al. QT interval prolongation in the patients receiving maintenance hemodialysis. Clin Nephrol. 1998;49:240–244. [PubMed] [Google Scholar]
- 27.Tong Y, Hou H. The alteration of QT dispersion in hemodialysis subjects. Kidney Blood Press Res. 2006;29:231–236. doi: 10.1159/000095738. [DOI] [PubMed] [Google Scholar]
- 28.Di Iorio BR, Bortone S, Piscopo C, et al. Cardiac vascular calcification and QT interval in ESRD patients: is there a link? Blood Purif. 2006;24:451–459. doi: 10.1159/000095362. [DOI] [PubMed] [Google Scholar]
- 29.Di Iorio B, Nargi O, Cucciniello E, et al. Coronary artery calcification progression is associated with arterial stiffness and cardiac repolarization deterioration in hemodialysis patients. Kidney Blood Press Res. 2011;34:180–187. doi: 10.1159/000325656. [DOI] [PubMed] [Google Scholar]
- 30.Claes KJ, Heye S, Nuyens D, et al. Impact of vascular calcification on corrected QT interval at the time of renal transplantation. Am J Nephrol. 2012;35:24–30. doi: 10.1159/000334597. [DOI] [PubMed] [Google Scholar]
- 31.Di Iorio BR, Cucciniello E, Bellizzi V. Vascular calcification and QT interval in incident hemodialysis patients. J Nephrol. 2009;22:694–698. [PubMed] [Google Scholar]
- 32.Kestenbaum B, Rudser KD, Shlipak MG, et al. Kidney function, electrocardiographic findings, and cardiovascular events among older adults. Clin J Am Soc Nephrol. 2007;2:501–508. doi: 10.2215/CJN.04231206. [DOI] [PubMed] [Google Scholar]
- 33.Morris ST, Galiatsou E, Stewart GA, et al. QT dispersion before and after hemodialysis. J Am Soc Nephrol. 1999;10:160–163. doi: 10.1681/ASN.V101160. [DOI] [PubMed] [Google Scholar]
- 34.Lorincz I, Matyus J, Zilahi Z, et al. QT dispersion in patients with end-stage renal failure and during hemodialysis. J Am Soc Nephrol. 1999;10:1297–1302. doi: 10.1681/ASN.V1061297. [DOI] [PubMed] [Google Scholar]
- 35.Yildiz A, Akkaya V, Sahin S, et al. QT dispersion and signal-averaged electrocardiogram in hemodialysis and CAPD patients. Perit Dial Int. 2001;21:186–192. [PubMed] [Google Scholar]
- 36.Covic A, Diaconita M, Gusbeth-Tatomir P, et al. Haemodialysis increases QTc interval but not QTc dispersion in ESRD patients without manifest cardiac disease. Nephrol Dial Transplant. 2002;17:2170–2177. doi: 10.1093/ndt/17.12.2170. [DOI] [PubMed] [Google Scholar]
- 37.Malhis M, Al-Bitar S, Farhood S, et al. Changes in QT intervals in patients with end-stage renal disease before and after hemodialysis. Saudi J Kidney Dis Transplant. 2010;21:460–465. [PubMed] [Google Scholar]
- 38.Bleyer AJ, Russell GB, Satko SG. Sudden and cardiac death rates in hemodialysis patients. Kidney Int. 1999;55:1553–1559. doi: 10.1046/j.1523-1755.1999.00391.x. [DOI] [PubMed] [Google Scholar]
- 39.Hage FG, de Mattos AM, Khamash H, et al. QT prolongation is an independent predictor of mortality in end-stage renal disease. Clin Cardiol. 2010;33:361–366. doi: 10.1002/clc.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beaubien ER, Pylypchuk GB, Akhtar J, et al. Value of corrected QT interval dispersion in identifying patients initiating dialysis at increased risk of total and cardiovascular mortality. Am J Kidney Dis. 2002;39:834–842. doi: 10.1053/ajkd.2002.32005. [DOI] [PubMed] [Google Scholar]
- 41.Schouten EG, Dekker JM, Meppelink P, et al. QT interval prolongation predicts cardiovascular mortality in an apparently healthy population. Circulation. 1991;84:1516–1523. doi: 10.1161/01.cir.84.4.1516. [DOI] [PubMed] [Google Scholar]
- 42.Dekker JM, Schouten EG, Klootwijk P, et al. Association between QT interval and coronary heart disease in middle-aged and elderly men. The Zutphen Study. Circulation. 1994;90:779–785. doi: 10.1161/01.cir.90.2.779. [DOI] [PubMed] [Google Scholar]
- 43.de Bruyne MC, Hoes AW, Kors JA, et al. QTc dispersion predicts cardiac mortality in the elderly: the Rotterdam Study. Circulation. 1998;97:467–472. doi: 10.1161/01.cir.97.5.467. [DOI] [PubMed] [Google Scholar]
- 44.Elming H, Holm E, Jun L, et al. The prognostic value of the QT interval and QT interval dispersion in all-cause and cardiac mortality and morbidity in a population of Danish citizens. Eur Heart J. 1998;19:1391–1400. doi: 10.1053/euhj.1998.1094. [DOI] [PubMed] [Google Scholar]
- 45.Algra A, Tijssen JG, Roelandt JR, et al. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991;83:1888–1894. doi: 10.1161/01.cir.83.6.1888. [DOI] [PubMed] [Google Scholar]
- 46.Straus SM, Kors JA, De Bruin ML, et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–367. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 47.Goldberg RJ, Bengtson J, Chen ZY, et al. Duration of the QT interval and total and cardiovascular mortality in healthy persons (The Framingham Heart Study experience) Am J Cardiol. 1991;67:55–58. doi: 10.1016/0002-9149(91)90099-7. [DOI] [PubMed] [Google Scholar]