Abstract
‘Acro-renal syndrome’ refers to co-occurrence of congenital renal and limb anomalies. The term acro-renal syndrome was coined by Curran et al. in 1972 though Dieker and Opitz were the first to report this phenomenon in three male patients in 1969. The common limb defects include oligodactyly, ectrodactyly, syndactyly or brachydactyly anomalies of the carpal and tarsal bones and the common renal anomalies observed are unilateral renal agenesis (URA), bilateral renal hypoplasia, ureteric hypoplasia, hydroureteronephrosis and duplication abnormalities. The acro-renal syndrome as originally described is rare, reported only in ∼20 patients in the international literature. We report a 23-year-old male patient with renal anomalies in the form of absent right kidney, left-sided vesicoureteric reflux (VUR) and skeletal anomalies viz short radius, absent first metacarpal ray in left hand and left undescended testis, consistent with Dieker's type acro-renal syndrome. Apart from the classical acro-renal syndrome, several anomalies of acro-renal patterns and the abnormal gene loci involved are described in the literature. This article is a comprehensive review of the development of kidneys, types of acro-renal syndromes, congenital anomalies of the kidney and urinary tract (CAKUT), syndromes associated with combined limb and renal anomalies, and anomalies associated with URA.
Keywords: acro-renal syndrome, CAKUT, URA, vesicoureteric reflux
Introduction
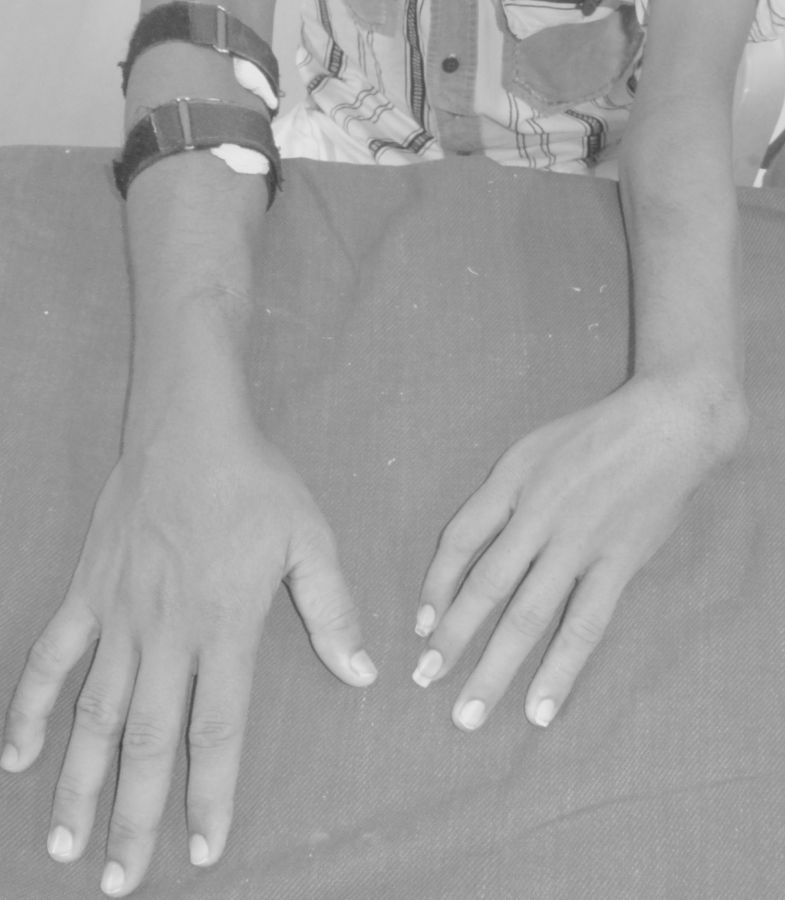
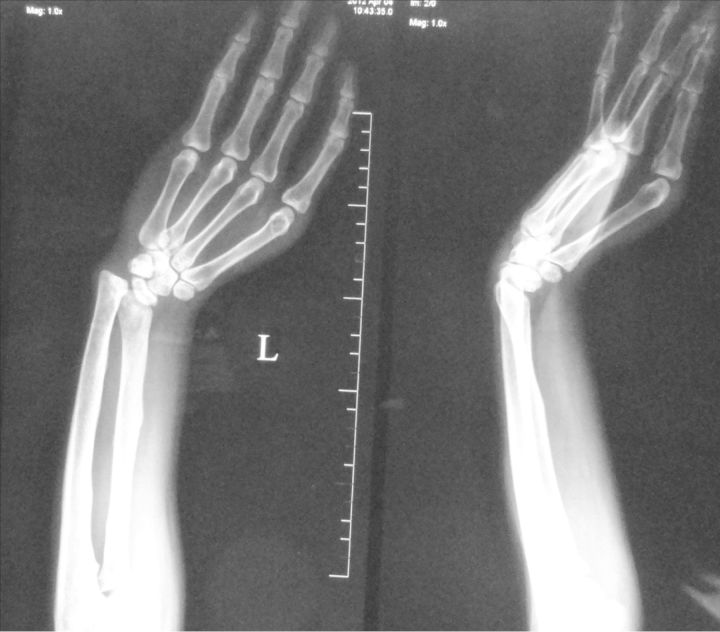
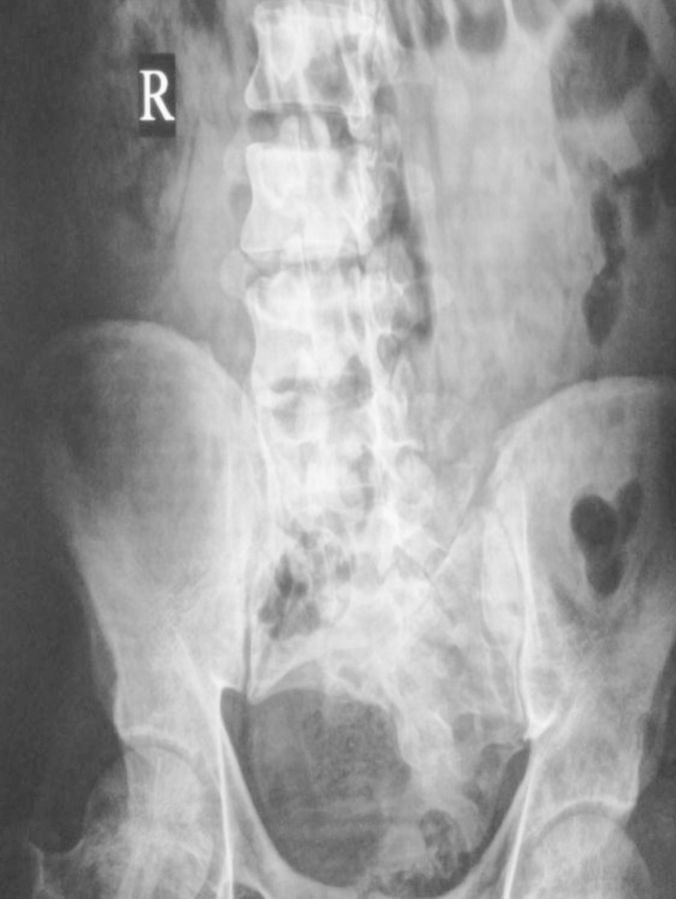
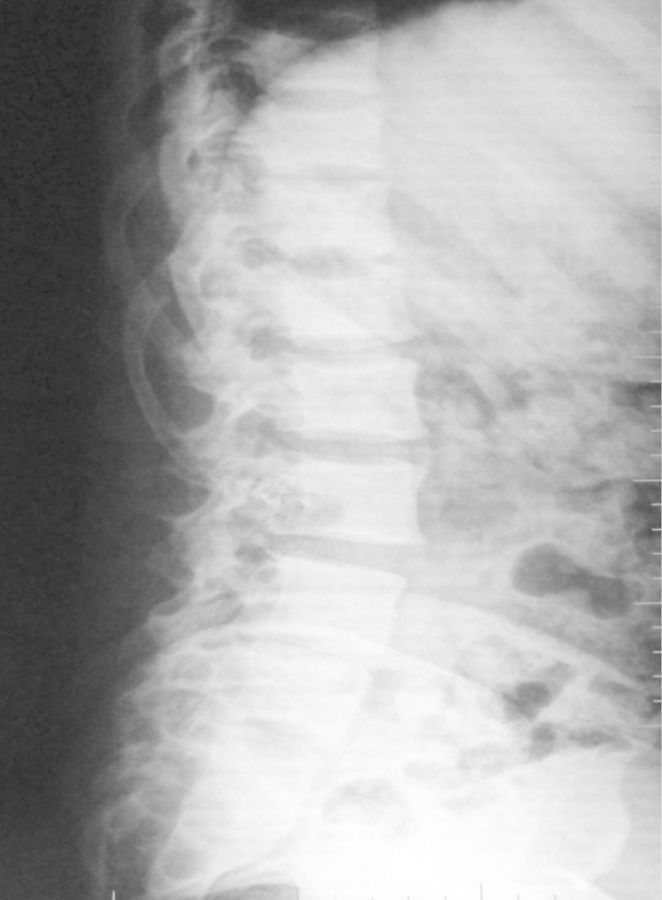
Limb and urinary tract anomalies have frequently been reported to occur together as components of a single acro-renal defect or multiple malformation syndromes [1]. The incidence of associated limb and renal anomalies is about 1 in 20 000 births. The acro-renal syndrome has a restrictive definition with limb defects usually bilateral, like cleft hands or feet and longitudinal defects involving radius or ulna, tibia or fibula [2]. Renal anomalies include agenesis (unilateral or bilateral), hypoplasia and rarely polycystic kidneys. Additional malformations may involve the oro-mandibular region, the trachea and lungs, skin derivatives including sweat glands, mammary glands, the uterus, vas deferens, the nasal placodes and the eyes. Our patient had left upper limb anomalies viz shortened radius, absent first metacarpal ray (thumb and first metacarpal bone) and absent trapezium and scaphoid carpal bones (Figures 1 and 2), axial skeletal anomalies like scoliosis and sacral hypoplasia (Figures 3 and 4) and renal anomalies (left solitary kidney with grade 5 VUR).
Fig. 1.
Clinical photograph showing hypoplastic left forearm with absent thumb. Compare with the normal right upper limb.
Fig. 2.
Radiography showing short radius, absent trapezium, scaphoid, first metacarpal and phalanges of thumb.
Fig. 3.
Radiography abdomen showing sacral hypoplasia.
Fig. 4.
Lateral abdomen radiography showing vertebral kyphoscoliosis.
Embryology of renal tract
The development of the kidney proceeds through a series of successive phases, which include pronephros, mesonephros, and metanephros that develop in a cranio-caudal fashion [3, 4]. During embryonic development, the pronephros appears in pair towards the cranial end of the intermediate mesoderm. The epithelial cells in this region arrange themselves in a series of tubules and join laterally with the pronephric duct. The pronephric duct induces nearby intermediate mesoderm in the thoracolumbar area to form epithelial tubules called mesonephric tubules which are drained into the continuation of the pronephric duct, now called the mesonephric duct or wolffian duct. During the fifth week of gestation, the mesonephric duct develops an outpouching, the so-called ureteric bud [3]. The elongated stalk of the ureteric bud called the metanephric duct later forms the ureter. The cranial end of the ureteric bud extends into the intermediate mesoderm and undergoes a series of branching to form the collecting duct system. It also forms the major and minor calyces and the renal pelvis. The essential step in the process of kidney development is the mutual induction between the metanephric mesenchyme and the ureteric bud [5]. As the foetus develops, the torso elongates and the kidneys rotate and migrate upward towards the lumbar region.
Congenital anomalies of kidney and urinary tract
Kidney malformations occur during organogenesis between 4 and 12 weeks of foetal life leading to congenital anomalies of the kidney and urinary tract (CAKUT) [6]. The incidence as detected in antenatal ultrasound examinations is 1:500 [7]. The urinary tract anomalies account for ∼20–30% of total congenital anomalies diagnosed during pregnancy [3]. CAKUT is phenotypically variable and results in significant renal problems in adulthood ranging from hypertension, proteinuria to end-stage renal disease [8]. The spectrum of CAKUT includes kidney hypoplasia/dysplasia, renal agenesis, multicystic, horseshoe or duplex kidneys, VUR, hydroureter, hydronephrosis and obstruction at the vesicoureteric or uretero-pelvic junction [5, 8].
Congenital renal anomalies can be sporadic or familial, syndromic (also affecting non-renal tissues) or non-syndromic. The primary insults believed to be associated with the development of CAKUT are environmental factors and genetic mechanisms [9, 10]. Animal studies have shown that any perturbations to the foetal environment that include maternal food restriction, low-protein diet, placental insufficiency, maternal vitamin A deficiency, use of alcohol and drugs like angiotensin-converting enzyme inhibitors and sodium valproate can result in reduced nephron endowment and renal anomalies. Various genetic loci associated with syndromic CAKUT are identified. In animal models, deletion of several of these genes results in low nephron endowment including PAX2, p53, GLI3R, GDNF, FGF 7, FRS2 and Six2 [11, 12]. The UK Renal Registry data show that dysplastic/hypoplastic kidneys together account for ∼40% of all children on renal replacement therapy and are six times more common than nephronophthisis, congenital nephrotic syndromes or metabolic diseases [13].
Acro-renal syndrome
The acro-renal syndrome of Dieker's type is sporadic in nature as in our patient. The anomalies include unilateral renal agenesis (URA), ectopic kidneys, urethral diverticulum, hydro ureteronephrosis, ectrodactyly, oligodactyly and hypoplastic carpal/tarsal bones [1, 14]. Acro-renal syndrome of Johnson-Munson is characterized by unilateral or bilateral renal agenesis, aphalangy, hemivertebrae and genital or intestinal dysgenesis [15]. The acro-renal syndrome of Siegler is characterized by renal ectopia, hydroureteronephrosis, ureteric atresia, short stature, hypoplastic radii/ulna and oligodactyly [1]. The mode of inheritance and the gene loci involved in acro-renal syndromes remain unclear, but it is believed they follow an autosomal recessive fashion [12]. A candidate gene for acro-renal disorders is the Formin gene that has been mapped to 15q13–14. Renal failure reported in patients with acro-renal syndrome is due to oligomeganephronic renal hypoplasia [16–18], bilateral renal agenesis, secondary focal segmental glomerulosclerosis [2] or other associated urological anomalies.
Syndromes with acro-renal anomalies
The phases of development with respect to kidney and limbs occur in a synchronized manner during the initial 3–8 weeks of gestation. There are a number of syndromes with combined anomalies of the renal system and limbs other than the classical acro-renal syndrome. Such acro-renal defects are clearly heterogeneous in terms of cause and specific morphological defects, but rarely observed without malformations in other systems [19]. Few such conditions are summarized below.
- Autosomal dominant inheritance
- Acro-renal-ocular syndrome (AROS) is characterized by ocular anomalies (optic nerve coloboma), radial ray abnormalities of the hand from mild thenar hypoplasia to hypoplastic thumbs or prominent upper limb abnormalities and urinary tract anomalies including URA, renal ectopia, malrotation, bilateral renal hypoplasia, horseshoe kidney, VUR and bladder diverticulum [20, 21]. In addition, sensorineural deafness, cardiac anomalies and anal stenosis are noted. Duane-Radial Ray syndrome allelic to AROS is characterized by Duane eye anomaly, radial ray malformations like triphalangeal thumb, preaxial polydactyly, hypoplasia/aplasia of the thumb and radii, shortening and radial deviation of the forearm. This is due to mutations in the SALL4 gene in chromosome (chr) 20 q [22].
- Pallister–Hall syndrome includes imperforate anus, renal anomalies (renal hypoplasia or agenesis), limb anomalies (polydactyly, short limbs, syndactyly and nail dysplasia) and genitourinary abnormalities (microphallus/cryptorchidism)[27, 28]. This is due to heterozygous mutations in GLI3 (Gli-Kruppel family member 3) in chr 7p [29, 30].
- Autosomal recessive inheritance
- Short-rib polydactyly syndrome types I and II: cystic dysplasia, hypoplasia of ureters, dwarfism, thoracic dystrophy, polydactyly, syndactyly and short limbs. Molecular basis is yet to be identified [38].
- Miscellaneous
- VACTERL association is typically defined by the presence of at least three of the following congenital malformations: vertebral defects, anal atresia, cardiac defects, tracheo-oesophageal fistula, renal anomalies and limb abnormalities [39]. This is one of the close differential diagnoses of acro-renal syndrome. The incidence is 1 in 10 000–40 000 live births. Vertebral anomalies typically include segmentation defects, such as hemivertebrae, butterfly vertebrae and vertebral fusions, supernumerary or absent vertebrae. Cardiac malformations are seen in 40–80% [40, 41]. Renal anomalies, which may include URA (or bilateral in severe cases), horseshoe kidney, and cystic and/or dysplastic kidneys, and ureteral anomalies are reported in 50–80%. Limb malformations are seen in 40–50% which include radial anomalies, including thumb aplasia/hypoplasia, polydactyly and lower limb anomalies. Approximately 90% of cases appear to be sporadic. The presumed gene involvement is Shh, Gli,HOXD13 and ZIC3 [40–43].
- Atrio-acro-renal syndrome [44] of partial trisomy 4q is characterised by mental retardation, dysplastic ears with large antihelix, anti-mongoloid palpebral fissures, malformations of the fingers and thumb, renal malformations (horseshoe kidney, renal hypoplasia, ureteral reflux with or without hydronephrosis), congenital heart disease and cryptorchidism [44, 45].
- Poland's syndrome: the incidence is 1 in 7000–10 000. Males are affected more frequently (M:F ratio is 3:1) [48–50], commonly involving the right side (60–75%). In addition to aplasia of the pectoralis, major renal anomalies such as URA or duplication of the urinary collecting system are noted [51, 52].
- Anomalies due to toxic and metabolic factors: foetal alcohol syndrome presents with small rotated kidneys, URA, growth retardation, microcephaly, short palpebral fissures, limb and cardiac defects. Renal agenesis/dysplasia, brain, heart and skeletal anomalies, caudal regression syndrome are seen in foetuses of diabetic mothers [53]. Thalidomide embryopathy is characterized by renal agenesis, hypoplasia, horseshoe kidney, cystic dysplasia, renal ectopia, anomalies of rotation, phocomelia, heart, intestinal and genitourinary anomalies [54].
Renal agenesis
Renal agenesis is the most profound renal tract malformation characterized by complete absence of kidney development and is often accompanied by an absent ureter [55]. Four theories have been proposed as the cause: (i) failure of the metanephric bud to appear in spite of a normally preceding mesonephros, (ii) early regression of the metanephros, (iii) imperfect development of mesonephros and (iv) non-development of pronephros leading to non-growth of mesonephros [56].
Renal agenesis is usually unilateral with the prevalence of 1 in 1500–3200 live births and more common in males [57]. Bilateral renal agenesis is invariably fatal. URA is often asymptomatic and is incidentally diagnosed by radiology [58, 59]. The mean age at diagnosis for renal agenesis is 2.8 years [60]. This anomaly is more common on the left side. An exception to this left-sided predominance is noted in females with combined genital anomalies and URA that commonly presents on the right side [61]. The mechanisms leading to this lateralization remain unclear. Nearly half of the patients with URA have associated urological anomalies, VUR being the commonest [62]. This disorder is often associated with ipsilateral absence of the vas deferens in men and hypoplasia of the uterine horn in women. Renal agenesis has also been associated with chromosomal abnormalities such as 21, 22, 7 and 10 trisomies, 45 X mosaicism and 22q11 microdeletion [63].
A review of 30 cases with a congenital solitary functioning kidney revealed an absent left kidney (67%), associated anomalies that include the ear, nose and throat (30%), the musculoskeletal system (27%), urological tract (47%), gastrointestinal tract (23%), cardiovascular (13%), dermatological and gynaecological (3%) anomalies. Proteinuria was observed in 20% and hypertension in 7% of patients. Chronic kidney disease was documented in 20% [64].
Syndromes with renal agenesis
Branchio-oto-renal syndrome (Melnick–Fraser syndrome): this autosomal dominant disease is characterized by coexistence of deafness, branchial fistulae, pre-auricular pits and renal anomalies, unilateral agenesis being more common. Most common mutation is present on EYA (Eyes-absent homologue 1) gene in chr 8q but also involves SIX1 and SIX6 genes [65–67]. The prevalence is 1: 40 000–70 000 [68].
Renal coloboma syndrome (papillorenal syndrome): it is characterized by renal hypoplasia and agenesis, vesicoureteral reflux and optic nerve coloboma [22] and is due to mutations in nuclear transcription factor PAired-boX gene 2 (PAX2) in chr 10q. Almost all patients develop end-stage kidney disease [69, 70].
Alagille syndrome (arteriohepatic dysplasia): marked arterionephrosclerosis with diffuse calcinosis, URA, vertebral anomalies (butterfly vertebrae), peripheral pulmonary stenosis, mental and growth retardation and neonatal cholestasis. This occurs due to mutations in NOTCH2 in chr 1p and JAG1 (Jagged 1) in chr 20p [71–74].
Winter syndrome: renal agenesis (unilateral/bilateral), middle-ear anomalies and internal genital malformations are noted in these patients. The mutation of the PAX-8 gene in chr 2q has been postulated [75].
Kallmann syndrome: URA, congenital anosmia, hypogonadism and cryptorchidism due to mutations in KAL1 in chr Xp [76].
CHARGE association: URA, duplicated upper pole of one kidney, coloboma, choanal atresia, cardiac, genital and ear defects are due to mutations in CHD7 (chromodomain helicase DNA-binding protein 7) in chr 8q [77].
Genetic testing
The benefit of genetic testing of patients and their relatives is not clear because of a general concern about the appropriateness and limitations of genetic testing in CAKUT. According to the standard guidelines, an appropriate genetic test should be accurate to identify a particular disease, which in most cases of CAKUT is not possible. This is due to uncertain phenotypical presentation and penetrance [3]. Thus, CAKUT is diagnosed through studying the foetal and postnatal imaging. Our knowledge of the genetic basis is mainly based on syndromic cases of CAKUT and animal models [78–80]. Genetic analyses are available only on a research basis [81].
Conclusions
The presence of one congenital anomaly is an indirect indicator of abnormalities in the other systems. Early diagnosis and treatment of urological anomalies are important to improve the long-term renal prognosis. Evaluation is advocated to patients with congenital skeletal deformities of the extremities for co-existing renal anomalies. Parental counselling is necessary in case of genetically determined syndromes. Specialized outpatient clinics for rare diseases are increasingly common and patients should seek out these institutions.
Conflict of interest statement
None declared.
References
- 1.Evans JA, Vitez M, Czeizel A. Patterns of acrorenal malformation associations. Am J Med Genet. 1992;44:413–419. doi: 10.1002/ajmg.1320440405. [DOI] [PubMed] [Google Scholar]
- 2.Zeier M, Tariverdian G, Waldherr R, et al. Acrorenal syndrome in an adult -presentation with proteinuria, hypertension and glomerular lesions. Am J Kidney Dis. 1989;14:221–224. doi: 10.1016/s0272-6386(89)80075-7. [DOI] [PubMed] [Google Scholar]
- 3.Hakan RT, Okan T, Ali H, et al. Congenital Anomalies of Kidney and Urinary Tract. Semin Nephrol. 2010;30:374–386. doi: 10.1016/j.semnephrol.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Guillaume R, Bressan M, Herzlinger D. Paraxial mesoderm contributes stromal cells to the developing kidney. Dev Biol. 2009;329:169–175. doi: 10.1016/j.ydbio.2009.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piscione TD, Rosenblum N. The molecular control of renal branching morphogenesis: current knowledge and emerging insights. Differentiation. 2002;70:227–246. doi: 10.1046/j.1432-0436.2002.700602.x. [DOI] [PubMed] [Google Scholar]
- 6.Krzemien G, Blaim MR, Kostro I, et al. Urological anomalies in children with renal agenesis or multicystic dysplastic kidney. J Appl Genet. 2006;47:171–176. doi: 10.1007/BF03194618. [DOI] [PubMed] [Google Scholar]
- 7.Elias HAM, Stoutenbeek PH, Visser GHA, et al. Concomitant anomalies in 100 children with unilateral multicystic kidney. Ultrasound Obstet Gynecol. 2005;25:384–388. doi: 10.1002/uog.1851. [DOI] [PubMed] [Google Scholar]
- 8.Zaffanello M, Brugnara M, Zuffante M, et al. Are children with con-genital solitary kidney at risk for lifelong complications? A lack of prediction demands caution. Int Urol Nephrol. 2009;41:127–135. doi: 10.1007/s11255-008-9437-5. [DOI] [PubMed] [Google Scholar]
- 9.Weber S, Moriniere V, Knuppel T, et al. Prevalence of mutations in renal developmental genes in children with renal hypodysplasia: results of the ESCAPE study. J Am Soc Nephrol. 2006;17:2864–2870. doi: 10.1681/ASN.2006030277. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen GL, Norgard B, Puho E, et al. Risk of specific congenital abnormalities in offspring of women with diabetes. Diabet Med. 2005;22:693–696. doi: 10.1111/j.1464-5491.2005.01477.x. [DOI] [PubMed] [Google Scholar]
- 11.Adalat S, Bockenhauer D, Sarah E, et al. Renal malformations associated with mutations of developmental genes: messages from the clinic. Pediatr Nephrol. 2010;25:2247–2255. doi: 10.1007/s00467-010-1578-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber S, Taylor JC, Winyard P, et al. SIX2 and BMP4 mutations associate with anomalous kidney development. J Am Soc Nephrol. 2008;19:891–803. doi: 10.1681/ASN.2006111282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis MA, Shaw J, Sinha M, et al. UK Renal Registry 11th Annual Report (December 2008): chapter 13 demography of the UK paediatric renal replacement therapy population. Nephron Clin Pract. 2009;111:257–267. doi: 10.1159/000210002. [DOI] [PubMed] [Google Scholar]
- 14.Zeier M, Ritz E. No stinkfinger and renal failure-do you see a link? Nephrol Dial Transplant. 1999;14:2763–2765. doi: 10.1093/ndt/14.11.2763. [DOI] [PubMed] [Google Scholar]
- 15.Curran AS, Curran JP. Associated acral and renal malformations: a new syndrome? Pediatrics. 1972;49:716–725. [PubMed] [Google Scholar]
- 16.Cascio S, Paran S, Puri P. Associated urological anomalies in children with unilateral renal agenesis. J Urol. 1999;162:1081–1083. doi: 10.1016/S0022-5347(01)68074-1. [DOI] [PubMed] [Google Scholar]
- 17.Kroes HY, Olney RS, Rosano A, et al. Renal defects and limb deficiencies in 197 infants: is it possible to define the “acrorenal syndrome”? Am J Med Genet. 2004;129:149–155. doi: 10.1002/ajmg.a.30176. [DOI] [PubMed] [Google Scholar]
- 18.Akl K. Acrorenal Syndrome in a Child With Renal Failure. Am J Med Genet. 1994;49:447. doi: 10.1002/ajmg.1320490419. [DOI] [PubMed] [Google Scholar]
- 19.Rosano A, Botto LD, Olney RS, et al. Limb defects associated with major congenital anomalies: clinical and epidemiological study from the International Clearinghouse for birth defects monitoring systems. Am J Med Genet. 2000;93:110–116. doi: 10.1002/1096-8628(20000717)93:2<110::aid-ajmg6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 20.Naito T, Kida H, Yokoyama H, et al. Nature of renal involvement in the acro-renal-ocular syndrome. Nephron. 1989;51:115. doi: 10.1159/000185264. [DOI] [PubMed] [Google Scholar]
- 21.Halal F, Homsy M, Perreault G. Acro-renal-ocular syndrome: autosomal dominant thumb hypoplasia, renal ectopia, and eye defect. Am J Med Genet. 1984;17:753. doi: 10.1002/ajmg.1320170406. [DOI] [PubMed] [Google Scholar]
- 22.Weaver RG, Cashwell LF, Lorentz W, et al. Optic nerve coloboma associated with renal disease. Am J Med Genet. 1988;29:597–602. doi: 10.1002/ajmg.1320290318. [DOI] [PubMed] [Google Scholar]
- 23.Albrecht B, Liebers M, Kohlhase J. Atypical phenotype and intrafamilial variability associated with a novel SALL1 mutation. Am J Med Genet A. 2004;125:102–104. doi: 10.1002/ajmg.a.20484. [DOI] [PubMed] [Google Scholar]
- 24.Buck A, Archangelo L, Dixkens C, et al. Molecular cloning, chromosomal localization, and expression of the murine SALL1 ortholog Sall1. Cytogenet Cell Gene. 2000;89:150–153. doi: 10.1159/000015598. [DOI] [PubMed] [Google Scholar]
- 25.Devriendt K, Fryns JP, Lemmens F, et al. Somatic mosaicism and variable expression of Townes–Brocks syndrome. Am J Med Genet. 2002;111:230–231. doi: 10.1002/ajmg.10485. [DOI] [PubMed] [Google Scholar]
- 26.Engels S, Kohlhase J, McGaughran J. A SALL1 mutation causes a branchio-oto-renal syndrome-like phenotype. J Med Genet. 2000;37:458–460. doi: 10.1136/jmg.37.6.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuo JS, Casey SO, Thompson L, et al. Pallister–Hall syndrome: clinical and MR features. Am J Neuroradiol. 1999;20:1839–1841. [PMC free article] [PubMed] [Google Scholar]
- 28.Biesecker LG, Graham JM., Jr Pallister–Hall syndrome. J Med Genet. 1996;33:585–589. doi: 10.1136/jmg.33.7.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall JG, Pallister PD, Clarren SK, et al. Congenital hypothalamic hamartoblastoma, hypopituitarism, imperforate anus, and postaxial polydactyly—a new syndrome? Clinical, causal and pathogenetic considerations. Am J Med Genet. 1980;7:47–74. doi: 10.1002/ajmg.1320070110. [DOI] [PubMed] [Google Scholar]
- 30.Low M, Moringlane JR, Reif J, et al. Polysyndactyly and asymptomatic hypothalamic hamartoma in mother and son: a variant of Pallister–Hall syndrome. Clin Genet. 1995;48:209–212. doi: 10.1111/j.1399-0004.1995.tb04090.x. [DOI] [PubMed] [Google Scholar]
- 31.Cheney WD. Acro-osteolysis. Am J Roentgenol. 1965;94:595–560. [PubMed] [Google Scholar]
- 32.Strassburg A, Schirg E, Enrich HJH. A child with polycystic kidney disease-do we care about associated malformations? Nephrol Dial Transplant. 2001;16:1942–1944. doi: 10.1093/ndt/16.9.1942. [DOI] [PubMed] [Google Scholar]
- 33.Evans JA, Phillips S, Reed M, et al. Severe acrorenal-uterine-mandibular syndrome. Am J Med Genet. 2000;93:67–73. doi: 10.1002/1096-8628(20000703)93:1<67::aid-ajmg11>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 34.Tobias ES, Patrick WJA, MacKenzie JR, et al. A case of Acro-renal-mandibular syndrome in an 18-week male foetus. Clin Dysmorphol. 2001;10:61–64. doi: 10.1097/00019605-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 35.Halal F, Desgranger MF, Ledec B, et al. Acrorenal mandibular syndrome. Am J Med Genet. 1980;5:277–284. doi: 10.1002/ajmg.1320050310. [DOI] [PubMed] [Google Scholar]
- 36.Slavotinek AM, Tifft CJ. Fraser syndrome and cryptophthalmos: a review of the diagnostic criteria and evidence of phenotypic modules. J Med Genet. 2002;39:623–633. doi: 10.1136/jmg.39.9.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGregor L, Makela V, Darling SM, et al. Fraser syndrome and mouse blebbed phenotype caused by mutations in FRAS1/Fras1 encoding a putative extracellular matrix protein. Nat Genet. 2003;34:203–208. doi: 10.1038/ng1142. [DOI] [PubMed] [Google Scholar]
- 38.Urioste M, Martínez-Frías ML, Bermejo E, et al. Short rib polydactyly syndrome and pericentric inversion of chromosome. Am J Med Genet. 1994;49:94–97. doi: 10.1002/ajmg.1320490118. [DOI] [PubMed] [Google Scholar]
- 39.Quan L, Smith DW. The VATER association. Vertebral defects, anal atresia, T-E fistula with esophageal atresia, radial and renal dysplasia: a spectrum of associated defects. J Pediatr. 1973;82:104–107. doi: 10.1016/s0022-3476(73)80024-1. [DOI] [PubMed] [Google Scholar]
- 40.Opitz JM. The developmental field concept. Am J Med Genet. 1985;21:1–11. doi: 10.1002/ajmg.1320210102. [DOI] [PubMed] [Google Scholar]
- 41.Temtamy SA, Miller JD. Extending the scope of the VATER association: definition of the VATER syndrome. J Pediatr. 1974;85:345–349. doi: 10.1016/s0022-3476(74)80113-7. [DOI] [PubMed] [Google Scholar]
- 42.Rittler M, Paz JE, Castilla EE. VACTERL association, epidemiologic definition and delineation. Am J Med Genet. 1996;63:529–536. doi: 10.1002/(SICI)1096-8628(19960628)63:4<529::AID-AJMG4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 43.Czeizel A, Ludanyi I. An aetiological study of the VACTERL-association. Eur J Pediatr. 1985;144:331–337. doi: 10.1007/BF00441773. [DOI] [PubMed] [Google Scholar]
- 44.Otsuka T, Fujinaka H, Immamura M, et al. Duplication of chromosome 4q: renal pathology of two siblings. Am J Med Genet A. 2005;134:330–333. doi: 10.1002/ajmg.a.30643. [DOI] [PubMed] [Google Scholar]
- 45.Gorlin RJ, Cohen MM, Hennekam RCM. Chromosomal syndromes: unusual variants. Syndromes of the head and neck. 4th edn. Oxford: Oxford University Press; 2001. pp. 84–85. [Google Scholar]
- 46.Duncan PA, Shapiro LR, Stangel JJ, et al. The MURCS association: Müllerian duct aplasia, renal aplasia, and cervicothoracic somite dysplasia. J Pediatr. 1979;95:399–402. doi: 10.1016/s0022-3476(79)80514-4. [DOI] [PubMed] [Google Scholar]
- 47.Lira CS, Forbin K, Martinez CJC. Rokitansky syndrome and MURCS association-clinical features and basis for diagnosis. Int J Fertil Women Med. 1999;45:250–255. [PubMed] [Google Scholar]
- 48.Gorlin RJ. Risk of recurrence in usually nongenetic malformation syndromes. Birth Defects Orig Artic Ser. 1979;15:181–188. [PubMed] [Google Scholar]
- 49.Stevens DB, Fink BA, Prevel C. Poland's syndrome in one identical twin. J Pediatr Orthop. 2000;20:392–395. [PubMed] [Google Scholar]
- 50.Hedge HR, Leung AKC. Aplasia of pectoralis major muscle and renal anomalies. Am J Med Genet. 1989;32:109–111. doi: 10.1002/ajmg.1320320123. [DOI] [PubMed] [Google Scholar]
- 51.Pranava VM, Rao PSSS, Neelachalam A, et al. Poland's syndrome with renal hypertension. JAPI. 2000;48:452–453. [PubMed] [Google Scholar]
- 52.Mace JW, Kaplan JM, Schanberger JE, et al. Poland's syndrome: report of seven cases and review of the literature. Clin Pediatr. 1972;11:98–102. doi: 10.1177/000992287201100217. [DOI] [PubMed] [Google Scholar]
- 53.Tran S, Chen YW, Chenier I, et al. Maternal diabetes modulates renal morphogenesis in offspring. J Am Soc Nephrol. 2008;19:943–952. doi: 10.1681/ASN.2007080864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nora AH, Nora JJ. A syndrome of multiple congenital anomalies associated with teratogenic exposure. Arch Environ Health. 1975;30:17–21. doi: 10.1080/00039896.1975.10666626. [DOI] [PubMed] [Google Scholar]
- 55.Boyden EA. Congenital absence of the kidney: an interpretation based on a 10-mm. human embryo exhibited unilateral renal agenesis. Anat Rec. 1932;52:325–349. [Google Scholar]
- 56.Hiraoka M, Tsukkahara H, Ohshima Y, et al. Renal aplasia is the predominant cause of congenital solitary kidneys. Kidney Int. 2002;61:1840–1844. doi: 10.1046/j.1523-1755.2002.00322.x. [DOI] [PubMed] [Google Scholar]
- 57.Robson WL, Leung AK, Rogers RC. Unilateral renal agenesis. Adv Pediatr. 1995;42:575–592. [PubMed] [Google Scholar]
- 58.Mishra A. Renal agenesis: report of an interesting case. Br J Radiol. 2007;80:e167–e169. doi: 10.1259/bjr/79912069. [DOI] [PubMed] [Google Scholar]
- 59.Woolf AS. Unilateral multicystic dysplastic kidney. Kidney Int. 2006;69:190–193. doi: 10.1038/sj.ki.5000015. [DOI] [PubMed] [Google Scholar]
- 60.de Lucas C, Nocea A, San Roman J, et al. Solitary kidney. Study of renal morphology and function in 95 children. Nefrología. 2006;26:56–63. [PubMed] [Google Scholar]
- 61.Michiel F. Schreude. Unilateral anomalies of kidney development: why is left not right? Kidney Int. 2011;80:740–745. doi: 10.1038/ki.2011.204. [DOI] [PubMed] [Google Scholar]
- 62.Atiyeh B, Husmann D, Baum M. Contralateral renal abnormalities in patients with renal agenesis and noncystic renal dysplasia. Pediatrics. 1993;91:812–815. [PubMed] [Google Scholar]
- 63.Woolf AS, Hillman KA. Unilateral renal agenesis and the congenital solitary functioning kidney: developmental, genetic and clinical perspectives. BJU Int. 2007;99:17–21. doi: 10.1111/j.1464-410X.2006.06504.x. [DOI] [PubMed] [Google Scholar]
- 64.Akl K. The anomalies associated with congenital solitary functioning kidney in children. Saudi J Kidney Dis Transplant. 2011;22:67–71. [PubMed] [Google Scholar]
- 65.Ruf RG, Xu PX, Silvius D, et al. SIX1 mutations cause branchio-oto-renal syndrome by disruption of EYA1-SIX1-DNA complexes. Proc Natl Acad Sci USA. 2004;101:8090–8095. doi: 10.1073/pnas.0308475101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoskins BE, Cramer CH, Silvius D, et al. Transcription factor SIX5 is mutated in patients with branchio-oto-renal syndrome. Am J Hum Genet. 2007;80:800–804. doi: 10.1086/513322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Misra M, Nolph KD. Renal failure and deafness: bronchio-oto renal syndrome. Am J Kidney Dis. 1998;32:334–337. doi: 10.1053/ajkd.1998.v32.pm9708623. [DOI] [PubMed] [Google Scholar]
- 68.Buller C, Xu X, Marquis V, et al. Molecular effects of Eya1 domain mutations causing organ defects in BOR syndrome. Hum Mol Genet. 2001;10:2775–2781. doi: 10.1093/hmg/10.24.2775. [DOI] [PubMed] [Google Scholar]
- 69.Sanyanusin P, Schimmenti LA, McNoe LA, et al. Mutation of the PAX2 gene in a family with optic nerve colobomas, renal anomalies, and vesicoureteral reflux. Nat Genet. 1995;9:358–364. doi: 10.1038/ng0495-358. [DOI] [PubMed] [Google Scholar]
- 70.Cunliffe HE, McNoe LA, Ward TA, et al. The prevalence of PAX2 mutations in patients with isolated colobomas or colobomas associated with urogenital anomalies. J Med Genet. 1998;35:806–812. doi: 10.1136/jmg.35.10.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oda T, Elkahloun AG, Pike BL, et al. Mutations in the human Jagged 1 gene are responsible for Alagille syndrome. Nat Genet. 1997;16:235–242. doi: 10.1038/ng0797-235. [DOI] [PubMed] [Google Scholar]
- 72.Li L, Krantz ID, Deng Y, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet. 1997;16:243–251. doi: 10.1038/ng0797-243. [DOI] [PubMed] [Google Scholar]
- 73.Krantz ID, Piccoli DA, Spinner NB. Clinical and molecular genetics of Alagille syndrome. Curr Opin Pediatr. 1999;11:558–564. doi: 10.1097/00008480-199912000-00015. [DOI] [PubMed] [Google Scholar]
- 74.McDaniell R, Warthen DM, Sanchez-Lara Pa, et al. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am J Hum Genet. 2006;79:169–173. doi: 10.1086/505332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maha MAH, Makia JM, Sawsan JA. Baraitser–Winter syndrome: an additional Arab patient. Egypt J Med Hum Genet. 2010;11:187–191. [Google Scholar]
- 76.Hardelin JP. Kallmann syndrome towards molecular pathogenesis. Mol Cell Endocrinol. 2001;179:75–81. doi: 10.1016/s0303-7207(01)00462-2. [DOI] [PubMed] [Google Scholar]
- 77.Zentner GE, Layman WS, Martin DM, et al. Molecular and phenotypic aspects of CHD7 mutation in CHARGE syndrome. Am J Med Genet. 2010;152:674–686. doi: 10.1002/ajmg.a.33323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Taranta A, Palma A, De Luca V, et al. Renal-coloboma syndrome: a single nucleotide deletion in the PAX2 gene at exon 8 is associated with a highly variable phenotype. Clin Nephrol. 2007;67:1. doi: 10.5414/cnp67001. [DOI] [PubMed] [Google Scholar]
- 79.Fletcher J, Hu M, Berman Y, et al. Multicystic dysplastic kidney and variable phenotype in a family with a novel deletion mutation of PAX2. J Am Soc Nephrol. 2005;16:2754. doi: 10.1681/ASN.2005030239. [DOI] [PubMed] [Google Scholar]
- 80.Decramer S, Parant O, Beaufils S, et al. Anomalies of the TCF2 gene are the main cause of fetal bilateral hyperechogenic kidneys. J Am Soc Nephrol. 2007;18:923. doi: 10.1681/ASN.2006091057. [DOI] [PubMed] [Google Scholar]
- 81.Kerecuk L, Schreuder MF, Woolf AS. Renal tract malformations: perspectives for nephrologists. Nat Clin Pract Nephrol. 2008;4:312–325. doi: 10.1038/ncpneph0807. [DOI] [PubMed] [Google Scholar]