Abstract
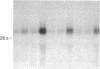
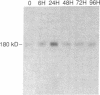
Neuroblastoma, a malignant neoplasm that arises in the adrenal medulla or sympathetic ganglion, is one of the most common solid tumors of childhood. Reports that neuroblastomas spontaneously mature to form benign ganglioneuromas have prompted investigations into the efficacy of using agents that induce neuronal differentiation in the treatment of this malignancy. Retinoic acid is one agent in particular that has been shown to induce growth inhibition and terminal differentiation of neuroblastoma cell lines in vitro. Using the human neuroblastoma cell line SMH-KCNR, we have investigated the role of the extracellular matrix protein thrombospondin in retinoic acid induced neuroblastoma differentiation. Treatment with retinoic acid results in a rapid induction (within 4 h) of thrombospondin (TSP) message which is independent of intervening protein synthesis and superinducible in the presence of cycloheximide. This suggests that TSP functions as a retinoic acid inducible immediate early response gene. A concomitant increase in both cell associated and soluble forms of TSP protein can be detected within 24 h of retinoic acid treatment. A functional role for TSP in SMH-KCNR differentiation was established in experiments which showed that exposure to anti-TSP monoclonal antibodies delay retinoic acid differentiation for 48 h. At the time the cells overcome the effects of TSP inhibition, laminin production becomes maximal. Treatment of the cells with a combination of anti-TSP and antilaminin antibodies results in complete inhibition of differentiation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almendral J. M., Sommer D., Macdonald-Bravo H., Burckhardt J., Perera J., Bravo R. Complexity of the early genetic response to growth factors in mouse fibroblasts. Mol Cell Biol. 1988 May;8(5):2140–2148. doi: 10.1128/mcb.8.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi A., Rossing T. H., Bennett J., Todd R. F., 3rd Surface membrane heterogeneity among human mononuclear phagocytes. J Immunol. 1984 Mar;132(3):1237–1243. [PubMed] [Google Scholar]
- Boot-Handford R. P., Kurkinen M., Prockop D. J. Steady-state levels of mRNAs coding for the type IV collagen and laminin polypeptide chains of basement membranes exhibit marked tissue-specific stoichiometric variations in the rat. J Biol Chem. 1987 Sep 15;262(26):12475–12478. [PubMed] [Google Scholar]
- Bornstein P., O'Rourke K., Wikstrom K., Wolf F. W., Katz R., Li P., Dixit V. M. A second, expressed thrombospondin gene (Thbs2) exists in the mouse genome. J Biol Chem. 1991 Jul 15;266(20):12821–12824. [PubMed] [Google Scholar]
- Carlin B. E., Durkin M. E., Bender B., Jaffe R., Chung A. E. Synthesis of laminin and entactin by F9 cells induced with retinoic acid and dibutyryl cyclic AMP. J Biol Chem. 1983 Jun 25;258(12):7729–7737. [PubMed] [Google Scholar]
- Castle V., Varani J., Fligiel S., Prochownik E. V., Dixit V. Antisense-mediated reduction in thrombospondin reverses the malignant phenotype of a human squamous carcinoma. J Clin Invest. 1991 Jun;87(6):1883–1888. doi: 10.1172/JCI115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C. L. Immortalization of neural cells via retrovirus-mediated oncogene transduction. Annu Rev Neurosci. 1989;12:47–65. doi: 10.1146/annurev.ne.12.030189.000403. [DOI] [PubMed] [Google Scholar]
- Cooper A. R., Taylor A., Hogan B. L. Changes in the rate of laminin and entactin synthesis in F9 embryonal carcinoma cells treated with retinoic acid and cyclic amp. Dev Biol. 1983 Oct;99(2):510–516. doi: 10.1016/0012-1606(83)90300-7. [DOI] [PubMed] [Google Scholar]
- De Luca L. M., Adamo S., Kato S. Retinoids and cell adhesion. Methods Enzymol. 1990;190:81–91. doi: 10.1016/0076-6879(90)90012-p. [DOI] [PubMed] [Google Scholar]
- De Luca L. M. Retinoids and their receptors in differentiation, embryogenesis, and neoplasia. FASEB J. 1991 Nov;5(14):2924–2933. [PubMed] [Google Scholar]
- Dinsmore J. H., Solomon F. Inhibition of MAP2 expression affects both morphological and cell division phenotypes of neuronal differentiation. Cell. 1991 Feb 22;64(4):817–826. doi: 10.1016/0092-8674(91)90510-6. [DOI] [PubMed] [Google Scholar]
- Dixit V. M., Grant G. A., Santoro S. A., Frazier W. A. Isolation and characterization of a heparin-binding domain from the amino terminus of platelet thrombospondin. J Biol Chem. 1984 Aug 25;259(16):10100–10105. [PubMed] [Google Scholar]
- Dixit V. M., Hennessy S. W., Grant G. A., Rotwein P., Frazier W. A. Characterization of a cDNA encoding the heparin and collagen binding domains of human thrombospondin. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5449–5453. doi: 10.1073/pnas.83.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit V. M., Marks R. M., Sarma V., Prochownik E. V. The antimitogenic action of tumor necrosis factor is associated with increased AP-1/c-jun proto-oncogene transcription. J Biol Chem. 1989 Oct 5;264(28):16905–16909. [PubMed] [Google Scholar]
- Engvall E., Davis G. E., Dickerson K., Ruoslahti E., Varon S., Manthorpe M. Mapping of domains in human laminin using monoclonal antibodies: localization of the neurite-promoting site. J Cell Biol. 1986 Dec;103(6 Pt 1):2457–2465. doi: 10.1083/jcb.103.6.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans A. E., D'Angio G. J., Randolph J. A proposed staging for children with neuroblastoma. Children's cancer study group A. Cancer. 1971 Feb;27(2):374–378. doi: 10.1002/1097-0142(197102)27:2<374::aid-cncr2820270221>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Hogan B. L., Taylor A., Adamson E. Cell interactions modulate embryonal carcinoma cell differentiation into parietal or visceral endoderm. Nature. 1981 May 21;291(5812):235–237. doi: 10.1038/291235a0. [DOI] [PubMed] [Google Scholar]
- Huang F. L., Roop D. R., De Luca L. M. Vitamin A deficiency and keratin biosynthesis in cultured hamster trachea. In Vitro Cell Dev Biol. 1986 Apr;22(4):223–230. doi: 10.1007/BF02623307. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Ebihara I., Killen P. D., Sasaki M., Cannon F. B., Yamada Y., Martin G. R. Genes for basement membrane proteins are coordinately expressed in differentiating F9 cells but not in normal adult murine tissues. Dev Biol. 1987 Aug;122(2):373–378. doi: 10.1016/0012-1606(87)90302-2. [DOI] [PubMed] [Google Scholar]
- LaRosa G. J., Gudas L. J. An early effect of retinoic acid: cloning of an mRNA (Era-1) exhibiting rapid and protein synthesis-independent induction during teratocarcinoma stem cell differentiation. Proc Natl Acad Sci U S A. 1988 Jan;85(2):329–333. doi: 10.1073/pnas.85.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa G. J., Gudas L. J. Early retinoic acid-induced F9 teratocarcinoma stem cell gene ERA-1: alternate splicing creates transcripts for a homeobox-containing protein and one lacking the homeobox. Mol Cell Biol. 1988 Sep;8(9):3906–3917. doi: 10.1128/mcb.8.9.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J., Derick L. H., Connolly J. E., Chen J. H., Chao F. C. The structure of human platelet thrombospondin. J Biol Chem. 1985 Mar 25;260(6):3762–3772. [PubMed] [Google Scholar]
- Lewis J., Martin P. Vertebrate development. Limbs: a pattern emerges. Nature. 1989 Dec 14;342(6251):734–735. doi: 10.1038/342734a0. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Ong E. S., Dyck J. A., Evans R. M. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990 May 17;345(6272):224–229. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- Marotti K. R., Brown G. D., Strickland S. Two-stage hormonal control of type IV collagen mRNA levels during differentiation of F9 teratocarcinoma cells. Dev Biol. 1985 Mar;108(1):26–31. doi: 10.1016/0012-1606(85)90005-3. [DOI] [PubMed] [Google Scholar]
- Mason I. J., Taylor A., Williams J. G., Sage H., Hogan B. L. Evidence from molecular cloning that SPARC, a major product of mouse embryo parietal endoderm, is related to an endothelial cell 'culture shock' glycoprotein of Mr 43,000. EMBO J. 1986 Jul;5(7):1465–1472. doi: 10.1002/j.1460-2075.1986.tb04383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell E. M., Ben T., Newkirk C., Chang S., De Luca L. M. Differentiation of tracheal mucociliary epithelium in primary cell culture recapitulates normal fetal development and regeneration following injury in hamsters. Am J Pathol. 1987 Dec;129(3):511–522. [PMC free article] [PubMed] [Google Scholar]
- Neugebauer K. M., Emmett C. J., Venstrom K. A., Reichardt L. F. Vitronectin and thrombospondin promote retinal neurite outgrowth: developmental regulation and role of integrins. Neuron. 1991 Mar;6(3):345–358. doi: 10.1016/0896-6273(91)90244-t. [DOI] [PubMed] [Google Scholar]
- Noji S., Nohno T., Koyama E., Muto K., Ohyama K., Aoki Y., Tamura K., Ohsugi K., Ide H., Taniguchi S. Retinoic acid induces polarizing activity but is unlikely to be a morphogen in the chick limb bud. Nature. 1991 Mar 7;350(6313):83–86. doi: 10.1038/350083a0. [DOI] [PubMed] [Google Scholar]
- O'Shea K. S., Dixit V. M. Unique distribution of the extracellular matrix component thrombospondin in the developing mouse embryo. J Cell Biol. 1988 Dec;107(6 Pt 2):2737–2748. doi: 10.1083/jcb.107.6.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea K. S., Liu L. H., Dixit V. M. Thrombospondin and a 140 kd fragment promote adhesion and neurite outgrowth from embryonic central and peripheral neurons and from PC12 cells. Neuron. 1991 Aug;7(2):231–237. doi: 10.1016/0896-6273(91)90261-w. [DOI] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Prater C. A., Plotkin J., Jaye D., Frazier W. A. The properdin-like type I repeats of human thrombospondin contain a cell attachment site. J Cell Biol. 1991 Mar;112(5):1031–1040. doi: 10.1083/jcb.112.5.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds C. P., Kane D. J., Einhorn P. A., Matthay K. K., Crouse V. L., Wilbur J. R., Shurin S. B., Seeger R. C. Response of neuroblastoma to retinoic acid in vitro and in vivo. Prog Clin Biol Res. 1991;366:203–211. [PubMed] [Google Scholar]
- Roberts D. D., Sherwood J. A., Ginsburg V. Platelet thrombospondin mediates attachment and spreading of human melanoma cells. J Cell Biol. 1987 Jan;104(1):131–139. doi: 10.1083/jcb.104.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe C. R. Retinoic acid can mimic endogenous signals involved in transformation of the Xenopus nervous system. Neuron. 1991 Aug;7(2):239–247. doi: 10.1016/0896-6273(91)90262-x. [DOI] [PubMed] [Google Scholar]
- Sidell N. Retinoic acid-induced growth inhibition and morphologic differentiation of human neuroblastoma cells in vitro. J Natl Cancer Inst. 1982 Apr;68(4):589–596. [PubMed] [Google Scholar]
- Simeone A., Acampora D., Arcioni L., Andrews P. W., Boncinelli E., Mavilio F. Sequential activation of HOX2 homeobox genes by retinoic acid in human embryonal carcinoma cells. Nature. 1990 Aug 23;346(6286):763–766. doi: 10.1038/346763a0. [DOI] [PubMed] [Google Scholar]
- Simeone A., Acampora D., Nigro V., Faiella A., D'Esposito M., Stornaiuolo A., Mavilio F., Boncinelli E. Differential regulation by retinoic acid of the homeobox genes of the four HOX loci in human embryonal carcinoma cells. Mech Dev. 1991 Mar;33(3):215–227. doi: 10.1016/0925-4773(91)90029-6. [DOI] [PubMed] [Google Scholar]
- Thompson J. N., Howell J. M., Pitt G. A., McLaughlin C. I. The biological activity of retinoic acid in the domestic fowl and the effects of vitamin A deficiency on the chick embryo. Br J Nutr. 1969 Aug;23(3):471–490. doi: 10.1079/bjn19690056. [DOI] [PubMed] [Google Scholar]
- Tsokos M., Scarpa S., Ross R. A., Triche T. J. Differentiation of human neuroblastoma recapitulates neural crest development. Study of morphology, neurotransmitter enzymes, and extracellular matrix proteins. Am J Pathol. 1987 Sep;128(3):484–496. [PMC free article] [PubMed] [Google Scholar]
- Varani J., Dixit V. M., Fligiel S. E., McKeever P. E., Carey T. E. Thrombospondin-induced attachment and spreading of human squamous carcinoma cells. Exp Cell Res. 1986 Dec;167(2):376–390. doi: 10.1016/0014-4827(86)90178-3. [DOI] [PubMed] [Google Scholar]
- Varani J., Nickoloff B. J., Riser B. L., Mitra R. S., O'Rourke K., Dixit V. M. Thrombospondin-induced adhesion of human keratinocytes. J Clin Invest. 1988 May;81(5):1537–1544. doi: 10.1172/JCI113486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M., Thaller C., Jessell T., Eichele G. Polarizing activity and retinoid synthesis in the floor plate of the neural tube. Nature. 1990 Jun 28;345(6278):819–822. doi: 10.1038/345819a0. [DOI] [PubMed] [Google Scholar]
- Wang S. Y., Gudas L. J. Isolation of cDNA clones specific for collagen IV and laminin from mouse teratocarcinoma cells. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5880–5884. doi: 10.1073/pnas.80.19.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Y., Gudas L. J. Protein synthesis inhibitors prevent the induction of laminin B1, collagen IV (alpha 1), and other differentiation-specific mRNAs by retinoic acid in F9 teratocarcinoma cells. J Cell Physiol. 1988 Aug;136(2):305–311. doi: 10.1002/jcp.1041360213. [DOI] [PubMed] [Google Scholar]
- Wedden S., Thaller C., Eichele G. Targeted slow-release of retinoids into chick embryos. Methods Enzymol. 1990;190:201–209. doi: 10.1016/0076-6879(90)90024-u. [DOI] [PubMed] [Google Scholar]