Case
A 50-year-old woman was brought to our emergency department as she could no longer walk, even to the bathroom. The weakness in her legs began 5 days earlier and was accompanied by diffuse muscle discomfort. She reported that her arms were weak as well. A physician had administered some ‘shots’ on the day prior to admission to alleviate a suspected neurological problem. A similar constellation of complaints had occurred 3 months earlier but apparently resolved without specific treatments. Five months earlier, she had been at another hospital with a urinary tract infection. At that time, a serum creatinine level of 176 µmol/L and a serum potassium level of 2.7 mmol/L were reported and supplemental oral potassium was recommended. The heart rate was 65 beats/min, blood pressure was 154/86 mmHg and the respiratory rate was 24 breaths/min. The patient was profoundly weak in both upper and lower extremities and could not stand. Breathing appeared shallow. The lungs were clear; there were no cardiac or abdominal findings. A neurologist observed depressed reflexes but could not discern a neurological disorder. The haemoglobin was 10.9 g/dL and haematocrit 31 vol%. Arterial blood gases disclosed PaO2 60, PaCO2 49 mmHg, pH 7.17 and HCO3 15 mmol/L. The sodium level was 139, chloride 122 and potassium 1.27 mmol/L. The creatinine level was 182 µmol/L. By test strip, the urine pH was found to be 6.0, proteinuria was 2+ and sediments revealed numerous erythrocytes and white blood cells without casts. The urine sodium was 70, potassium 24 and chloride 67 mmol/L.
Question
What are the molecular mechanisms responsible for the hypokalaemia in this patient?
Discussion
The patient presented with a combined metabolic and respiratory acidosis. With a serum HCO3 level of 15 mmol/L, we would expect her respiratory compensation to achieve a PaCO2 of ∼30 mmHg. However, she did not compensate her metabolic acidosis at all. Instead, she exhibited alveolar hypoventilation at a PaCO2 of 49 mmHg. In addition to tetraplegia, the respiratory musculature was apparently so weakened in our patient that she could not hyperventilate appropriately. Her respiratory drive was intact as documented by a respiratory rate of 24 breaths/min; however, her ventilation was too shallow to reach her alveolae. The urine pH was inappropriately alkaline. Furthermore, the sum of urinary sodium and potassium exceeded the urinary chloride concentration, demonstrating that very little ammonium chloride was being excreted. This constellation is consistent with a urinary acidification defect in the distal nephron (‘classical’ renal tubular acidosis type I). The inappropriately high urinary potassium concentration is strong evidence, suggesting that urinary potassium losses were responsible for the hypokalaemia [1].
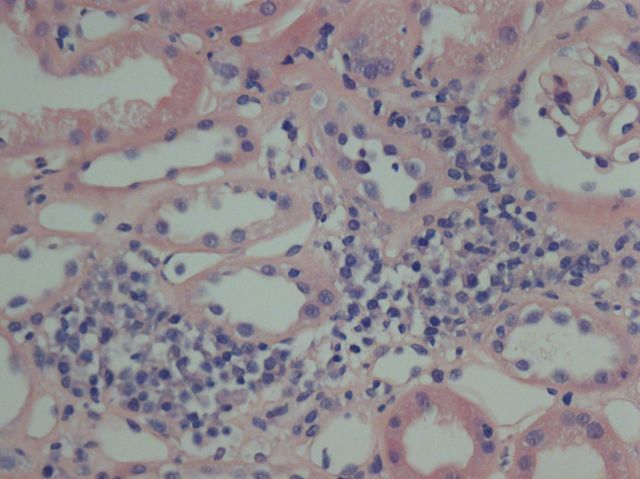
A central venous catheter was placed and 1 L balanced electrolyte solution containing an additional 40 mmol/L KCl was infused over 2 h. KCl infusions were continued for 24 h until her serum potassium increased to 3.9 mmol/L. By that time, the PaO2 was 91 mmHg, the PaCO2 was 27 mmHg, the pH 7.3, while there was little change in HCO3 at 12 mmol/L. The tetraplegia resolved and now we observe that the patient compensates her metabolic acidosis perfectly. We noted that the antinuclear antibody levels exceeded 1:5120. The patient also had anti- SS antibodies >2400 kU/L and anti-Ro-60 antibodies 2214 kU/L. Both titres were markedly elevated for our laboratory. Consultants performed a Schirmer test that was positive and a renal biopsy was performed revealing intense interstitial nephritis (Figure 1). We diagnosed Sjögren's syndrome in our patient. Corticosteroids and azathioprine resulted in an all-round improvement.
Fig. 1.
Haematoxylin and eosin stain of renal biopsy documenting intense interstitial nephritis.
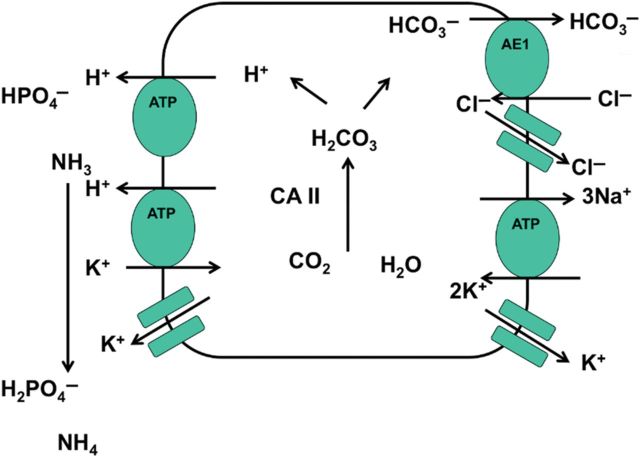
The mechanism by which the hyperglobulinaemia in Sjögren's syndrome causes distal RTA is not known; however, the RTA is independent of the class or quantity of abnormal circulating globulin [2]. In patients with Sjögren's syndrome, high levels of serum gamma globulin, serum protein and serum β-2 microglobulin are the best predictors of the development of classic distal RTA. A defect in hydrogen ion secretion is the major pathophysiological mechanism leading to distal RTA. In overt distal RTA, as in our patient, metabolic acidosis is found, while the urine is alkaline. The mechanisms involved in urinary acidification largely rely on α-intercalated cells that are not only abundant in the outer-medullary collecting duct but also present in the cortical collecting duct. The α-intercalated cell has an H+-ATPase in the apical membrane and a Cl−/HCO3− exchanger (AE1) in the basolateral membrane (Figure 2). A particularly attractive mechanism that could account for hypokalaemia is the existence of a defect in the renal K+/H+ ATPase pump. Renal K+ excretion is determined not only by active K+ secretion localized largely to the distal tubule and the cortical collecting tubule, but also by active K+ absorption localized, at least in part, to the outer medullary collecting tubule. Were the K+/H+ ATPase pump in the medullary collecting tubule defective, we would expect the development of both hypokalaemia and metabolic acidosis.
Fig. 2.
Schematic model of H+ secretion in the cortical collecting tubule. The main pump for luminal H+ secretion in the alpha type-intercalated cell is a vacuolar H+-ATPase. A H+,K+-ATPase is also involved in H+ secretion. Intracellularly formed HCO3− leaves the cell via Cl–HCO3− exchange, facilitated by an anion exchanger (AE1). Cytoplasmic carbonic anhydrase II (CA II) is necessary to secrete H+ (adapted) [2]. CA: Carbonic anhydrase, AE: anion exchanger.
Also critical for urinary acidification are carbonic anhydrases, key enzymes in the regulation of acid–base balance. They catalyze the reversible hydration of carbonic dioxide according to the reaction: CO2 + H2O↔HCO3–H+. Autoantibodies to carbonic anhydrase II have been observed in the sera of patients with Sjögren's syndrome and systemic lupus erythematosus, as well as in the distal tubules of the kidney. Pertovaara et al. recently described antibodies directed at carbonic anhydrases II, VI and XIII that were associated with renal acidification capacity in patients with Sjögren's syndrome [3].
Sjögren's syndrome with distal RTA and hypokalaemia is not uncommon; however, paralysis from hypokalaemia is unusual [4]. Our patient was particularly illustrative as she led us to review non-anion gap metabolic acidosis, appropriate compensation, urinary acidification mechanisms, renal tubular acidosis, hypokalaemia and management issues; these items were enough for a rainy afternoon.
Conflict of interest statement. None declared.
References
- 1.Rhee EP, Scott JA, Dighe AS. A 37-year-old man with muscle pain, weakness, and weight loss. N Engl J Med. 2012;366:553–560. doi: 10.1056/NEJMcpc1110051. [DOI] [PubMed] [Google Scholar]
- 2.Batlle D, Moorthi KM, Schlueter W, et al. Distal renal tubular acidosis and the potassium enigma. Semin Nephrol. 2006;26:471–478. doi: 10.1016/j.semnephrol.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Pertovaara M, Bootorabi F, Kuuslahti M, et al. Novel carbonic anhydrase autoantibodies and renal manifestations in patients with primary Sjogren's syndrome. Rheumatology. 2011;50:1453–1457. doi: 10.1093/rheumatology/ker118. [DOI] [PubMed] [Google Scholar]
- 4.Rehman HU. A woman with generalised weakness, hypokalaemia, and metabolic acidosis. BMJ. 2012;344:e2545. doi: 10.1136/bmj.e2545. [DOI] [PubMed] [Google Scholar]