Abstract
Insulin rapidly stimulates tyrosine phosphorylation of a protein of approximately 185 kD in most cell types. This protein, termed insulin receptor substrate-1 (IRS-1), has been implicated in insulin signal transmission based on studies with insulin receptor mutants. In the present study we have examined the levels of IRS-1 and the phosphorylation state of insulin receptor and IRS-1 in liver and muscle after insulin stimulation in vivo in two rat models of insulin resistance, i.e., insulinopenic diabetes and fasting, and a mouse model of non-insulin-dependent diabetes mellitus (ob/ob) by immunoblotting with anti-peptide antibodies to IRS-1 and anti-phosphotyrosine antibodies. As previously described, there was an increase in insulin binding and a parallel increase in insulin-stimulated receptor phosphorylation in muscle of fasting and streptozotocin-induced (STZ) diabetic rats. There was also a modest increase in overall receptor phosphorylation in liver in these two models, but when normalized for the increase in binding, receptor phosphorylation was decreased, in liver and muscle of STZ diabetes and in liver of 72 h fasted rats. In the hyperinsulinemic ob/ob mouse there was a decrease in insulin binding and receptor phosphorylation in both liver and muscle. The tyrosyl phosphorylation of IRS-1 after insulin stimulation reflected an amplification of the receptor phosphorylation in liver and muscle of hypoinsulinemic animals (fasting and STZ diabetes) with a twofold increase, and showed a significant reduction (approximately 50%) in liver and muscle of ob/ob mouse. By contrast, the levels of IRS-1 protein showed a tissue specific regulation with a decreased level in muscle and an increased level in liver in hypoinsulinemic states of insulin resistance, and decreased levels in liver in the hyperinsulinemic ob/ob mouse. These data indicate that: (a) IRS-1 protein levels are differentially regulated in liver and muscle; (b) insulin levels may play a role in this differential regulation of IRS-1; (c) IRS-1 phosphorylation depends more on insulin receptor kinase activity than IRS-1 protein levels; and (d) reduced IRS-1 phosphorylation in liver and muscle may play a role in insulin-resistant states, especially of the ob/ob mice.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almira E. C., Reddy W. J. Effect of fasting on insulin binding to hepatocytes and liver plasma membranes from rats. Endocrinology. 1979 Jan;104(1):205–211. doi: 10.1210/endo-104-1-205. [DOI] [PubMed] [Google Scholar]
- Amatruda J. M., Roncone A. M. Normal hepatic insulin receptor autophosphorylation in nonketotic diabetes mellitus. Biochem Biophys Res Commun. 1985 May 31;129(1):163–170. doi: 10.1016/0006-291x(85)91417-2. [DOI] [PubMed] [Google Scholar]
- Balage M., Grizard J., Sornet C., Simon J., Dardevet D., Manin M. Insulin binding and receptor tyrosine kinase activity in rat liver and skeletal muscle: effect of starvation. Metabolism. 1990 Apr;39(4):366–373. doi: 10.1016/0026-0495(90)90250-g. [DOI] [PubMed] [Google Scholar]
- Block N. E., Buse M. G. Effects of hypercortisolemia and diabetes on skeletal muscle insulin receptor function in vitro and in vivo. Am J Physiol. 1989 Jan;256(1 Pt 1):E39–E48. doi: 10.1152/ajpendo.1989.256.1.E39. [DOI] [PubMed] [Google Scholar]
- Block N. E., Komori K., Robinson K. A., Dutton S. L., Lam C. F., Buse M. G. Diabetes-associated impairment of hepatic insulin receptor tyrosine kinase activity: a study of mechanisms. Endocrinology. 1991 Jan;128(1):312–322. doi: 10.1210/endo-128-1-312. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Charron M. J., Kahn B. B. Divergent molecular mechanisms for insulin-resistant glucose transport in muscle and adipose cells in vivo. J Biol Chem. 1990 May 15;265(14):7994–8000. [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- DeFronzo R. A., Hendler R., Simonson D. Insulin resistance is a prominent feature of insulin-dependent diabetes. Diabetes. 1982 Sep;31(9):795–801. doi: 10.2337/diab.31.9.795. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Araki E., Taira M., Shimada F., Mori M., Craik C. S., Siddle K., Pierce S. B., Roth R. A., Rutter W. J. Replacement of lysine residue 1030 in the putative ATP-binding region of the insulin receptor abolishes insulin- and antibody-stimulated glucose uptake and receptor kinase activity. Proc Natl Acad Sci U S A. 1987 Feb;84(3):704–708. doi: 10.1073/pnas.84.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Fehlmann M., Peyron J. F., Samson M., Van Obberghen E., Brandenburg D., Brossette N. Molecular association between major histocompatibility complex class I antigens and insulin receptors in mouse liver membranes. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8634–8637. doi: 10.1073/pnas.82.24.8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flier J. S. Insulin receptors and insulin resistance. Annu Rev Med. 1983;34:145–160. doi: 10.1146/annurev.me.34.020183.001045. [DOI] [PubMed] [Google Scholar]
- Freidenberg G. R., Klein H. H., Cordera R., Olefsky J. M. Insulin receptor kinase activity in rat liver. Regulation by fasting and high carbohydrate feeding. J Biol Chem. 1985 Oct 15;260(23):12444–12453. [PubMed] [Google Scholar]
- Gibbs E. M., Allard W. J., Lienhard G. E. The glucose transporter in 3T3-L1 adipocytes is phosphorylated in response to phorbol ester but not in response to insulin. J Biol Chem. 1986 Dec 15;261(35):16597–16603. [PubMed] [Google Scholar]
- Kadowaki T., Kasuga M., Akanuma Y., Ezaki O., Takaku F. Decreased autophosphorylation of the insulin receptor-kinase in streptozotocin-diabetic rats. J Biol Chem. 1984 Nov 25;259(22):14208–14216. [PubMed] [Google Scholar]
- Kadowaki T., Koyasu S., Nishida E., Tobe K., Izumi T., Takaku F., Sakai H., Yahara I., Kasuga M. Tyrosine phosphorylation of common and specific sets of cellular proteins rapidly induced by insulin, insulin-like growth factor I, and epidermal growth factor in an intact cell. J Biol Chem. 1987 May 25;262(15):7342–7350. [PubMed] [Google Scholar]
- Kahn C. R., White M. F. The insulin receptor and the molecular mechanism of insulin action. J Clin Invest. 1988 Oct;82(4):1151–1156. doi: 10.1172/JCI113711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasik A., Kahn C. R. Dexamethasone-induced changes in phosphorylation of the insulin and epidermal growth factor receptors and their substrates in intact rat hepatocytes. Endocrinology. 1988 Nov;123(5):2214–2222. doi: 10.1210/endo-123-5-2214. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Karlsson F. A., Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science. 1982 Jan 8;215(4529):185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- Kathuria S., Hartman S., Grunfeld C., Ramachandran J., Fujita-Yamaguchi Y. Differential sensitivity of two functions of the insulin receptor to the associated proteolysis: kinase action and hormone binding. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8570–8574. doi: 10.1073/pnas.83.22.8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maegawa H., Olefsky J. M., Thies S., Boyd D., Ullrich A., McClain D. A. Insulin receptors with defective tyrosine kinase inhibit normal receptor function at the level of substrate phosphorylation. J Biol Chem. 1988 Sep 5;263(25):12629–12637. [PubMed] [Google Scholar]
- Meyerovitch J., Backer J. M., Kahn C. R. Hepatic phosphotyrosine phosphatase activity and its alterations in diabetic rats. J Clin Invest. 1989 Sep;84(3):976–983. doi: 10.1172/JCI114261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momomura K., Tobe K., Seyama Y., Takaku F., Kasuga M. Insulin-induced tyrosine-phosphorylation in intact rat adipocytes. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1181–1186. doi: 10.1016/s0006-291x(88)81264-6. [DOI] [PubMed] [Google Scholar]
- Okamoto M., White M. F., Maron R., Kahn C. R. Autophosphorylation and kinase activity of insulin receptor in diabetic rats. Am J Physiol. 1986 Nov;251(5 Pt 1):E542–E550. doi: 10.1152/ajpendo.1986.251.5.E542. [DOI] [PubMed] [Google Scholar]
- Pang D. T., Sharma B. R., Shafer J. A. Purification of the catalytically active phosphorylated form of insulin receptor kinase by affinity chromatography with O-phosphotyrosyl-binding antibodies. Arch Biochem Biophys. 1985 Oct;242(1):176–186. doi: 10.1016/0003-9861(85)90491-6. [DOI] [PubMed] [Google Scholar]
- Rosen O. M. After insulin binds. Science. 1987 Sep 18;237(4821):1452–1458. doi: 10.1126/science.2442814. [DOI] [PubMed] [Google Scholar]
- Rothenberg P. L., Lane W. S., Karasik A., Backer J., White M., Kahn C. R. Purification and partial sequence analysis of pp185, the major cellular substrate of the insulin receptor tyrosine kinase. J Biol Chem. 1991 May 5;266(13):8302–8311. [PubMed] [Google Scholar]
- Shemer J., Adamo M., Wilson G. L., Heffez D., Zick Y., LeRoith D. Insulin and insulin-like growth factor-I stimulate a common endogenous phosphoprotein substrate (pp185) in intact neuroblastoma cells. J Biol Chem. 1987 Nov 15;262(32):15476–15482. [PubMed] [Google Scholar]
- Soll A. H., Kahn C. R., Neville D. M., Jr Insulin binding to liver plasm membranes in the obese hyperglycemic (ob/ob) mouse. Demonstration of a decreased number of functionally normal receptors. J Biol Chem. 1975 Jun 25;250(12):4702–4707. [PubMed] [Google Scholar]
- Sun X. J., Rothenberg P., Kahn C. R., Backer J. M., Araki E., Wilden P. A., Cahill D. A., Goldstein B. J., White M. F. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991 Jul 4;352(6330):73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- Sweet L. J., Dudley D. T., Pessin J. E., Spector A. A. Phospholipid activation of the insulin receptor kinase: regulation by phosphatidylinositol. FASEB J. 1987 Jul;1(1):55–59. doi: 10.1096/fasebj.1.1.3038645. [DOI] [PubMed] [Google Scholar]
- Tanti J. F., Grémeaux T., Brandenburg D., Van Obberghen E., Le Marchand-Brustel Y. Brown adipose tissue in lean and obese mice. Insulin-receptor binding and tyrosine kinase activity. Diabetes. 1986 Nov;35(11):1243–1248. doi: 10.2337/diab.35.11.1243. [DOI] [PubMed] [Google Scholar]
- Thies R. S., Molina J. M., Ciaraldi T. P., Freidenberg G. R., Olefsky J. M. Insulin-receptor autophosphorylation and endogenous substrate phosphorylation in human adipocytes from control, obese, and NIDDM subjects. Diabetes. 1990 Feb;39(2):250–259. doi: 10.2337/diab.39.2.250. [DOI] [PubMed] [Google Scholar]
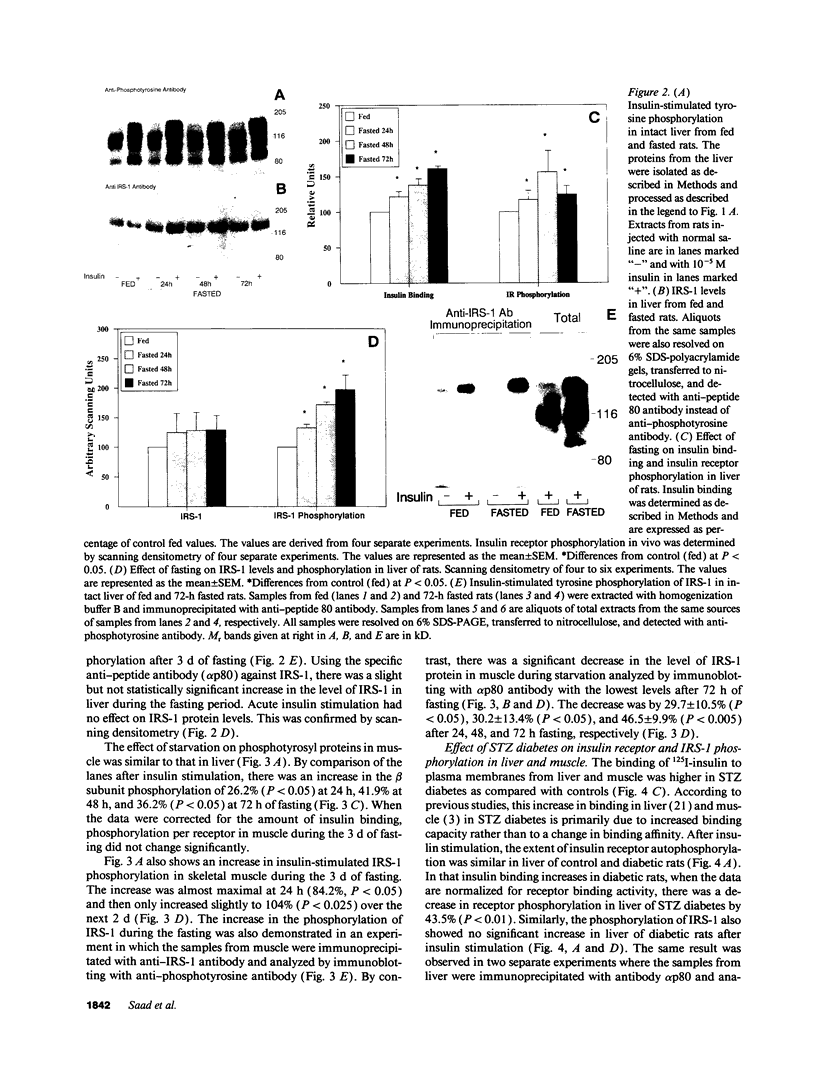
- Tobe K., Koshio O., Tashiro-Hashimoto Y., Takaku F., Akanuma Y., Kasuga M. Immunological detection of phosphotyrosine-containing proteins in rat livers after insulin injection. Diabetes. 1990 May;39(5):528–533. doi: 10.2337/diab.39.5.528. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truglia J. A., Hayes G. R., Lockwood D. H. Intact adipocyte insulin-receptor phosphorylation and in vitro tyrosine kinase activity in animal models of insulin resistance. Diabetes. 1988 Feb;37(2):147–153. doi: 10.2337/diab.37.2.147. [DOI] [PubMed] [Google Scholar]
- Vicario P., Brady E. J., Slater E. E., Saperstein R. Insulin receptor tyrosine kinase activity is unaltered in ob/ob and db/db mouse skeletal muscle membranes. Life Sci. 1987 Sep 7;41(10):1233–1241. doi: 10.1016/0024-3205(87)90201-3. [DOI] [PubMed] [Google Scholar]
- White M. F., Livingston J. N., Backer J. M., Lauris V., Dull T. J., Ullrich A., Kahn C. R. Mutation of the insulin receptor at tyrosine 960 inhibits signal transmission but does not affect its tyrosine kinase activity. Cell. 1988 Aug 26;54(5):641–649. doi: 10.1016/s0092-8674(88)80008-4. [DOI] [PubMed] [Google Scholar]
- White M. F., Maron R., Kahn C. R. Insulin rapidly stimulates tyrosine phosphorylation of a Mr-185,000 protein in intact cells. Nature. 1985 Nov 14;318(6042):183–186. doi: 10.1038/318183a0. [DOI] [PubMed] [Google Scholar]
- White M. F., Stegmann E. W., Dull T. J., Ullrich A., Kahn C. R. Characterization of an endogenous substrate of the insulin receptor in cultured cells. J Biol Chem. 1987 Jul 15;262(20):9769–9777. [PubMed] [Google Scholar]