Abstract
Aim
A genetic variant (rs20417) of the PTGS2 gene, encoding for COX-2, has been associated with decreased COX-2 activity and a decreased risk of cardiovascular disease (CVD). However, this genetic association and the role of COX-2 in CVD remain controversial.
Methods and results
The association of rs20417 with CVD was prospectively explored in 49 232 subjects (ACTIVE-A, CURE, epiDREAM/DREAM, ONTARGET, RE-LY, and WGHS) and the effect of potentially modifiable risk factors on the genetic association was further explored in 9363 INTERHEART participants. The effect of rs20417 on urinary thromboxane and prostacyclin metabolite concentrations was measured in 117 healthy individuals. Carriage of the rs20417 minor allele was associated with a decreased risk of major CVD outcomes (OR = 0.78, 95% CI: 0.70–0.87; P = 1.2 × 10−5). The genetic effect was significantly stronger in aspirin users (OR: 0.74, 95% CI: 0.64−0.84; P = 1.20 × 10−5) than non-users (OR: 0.87, 95% CI: 0.72−1.06; P = 0.16) (interaction P-value: 0.0041). Among patients with previous coronary artery disease (CAD), rs20417 carriers had a stronger protective effect on risk of major adverse events when compared with individuals without previous CAD (interaction P-value: 0.015). Carriers had significantly lower urinary levels of thromboxane (P = 0.01) and prostacyclin (P = 0.01) metabolites when compared with non-carriers.
Conclusion
The rs20417 polymorphism is associated with a reduced risk of major cardiovascular events and lower levels of thromboxane and prostacyclin. Our results suggest that a genetic decrease in COX-2 activity may be beneficial with respect to CVD risk, especially, in higher risk patients on aspirin.
Keywords: Pharmacogenetics, Genetics, Aspirin, Myocardial infarction, Stroke
See page 2208 for the editorial comment on this article (doi:10.1093/eurheartj/ehu219)
Introduction
Cyclooxygenase (COX) enzymes are responsible for converting arachidonic acid into prostaglandin (PG) H2,1 which acts as a metabolic precursor of PGs, prostacyclin, and thromboxane. Three isoforms of the COX enzyme have been identified (COX-1, COX-2, and COX-3), but only the COX-1 and COX-2 isoforms are functional. The COX-1 enzyme is constitutively expressed in most tissues, including platelets, where it is involved in the formation of thromboxane A2 through an intermediate. Low-dose aspirin decreases platelet activity by irreversibly acetylating COX-1 and inhibiting the production of platelet-derived thromboxane A2.2 COX-2 is an inducible enzyme that is expressed by cells involved in inflammation (i.e. endothelial cells, monocytes, and macrophages). It is believed to have cardioprotective effects by facilitating the production of prostacyclin, which is a potent vasodilator and inhibits platelet activation and smooth muscle cell proliferation.3
The role of COX-2 in atherothrombosis remains controversial. Some animal studies suggest that genetic inhibition of the COX-2 enzyme decreases the risk of atherosclerosis,4 whereas others demonstrate an increased risk of thrombosis.5 Most clinical studies have linked pharmacological inhibition by selective COX-2 inhibitors with an increased risk of cardiovascular events,6,7 presumably because COX-2 inhibition leads to unopposed COX-1-dependent thromboxane production and subsequent platelet activation and vasoconstriction.7 Additionally, higher doses of aspirin with shorter dosing intervals are required to inhibit the COX-2-dependent pathways since nucleated cells rapidly resynthesize this enzyme.8 In contrast with these results, a genetic polymorphism (rs20417) in the promoter of the PTGS2 gene (COX-2) has been associated with lower COX-2 activity in atherosclerotic plaque and a decreased risk of myocardial infarction and stroke.9 Furthermore, an interaction between aspirin use and carriage of the COX-2 polymorphism has been reported, whereby the genetic effect is stronger in aspirin users than non-users.10,11 However, previous studies mostly had small sample sizes, and only some10–14 but not all15–19 have replicated these findings. Thus confirmation and characterization of the genetic association between rs20417 and major adverse cardiovascular outcomes may provide greater insights into the biological role of COX-2 in cardiovascular disease (CVD), and may also improve risk stratification of CVD patients.
Given the contradictory evidence on the role of COX-2 and CVD, we undertook to (i) test the association of the rs20417 polymorphism with CVD, (ii) examine whether the genetic association is modified by aspirin use, or the presence or absence of major CVD risk factors, and (iii) explore the functional mechanisms of the polymorphism by examining its impact on thromboxane and prostacyclin urine levels.
Methods
Study populations overview
The objective of this paper was to assess the effect of rs20417 carrier status with risk of CVD outcomes by combing the genetic effect estimates across six diverse patient populations. Events were classified according to definitions from each parent study. Our primary outcome was major adverse vascular events, defined, unless otherwise specified, as the composite of CVD death, non-fatal myocardial infarction, or non-fatal stroke.
Further details of the study population characteristics, genotyping, and imputation are described in Supplementary material online. In brief, ACTIVE-A was a randomized, double-blind, placebo-controlled trial comparing clopidogrel (75 mg/day) with placebo in patients with high-risk atrial fibrillation (AF).20 CURE was a randomized, double-blind, placebo-controlled trial comparing clopidogrel (75 mg/day) with placebo in patients with acute coronary syndrome (ACS) without ST-segment elevation.21 DREAM was a randomized, double-blind trial with a 2×2 factorial design that assigned participants at high risk for or who had diabetes to receive either ramipril (15 mg/day) vs. placebo or rosiglitazone (8 mg/day) vs. placebo.22 The epiDREAM trial was an epidemiological arm of the DREAM trial and is comprised of participants who were either screened for eligibility to enter the DREAM clinical trial but were not eligible or who did not want to enter the trial but agreed to a long-term prospective follow-up.23 ONTARGET was a randomized, double-blind, parallel trial comparing the effects of ramipril (10 mg/day), telmisartan (80 mg/day), and combination therapy in patients with vascular disease or high-risk diabetes patients.24 RE-LY was a prospective, open-label, randomized trial that compared two fixed doses of dabigatran (110 or 150 mg twice daily) with open-label use of warfarin in patients with high-risk AF.25 The WGHS study26 is a subset of the Women's Health Study (WHS),27 which consists of healthy female participants who were randomized either to an aspirin intervention arm (100 mg of aspirin every other day) or placebo. In addition, the INTERHEART study was a large, international, standardized case–control study consisting of non-fatal acute myocardial infarction cases and controls from 52 countries.28 Finally, MARS was an open-label, two phase case–control study of individuals with CVD; however, for the purposes of this analysis, only healthy controls were considered. Urine was collected into preservative free tubes for the measurement of 11-dehydrothromboxane B2 and urinary 2,3-dinor-6-keto PGF1α using the standard method (Cayman Chemical, Ann Arbor, MI, USA).
Statistical analysis
Deviation from the Hardy–Weinberg equilibrium was tested in each ethnic group for each study (P > 0.05 for all). Owing to the limited number of individuals homozygous for the minor allele of rs20417, a dominant genetic model was used throughout, whereby individuals carrying either 1 or 2 minor alleles were pooled together, and thereafter referred as ‘carriers’ (unless otherwise specified). Logistic regression models were used for each individual study, with adjustment for age, sex, randomization status (when appropriate), and self-reported ethnicity. Results from each study were then combined using fixed-effect meta-analysis. Effect of rs20417 on outcomes was also assessed using Cox proportional hazard regression, without further adjustment. Association of rs20417 carrier status with urinary 11-dehydro thromboxane B2 and 2,3-dinor-6-keto PGF1α concentrations was performed using a non-parametric Kruskal–Wallis test. The statistical significance threshold was set at 0.05 (two-sided) for all analyses. All analyses were performed using R Statistical Package.
Results
Characteristics of study populations
The baseline demographics of the prospective study populations (ACTIVE-A, CURE, epiDREAM/DREAM, ONTARGET, RE-LY, and WGHS) are presented in Table 1. The baseline demographics of the INTERHEART and MARS study populations are presented in Supplementary material online, Tables S1 and S2.
Table 1.
Baseline characteristics of prospective study populations
| ACTIVE-A | CURE | epiDREAM/DREAM | ONTARGET | RE-LY | WGHS | |
|---|---|---|---|---|---|---|
| n | 1061 | 4662 | 14 104 | 3610 | 2501 | 23 294 |
| Mean age (SD) | 71.0 (9.9) | 63.6 (11) | 52.0 (11) | 67.0 (7.3) | 71.9 (7.4) | 54.2 (7.1) |
| Female (%) | 483 (45.5) | 1921 (41.2) | 8589 (60.9) | 998 (27.6) | 804 (32.1) | 23 294 (100) |
| BMI (kg/m2) (SD) | 29.1 (5.6) | 27.7 (4.2) | 30.0 (5.8) | 29.8 (5.2) | 29.2 (5.5) | 25.9 (5.0) |
| Previous CAD (%) | 314 (29.6) | 4662 (100) | 114 (0.81) | 2882 (79.8) | 799 (31.9) | 0 (0) |
| Diabetes (%) | 222 (20.9) | 994 (21.3) | 1842 (13.1) | 1909 (52.9) | 495 (19.8) | 586 (2.5) |
| High blood pressure (%) | 908 (85.6) | 2852 (61.2) | 5011 (35.5) | 529 (14.7) | 1884 (75.3) | 5730 (24.6) |
| Current smoking (%) | 81 (7.6) | 1048 (22.5) | 2049 (14.5) | 393 (10.9) | 226 (9.0) | 2710 (11.6) |
| Aspirin use (%) | 1061 (100) | 4662 (100) | 1535 (10.9) | 2843 (78.8) | 727 (29.1) | 11 617 (50) |
| rs20417 carrier status, n (%) | ||||||
| Carriers | 318 (30.0) | 1233 (26.4) | 4802 (34.0) | 857 (23.7) | 581 (23.2) | 6794 (29.2) |
| Non-carriers | 743 (70.0) | 3429 (73.6) | 9301 (66.0) | 2753 (76.3) | 1920 (76.8) | 16 490 (70.8) |
| Median follow-up (years) | 3.5 | 0.8 | 3.6 | 2.6 | 2.1 | 10.2 |
| Major cardiovascular events | ||||||
| n events | 247 | 456 | 131 | 565 | 87 | 518 |
| Events per 100 person-years (95% CI) | 7.3 (6.3–8.3) | 16.2 (14.7–17.7) | 0.3 (0.2–0.3) | 3.5 (3.2–3.8) | 1.7 (1.3–2.1) | 0.2 (0.2–0.2) |
| Vascular death | ||||||
| n events | 165 | 239 | 20 | 228 | 46 | 135 |
| Events per 1000 person-years (95% CI) | 4.8 (4.0–5.5) | 7.7 (6.7–8.8) | 0.05 (0.03–0.07) | 1.3 (1.2–1.5) | 0.8 (0.5–1.1) | 0.58 (0.48–0.68) |
| Myocardial infarction | ||||||
| n events | 43 | 255 | 86 | 269 | 43 | 217 |
| Events per 100 person-years (95% CI) | 1.2 (0.8–1.6) | 9.5 (8.3–10.7) | 0.2 (0.1–0.2) | 1.6 (1.4–1.9) | 0.8 (0.5–1.1) | 0.09 (0.08–0.11) |
| Stroke | ||||||
| n events | 105 | 55 | 48 | 157 | 46 | 270 |
| Events per 100 person-years (95% CI) | 3.1 (2.5–3.8) | 2.0 (1.4–2.5) | 0.1 (0.08–0.1) | 1.0 (0.8–1.1) | 0.8 (0.5–1.1) | 0.12 (0.10–0.13) |
Association of the rs20417 polymorphism with major adverse cardiovascular events
Overall, 29.6% of participants were carriers of at least one rs20417 minor allele, similar to that of European Caucasian (CEU) individuals from HapMap (31.8%). Carrier status ranged from 23.2% in RE-LY to 34% in epiDREAM/DREAM. However, a proxy was used in ONTARGET and RE-LY, explaining the lower frequencies.
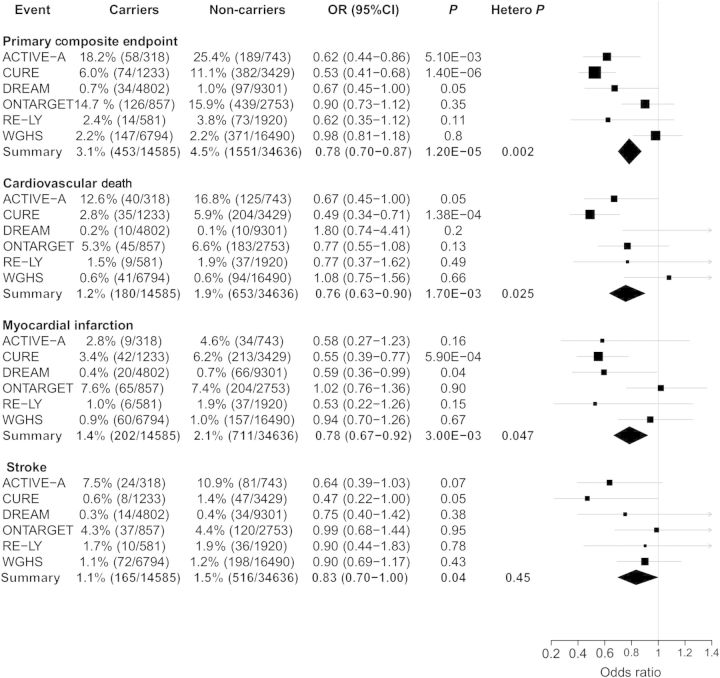
Among the six prospective study populations (ACTIVE-A, CURE, epiDREAM/DREAM, ONTARGET, RE-LY, and WGHS), rs20417 carrier status was significantly associated with a reduced risk of major cardiovascular outcomes [odds ratio (OR) = 0.78, 95% confidence interval (CI) 0.70–0.87; P = 1.20 × 10−5], vascular death (OR = 0.76, 95% CI: 0.63–0.90; P = 0.0017), myocardial infarction (OR = 0.78, 95% CI: 0.67–0.92; P = 0.003), and stroke (OR = 0.83, 95% CI: 0.70–1.00; P = 0.04) (Figure 1). Heterogeneity across study populations was observed for major adverse cardiovascular events (heterogeneity P = 0.0017), as well as vascular death (heterogeneity P = 0.025) and myocardial infarction (heterogeneity P = 0.047). However, once CURE was removed from the pooled analyses, there was no longer evidence of heterogeneity (heterogeneity P > 0.05 for all). No interaction with randomized treatment was observed in each trial (ACTIVE-A, CURE, epiDREAM/DREAM, ONTARGET, and RE-LY; P > 0.05 for all). As a sensitivity analysis, we also assessed rs20417 carrier status with risk of major CVD outcomes using an additive and unadjusted recessive model. Similar results were observed for the additive model (Supplementary material online, Figure S1), while associations were non-significant in the recessive model (data not shown).
Figure 1.
Association of rs20417 carrier status with major cardiovascular events in six prospective patient populations. Analyses were adjusted for age, sex, randomization status (when appropriate), and self-reported ethnicity. ACTIVE-A, CURE, epiDREAM/DREAM, ONTARGET, RE-LY, and WGHS data were included in the meta-analysis. DREAM represents epiDREAM/DREAM. Hetero. P. represents heterogeneity P-value.
Effect modification by aspirin use
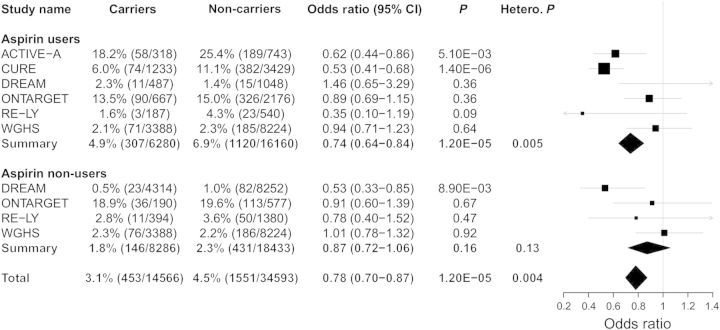
Owing to the close biological relationship between COX-2 and aspirin, and previous reports of a genetic interaction with aspirin use, we tested rs20417 carrier status for association with cardiovascular events stratified by aspirin use. Aspirin use appeared to modify the relationship between rs20417 carrier status and risk of major cardiovascular events (heterogeneity P: 0.0041) (Figure 2). Among participants using aspirin, carrier status was associated with a lower risk of CVD outcomes (OR: 0.74, 95% CI: 0.64−0.84; P = 1.20 × 10−5), while this relationship was attenuated in aspirin non-users (OR: 0.87, 95% CI: 0.72−1.06; P = 0.16).We also observed significant heterogeneity among the pooled estimate of aspirin users, which points to differences between studies (heterogeneity P = 0.0053).
Figure 2.
Analysis of association of rs20417 carrier status with major cardiovascular events stratified by aspirin use in six prospective patient populations. Analyses were adjusted for age, sex, randomization status (when appropriate), and self-reported ethnicity. ACTIVE-A, CURE, epiDREAM/DREAM, ONTARGET, RE-LY, and WGHS data were included in the meta-analysis. DREAM represents epiDREAM/DREAM. Hetero. P. represents heterogeneity P-value.
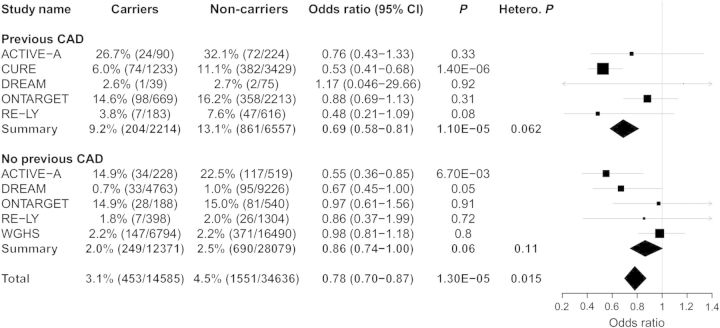
As most patients on aspirin have established coronary artery disease (CAD), we also assessed the genetic association between rs20417 carrier statuses for adverse CVD events stratified by previous CAD (Figure 3). Previous CAD was defined as established ACSs or angina. Previous CAD also appeared to modify the relationship between rs20417 carrier status and risk of cardiovascular outcomes (heterogeneity P: 0.015), where carriage of the rs20417 polymorphism had a stronger effect on risk of major adverse events among patients with previous CAD (OR: 0.69, 95% CI: 0.58−0.81; P = 1.10 × 10−5) when compared with individuals without previous CAD (OR: 0.86, 95% CI: 0.74 −1.00; P = 0.06). No significant interaction with aspirin use was observed when restricting the analysis to individuals with previous CAD, although a trend towards a stronger effect was observed in aspirin users. However, only 186 rs20417 carriers with previous CAD were classified as non-aspirin users (Supplementary material online, Figure S2).
Figure 3.
Analysis of association of rs20417 carrier status with major cardiovascular events stratified by previous coronary artery disease in six prospective patient populations. Analyses were adjusted for age, sex, randomization status (when appropriate), and self-reported ethnicity. ACTIVE-A, CURE, epiDREAM/DREAM, ONTARGET, RE-LY, and WGHS data were included in the meta-analysis. DREAM represents epiDREAM/DREAM. Hetero. P. represents heterogeneity P-value.
Genetic association in relation to other major cardiovascular disease risk factors
To evaluate the strength of the rs20417 carrier status association with CVD in relation to conventional risk factors, we utilized a multivariate analysis including rs20417 carrier status and other major CVD risk factors. Analyses were performed in ACTIVE-A and CURE because both studies were significantly associated with rs20417 carrier status in a univariate analyses and both had all participants on aspirin by design. Kaplan–Meier survival curves for ACTIVE-A and CURE are shown in Supplementary material online, Figure S3, along with hazard ratios calculated using unadjusted Cox proportional hazard models. For ease of interpretation, individuals with two major rs20417 alleles were compared with individuals with one or more minor rs20417 alleles, such that carriage of two major rs20417 alleles is presented as a risk factor (Table 2). No conventional risk factors were consistently associated with a larger effect estimate than carriage of two major rs20417 alleles. In fact, carriage of two major rs20417 alleles had the largest effect size of all risk factors (excluding age) among participants in CURE (OR = 1.90, 95% CI: 1.46–2.48; P = 2.10 × 10−6).
Table 2.
Association of rs20417 carrier status and conventional risk factors with major cardiovascular events in ACTIVE-A and CURE
| Risk factor | CURE |
ACTIVE-A |
||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Carriage of two major rs20417 alleles | 1.90 (1.46–2.48) | 2.10E−06 | 1.63 (1.16–2.30) | 5.10E−03 |
| 10 years of age | 1.62 (1.45–1.81) | 1.10E−17 | 1.84 (1.53–2.21) | 1.10E−10 |
| Male sex | 1.52 (1.23–1.89) | 1.20E−04 | 1.15 (0.84–1.57) | 0.38 |
| Presence of diabetes | 1.54 (1.23–1.93) | 1.50E−04 | 2.05 (1.44–2.91) | 6.70E−05 |
| Current smoking | 1.02 (0.76–1.35) | 0.90 | 1.66 (0.92–2.98) | 0.09 |
| High blood pressure | 1.31 (1.05–1.64) | 0.02 | 0.99 (0.65–1.52) | 0.98 |
| Obesity (BMI >30) | 0.94 (0.74–1.19) | 0.59 | 0.89 (0.64–1.24) | 0.50 |
Odds ratios of conventional risk factors for the risk of adverse events in CVD risk patient populations from multivariate models also including randomization status (when appropriate) and self-reported ethnicity.
Effect modification by major cardiovascular disease risk factors
To explore whether the presence of specific CVD risk factors modified the association of rs20417 carrier status with cardiovascular outcomes, we performed subgroup analyses within the prospective populations (Supplementary material online, Figure S4). WGHS was excluded as there were no males and the prevalence of many risk factors (e.g. diabetes, advanced age) was low in this study of apparently healthy middle-aged women. None of the risk factors tested showed a significant interaction with carriage of the rs20417 minor allele (P > 0.05 for all).
We also explored the association of rs20417 with myocardial infarction and its relation with major CVD risk factors in INTERHEART. Overall, 32.3% of INTERHEART participants were carriers of the rs20417 alternate allele (Supplementary material online, Table S1). There was no association between carrier status and non-fatal myocardial infarction when using a dominant genetic model (OR = 0.93, 95% CI: 0.85–1.02; P = 0.115) but a weak and consistent association was observed using an additive genetic model (OR = 0.92, 95% CI: 0.85–0.99, P = 0.02). There was a marginally significant interaction between apolipoprotein A1 (ApoA1) levels and carrier status (interaction P = 0.017); whereby, carrier status was significantly associated with CVD among individuals with lower than median ApoA1 levels (OR = 0.82, 95% CI: 0.73–0.93, P = 0.002) but not among those with greater than median ApoA1 levels (OR = 1.05, 95% CI 0.93–1.20, P = 0.42) (Supplementary material online, Figure S5). None of the other modifiable risk factors or clinical characteristics showed a significant interaction with carrier status (P > 0.05 for all). We also assessed whether the rs20417 polymorphism was associated with CVD risk factors using a model adjusted for age, sex, and ethnicity. None of the risk factors was significantly associated with carrier status (P > 0.05 for all) (Supplementary material online, Table S3).
Association of rs20417 with urinary metabolites of thromboxane and prostacyclin
Finally, we tested whether rs20417 carrier status was associated with COX-1 and COX-2 derived urinary 11-dehydrothromboxane B2 and urinary 2,3-dinor-6-keto PGF1α in 117 healthy European participants (not taking aspirin or other COX inhibitors). Minor allele carriers had decreased 11-dehydrothromboxane B2 urine concentration (P = 0.01), with median values of 97.0 ng/mmol creatinine and 126.0 ng/mmol creatinine in carriers (n = 32) and non-carriers (n = 85), respectively. In addition, minor allele carriers also had decreased urinary 2,3-dinor-6-keto PGF1α concentration (P = 0.01), with median values of 3335.8 pg/mg creatinine and 4790.4 pg/mg creatinine in carriers (n = 32) and non-carriers (n = 85), respectively (Supplementary material online, Figure S6).
Discussion
We found that the COX-2 (PTGS2) genetic variant rs20417 is associated with a decreased risk of major cardiovascular events (OR = 0.78, 95% CI: 0.70–0.87, P = 1.20 × 10−5), with consistent effects for cardiovascular death, myocardial infarction, and stroke. We also observed significant interactions with aspirin use (Heterogeneity P: 0.0041) and previous CAD (Heterogeneity P: 0.015). Indeed, in ACTIVE-A and CURE, the magnitude of CVD risk associated with carriage of two major rs20417 alleles was similar to that of traditional risk factors, such as sex, diabetes, smoking status, high blood pressure, and obesity.
Similar to previous reports, we observed an interaction between the non-selective COX inhibitor aspirin and rs20417 carrier status.10,11 While the possibility of a biological interaction is compelling, this association may also be confounded since aspirin users are also more likely to represent those with established CVD and thereby reflect a stronger genetic effect in higher risk populations29 rather than an interaction with aspirin use. Indeed, the clinical benefit of aspirin itself parallels the baseline risk of study populations, with a 12% proportional reduction in CVD events in primary prevention population,30 19% in secondary prevention,30 and 23% relative reduction in death in the acute ACS setting.31 Consistent with this hypothesis, previous CAD appeared to enhance the association between rs20417 carrier status and risk of CVD outcomes (Heterogeneity P: 0.015). Similarly, we found no association between rs20417 and CVD risk in WGHS, a population in which the originally reported aspirin benefit was modest,27 and we observed a strong association in ACS without ST-segment elevation patients enrolled in the CURE trial. This strong association may have also been driven by the fact that CURE participants were assigned a standard dose aspirin (75–325 mg daily) when compared with the healthy female participants in WGHS who were randomly allocated to either treatment with low-dose aspirin (100 mg every other day) or placebo. Thus, the net clinical benefit of aspirin could vary according to carrier status. For instance, the difference in major adverse cardiovascular event rate between rs20417 carriers and non-carriers (6.0 vs. 11.1%) was larger than the absolute risk of major and minor bleeds (2.8 and 2.1%) in CURE participants randomized to aspirin alone.
In agreement with the previous work showing that the rs20417 minor allele is linked to decreased COX-2 expression,9,19 we demonstrated that minor allele carriers had lower levels of both urinary 11-dehydrothromboxane B2 and urinary 2,3-dinor-6-keto PGF1α excretion. We posit that tissue-specific effects of the genetic variant could explain the apparent discrepancy between genetic and pharmacological inhibition of COX-2 activity with respect to cardiovascular risk. Indeed, Cipollone et al.9 demonstrated that the rs20417 genotype modified COX-2 activity in carotid plaques, whereby carrier status was associated with lower levels of COX-2 expression in plaque-derived macrophages; however, carrier status did not influence COX-2 activity in endothelial tissue. Furthermore, macrophage-specific COX-2 knock-out mice models have decreased atherosclerosis;4 whereas, deletion of COX-2 in endothelial cells and vascular smooth muscle cells in double knock-out mice leads to an increased risk of thrombosis.5 This may also explain the apparent heterogeneity among studies since participants with more advanced stages of CVD are likely to have complex, macrophage-rich plaques. Indeed, the largest genetic effect was observed among the secondary prevention patients in CURE, which had a dominant effect in our meta-analysis. Thus, CURE participants are expected to have more advanced atherosclerosis and may be more susceptible to the COX-2 inhibiting effect of rs20417. However, further research is required to fully assess the functional role of COX-2 in primary and secondary prevention patients.
Several factors likely contributed to the previously inconsistent reports of an association between rs20417 and risk of CVD.15–19 For instance, the sample size among several studies exploring the rs20417 association ranged from 22015 to 4994,11 which indicates that some of these studies may not have had enough statistical power to detect a genetic effect. Also, case–control studies may have underestimated the reported association between rs20417 minor allele carriers and CVD outcomes since these studies are more likely to include COX-2 carriers who experienced non-fatal vascular events as opposed to those who experienced vascular death. Indeed, inclusion of a large number of individuals from prospective studies strengthens our meta-analysis. Finally, it is possible that the genetic effect varies according to study population and aspirin exposure such that heterogeneous estimates reflect true genetic risks. Nonetheless, we did not observe any significant interactions among the clinical risk factors tested in INTERHEART with the exception of ApoA1 (interaction P-value: 0.017). High-density lipoproteins and ApoA1 have been both shown to up-regulate COX-2 expression and induce prostacyclin synthesis in endothelial cells.28 High-density lipoproteins and ApoA1 have been both shown to up-regulate COX-2 expression and induce prostacyclin synthesis in endothelial cells.32–34 However, the observed interaction was of modest statistical significance and should be considered exploratory since it would not withstand adjustment for multiple testing.
A few limitations of our study warrant discussion. First, tissue-specific gene expression would ideally be needed to delineate the effects of rs20417 on COX-2 gene expression in relevant tissues such as endothelial cells, atherosclerosis plaques, and macrophages. Second, rs20417 was not directly genotyped in ONTARGET or in RE-LY and could not be imputed such that a proxy was used.35 However, use of a proxy should bias our results towards the null without invalidating our conclusions. Third, while the genetic effect was consistent across ethnic groups in both the epiDREAM/DREAM and INTERHEART patient populations, our study populations were predominantly European and further studies will be needed to confirm in other ethnic groups. Finally, although our results suggest an interaction between ApoA1 levels and carriers of the rs20417 genotype with CVD, this was an exploratory analysis and further work is needed.
In summary, we confirmed the protective effect of the minor allele of the COX-2 (PTGS2) SNP, providing a genetic link between COX-2 activity and cardiovascular risk. In particular, our data suggest that decreased COX-2 activity is not universally deleterious in humans with respect to risk of adverse CVD outcomes, and taken together with observations in human atherosclerosis plaques and model systems implies that inhibition of COX-2 in macrophages could be beneficial. Additionally, the biologically compelling interaction between rs20417 carrier status and aspirin use suggests that widely prescribed non-selective COX inhibitors may be more beneficial among rs20417 carriers. Our results also highlight the complex genetic epidemiology of CVD and argue that genetic determinants may have different effect sizes according to study population characteristics, such as aspirin use or presence of vascular disease. Further research will be needed to fully delineate the clinical, epidemiological, and pathophysiological implications of the observed genetic association.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by grants from the following studies: ACTIVE-A (Sanofi-Aventis and Bristol-Myers Squibb), CURE (Sanofi-Synthelabo and Bristol-Myers Squibb), epiDREAM/DREAM [Canadian Institutes of Health Research (CIHR) ,Sanofi-Aventis, GlaxoSmithKline, and King Pharmaceuticals], INTERHEART [CIHR, the Heart and Stroke Foundation of Ontario, and the International Clinical Epidemiology Network (INCLEN), as well as through unrestricted grants from several pharmaceutical companies (with major contributions from AstraZeneca, Novartis, Sanofi-Aventis, Knoll Pharmaceuticals (now Abbott), Bristol-Myers Squibb and KingPharma)], MARS (Heart and Stroke Foundation), ONTARGET (Boehringer-Ingelheim), RE-LY (Boehringer-Ingelheim), and WGHS (National Heart Lung and Blood Institute and the National Cancer Institute). J.E., receiving support from Canada Research Chair in Cardiovascular Medicine; S.S.A., receiving support from Canada Research Chair in Ethnic Diversity and Cardiovascular Disease, Eli Lilly Canada/May Cohen Chair in Women's Health, and Heart and Stroke Foundation of Ontario/Michael G. DeGroote Chair in Population Health Research; H.C.G., receiving support from Population Health Institute Chair in Diabetes Research and Care; S.J.C., receiving support from Salim Yusuf Chair in Cardiology; S.Y., receiving support from Heart and Stroke Foundation of Ontario/Marion W. Burke Chair in Cardiovascular Disease; and G.P., receiving support from Canada Research Chair in Genetic and Molecular Epidemiology, CISCO Professorship in Integrated Health Systems and grant support from CIHR (MOP-106715).
Conflict of interest: none declared.
Acknowledgements
We are thankful to all the participants having agreed to contribute to this project.
References
- 1.Fritsche E, Baek SJ, King LM, Zeldin DC, Eling TE, Bell DA. Functional characterization of cyclooxygenase-2 polymorphisms. J Pharmacol Exp Ther. 2001;299:468–476. [PubMed] [Google Scholar]
- 2.Hankey GJ, Eikelboom JW. Aspirin resistance. Lancet. 2006;367:606–617. doi: 10.1016/S0140-6736(06)68040-9. [DOI] [PubMed] [Google Scholar]
- 3.FitzGerald GA, Smith B, Pedersen AK, Brash AR. Increased prostacyclin biosynthesis in patients with severe atherosclerosis and platelet activation. N Engl J Med. 1984;310:1065–1068. doi: 10.1056/NEJM198404263101701. [DOI] [PubMed] [Google Scholar]
- 4.Hui Y, Ricciotti E, Crichton I, Yu Z, Wang D, Stubbe J, Wang M, Pure E, FitzGerald GA. Targeted deletions of cyclooxygenase-2 and atherogenesis in mice. Circulation. 2010;121:2654–2660. doi: 10.1161/CIRCULATIONAHA.109.910687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu Y, Ricciotti E, Scalia R, Tang SY, Grant G, Yu Z, Landesberg G, Crichton I, Wu W, Pure E, Funk CD, FitzGerald GA. Vascular COX-2 modulates blood pressure and thrombosis in mice. Sci Transl Med. 2012;4:132ra54. doi: 10.1126/scitranslmed.3003787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. Br Med J. 2006;332:1302–1308. doi: 10.1136/bmj.332.7553.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286:954–959. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- 8.Eikelboom JW, Hirsh J, Spencer FA, Baglin TP, Weitz JI. Antiplatelet drugs: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e89S–119S. doi: 10.1378/chest.11-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cipollone F, Toniato E, Martinotti S, Fazia M, Iezzi A, Cuccurullo C, Pini B, Ursi S, Vitullo G, Averna M, Arca M, Montali A, Campagna F, Ucchino S, Spigonardo F, Taddei S, Virdis A, Ciabattoni G, Notarbartolo A, Cuccurullo F, Mezzetti A. A polymorphism in the cyclooxygenase 2 gene as an inherited protective factor against myocardial infarction and stroke. JAMA. 2004;291:2221–2228. doi: 10.1001/jama.291.18.2221. [DOI] [PubMed] [Google Scholar]
- 10.Lee CR, North KE, Bray MS, Couper DJ, Heiss G, Zeldin DC. Cyclooxygenase polymorphisms and risk of cardiovascular events: the Atherosclerosis Risk in Communities (ARIC) study. Clin Pharmacol Ther. 2008;83:52–60. doi: 10.1038/sj.clpt.6100221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemaitre RN, Rice K, Marciante K, Bis JC, Lumley TS, Wiggins KL, Smith NL, Heckbert SR, Psaty BM. Variation in eicosanoid genes, non-fatal myocardial infarction and ischemic stroke. Atherosclerosis. 2009;204:e58–e63. doi: 10.1016/j.atherosclerosis.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colaizzo D, Fofi L, Tiscia G, Guglielmi R, Cocomazzi N, Prencipe M, Margaglione M, Toni D. The COX-2 G/C -765 polymorphism may modulate the occurrence of cerebrovascular ischemia. Blood Coagul Fibrinolysis. 2006;17:93–96. doi: 10.1097/01.mbc.0000214706.88621.da. [DOI] [PubMed] [Google Scholar]
- 13.Vogel U, Segel S, Dethlefsen C, Tjonneland A, Saber AT, Wallin H, Jensen MK, Schmidt EB, Andersen PS, Overvad K. Associations between COX-2 polymorphisms, blood cholesterol and risk of acute coronary syndrome. Atherosclerosis. 2010;209:155–162. doi: 10.1016/j.atherosclerosis.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 14.Maguire J, Thakkinstian A, Levi C, Lincz L, Bisset L, Sturm J, Scott R, Whyte S, Attia J. Impact of COX-2 rs5275 and rs20417 and GPIIIa rs5918 polymorphisms on 90-day ischemic stroke functional outcome: a novel finding. J Stroke Cerebrovasc Dis. 2011;20:134–144. doi: 10.1016/j.jstrokecerebrovasdis.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Orbe J, Beloqui O, Rodriguez JA, Belzunce MS, Roncal C, Paramo JA. Protective effect of the G-765C COX-2 polymorphism on subclinical atherosclerosis and inflammatory markers in asymptomatic subjects with cardiovascular risk factors. Clin Chim Acta. 2006;368:138–143. doi: 10.1016/j.cca.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 16.Lahteela K, Kunnas T, Lyytikainen LP, Mononen N, Taittonen L, Laitinen T, Kettunen J, Juonala M, Hutri-Kahonen N, Kahonen M, Viikari JS, Raitakari OT, Lehtimaki T, Nikkari ST. No association of nineteen COX-2 gene variants to preclinical markers of atherosclerosis The Cardiovascular Risk in Young Finns Study. BMC Med Genet. 2012;13:32. doi: 10.1186/1471-2350-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montali A, Barilla F, Tanzilli G, Vestri A, Fraioli A, Gaudio C, Martino F, Mezzetti A, Cipollone F, Arca M. Functional rs20417 SNP (-765G>C) of cyclooxygenase-2 gene does not predict the risk of recurrence of ischemic events in coronary patients: results of a 7-year prospective study. Cardiology. 2010;115:236–242. doi: 10.1159/000298880. [DOI] [PubMed] [Google Scholar]
- 18.Sharma V, Kaul S, Al-Hazzani A, Alshatwi AA, Jyothy A, Munshi A. Association of COX-2 rs20417 with aspirin resistance. J Thromb Thrombolysis. 2013;35:95–99. doi: 10.1007/s11239-012-0777-8. [DOI] [PubMed] [Google Scholar]
- 19.Papafili A, Hill MR, Brull DJ, McAnulty RJ, Marshall RP, Humphries SE, Laurent GJ. Common promoter variant in cyclooxygenase-2 represses gene expression: evidence of role in acute-phase inflammatory response. Arterioscler Thromb Vasc Biol. 2002;22:1631–1636. doi: 10.1161/01.ATV.0000030340.80207.C5. [DOI] [PubMed] [Google Scholar]
- 20.Connolly SJ, Pogue J, Hart RG, Hohnloser SH, Pfeffer M, Chrolavicius S, Yusuf S. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med. 2009;360:2066–2078. doi: 10.1056/NEJMoa0901301. [DOI] [PubMed] [Google Scholar]
- 21.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 22.Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman RR. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368:1096–1105. doi: 10.1016/S0140-6736(06)69829-2. [DOI] [PubMed] [Google Scholar]
- 23.Anand SS, Dagenais GR, Mohan V, Diaz R, Probstfield J, Freeman R, Shaw J, Lanas F, Avezum A, Budaj A, Jung H, Desai D, Bosch J, Yusuf S, Gerstein HC. Glucose levels are associated with cardiovascular disease and death in an international cohort of normal glycaemic and dysglycaemic men and women: the EpiDREAM cohort study. Eur J Prev Cardiol. 2012;19:755–764. doi: 10.1177/1741826711409327. [DOI] [PubMed] [Google Scholar]
- 24.Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 25.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 26.Ridker PM, Chasman DI, Zee RY, Parker A, Rose L, Cook NR, Buring JE. Rationale, design, and methodology of the Women's Genome Health Study: a genome-wide association study of more than 25,000 initially healthy American women. Clin Chem. 2008;54:249–255. doi: 10.1373/clinchem.2007.099366. [DOI] [PubMed] [Google Scholar]
- 27.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 28.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case–control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 29.Reilly MP, Li M, He J, Ferguson JF, Stylianou IM, Mehta NN, Burnett MS, Devaney JM, Knouff CW, Thompson JR, Horne BD, Stewart AF, Assimes TL, Wild PS, Allayee H, Nitschke PL, Patel RS, Martinelli N, Girelli D, Quyyumi AA, Anderson JL, Erdmann J, Hall AS, Schunkert H, Quertermous T, Blankenberg S, Hazen SL, Roberts R, Kathiresan S, Samani NJ, Epstein SE, Rader DJ. Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: two genome-wide association studies. Lancet. 2011;377:383–392. doi: 10.1016/S0140-6736(10)61996-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Roncaglioni MC, Zanchetti A. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet. 1988;2:349–360. [PubMed] [Google Scholar]
- 32.Martinez-Gonzalez J, Escudero I, Badimon L. Simvastatin potenciates PGI(2) release induced by HDL in human VSMC: effect on Cox-2 up-regulation and MAPK signalling pathways activated by HDL. Atherosclerosis. 2004;174:305–313. doi: 10.1016/j.atherosclerosis.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 33.Norata GD, Callegari E, Inoue H, Catapano AL. HDL3 induces cyclooxygenase-2 expression and prostacyclin release in human endothelial cells via a p38 MAPK/CRE-dependent pathway: effects on COX-2/PGI-synthase coupling. Arterioscler Thromb Vasc Biol. 2004;24:871–877. doi: 10.1161/01.ATV.zhq0504.1403. [DOI] [PubMed] [Google Scholar]
- 34.Liu D, Ji L, Tong X, Pan B, Han JY, Huang Y, Chen YE, Pennathur S, Zhang Y, Zheng L. Human apolipoprotein A-I induces cyclooxygenase-2 expression and prostaglandin I-2 release in endothelial cells through ATP-binding cassette transporter A1. Am J Physiol Cell Physiol. 2011;301:C739–C748. doi: 10.1152/ajpcell.00055.2011. [DOI] [PubMed] [Google Scholar]
- 35.Carlson CS, Eberle MA, Rieder MJ, Yi Q, Kruglyak L, Nickerson DA. Selecting a maximally informative set of single-nucleotide polymorphisms for association analyses using linkage disequilibrium. Am J Hum Genet. 2004;74:106–120. doi: 10.1086/381000. [DOI] [PMC free article] [PubMed] [Google Scholar]