Abstract
Background
Type 2 diabetes mellitus is associated with dementia risk, however evidence is limited for possible associations of diabetes and pre-diabetes with cognitive decline.
Objective
To determine if diabetes in mid-life is associated with 20-year cognitive decline, and to characterize long-term cognitive decline across clinical categories of hemoglobin A1c (HbA1c).
Design
Prospective cohort.
Setting
The community-based Atherosclerosis Risk in Communities (ARIC) Study.
Participants
13351 black and white adults aged 48-67 years at baseline (1990-1992).
Measurements
Diabetes was defined by self-report of physician diagnosis or medication use or HbA1c≥6.5%. Undiagnosed diabetes, pre-diabetes, and glucose control in persons with diagnosed diabetes were defined using clinical categories of HbA1c. Delayed Word Recall, Digit Symbol Substitution, and Word Fluency tests were used to assess cognitive performance, and were summarized using a global Z-score.
Results
Diabetes in midlife was associated with significantly greater cognitive decline over 20 years (adjusted global Z-score difference=-0.15, 95% CI:-0.22,-0.08), representing a 19% greater decline than those without diabetes. Cognitive decline was significantly greater among persons with pre-diabetes (HbA1c 5.7-6.4%) than those without diabetes and HbA1c<5.7%. Participants with poorly controlled diabetes (HbA1c≥7.0%) had a larger decline compared to persons whose diabetes was controlled (adjusted global Z-score difference=-0.16,p-value=0.071). Longer duration of diabetes was also associated with greater late-life cognitive decline (p-value-for-trend=<0.001). No significant differences in the rates of declines were seen in whites compared to blacks (p-value-for-interaction=0.4357).
Limitations
Single measurement of HbA1c at baseline, only one test to per cognitive domain, potential geographic confounding of race comparisons.
Conclusions
These findings suggest that diabetes prevention and glucose control in midlife may protect against late-life cognitive decline.
Introduction
The prevalence of diabetes has increased substantially over the past several decades, with a current prevalence of approximately 10%, affecting 21 million adults in the U.S.(1). Type 2 diabetes is an established risk factor for heart disease, stroke, hypertension, blindness, and kidney disease(2-4). The association of diabetes with dementia risk is well established(5-7). The association of diabetes with cognitive decline, however, is less well characterized. Because cognitive decline is a precursor to dementia, strong risk factors for decline can help identify persons who may realize the benefits of early intervention. The effects of diabetes and early hyperglycemic states assessed in mid-life on long-term cognitive decline are relatively uncharacterized(6). Previous studies have been limited by short duration of follow-up, lack of rigorous adjustment for potential confounding variables, and most were limited to whites and conducted in elderly populations, where associations tend to be weaker(8, 9).
Hemoglobin A1c (HbA1c) is a measure of average circulating glucose in the blood over the preceding 2 to 3 months. HbA1c is the standard measure used in the clinical management of diabetes control and is now recommended for the use for diagnosis of diabetes and identification of persons at risk for future diabetes(10). Studies have shown cross-sectional associations between HbA1c and cognitive scores in persons with diabetes(11, 12). However there is little evidence prospectively linking better glycemic control to slower cognitive decline and few studies have examined the association of chronic hyperglycemia below the threshold for a diagnosis of diabetes with long-term cognitive impairment(13-15).
Our objective was to examine the association of diabetes assessed in middle-age with subsequent 20-year cognitive decline in a community-based population of black and white adults. We also examined the associations of hyperglycemia below the threshold for a diagnosis of diabetes (i.e. “pre-diabetes”) and glycemic control in the setting of diabetes with 20-year cognitive decline. An inherent challenge to accurately quantifying the long-term risk factor associations in observational studies is that participants who are ill are less likely to return for study visits. In this study, we use methods to account for this attrition, which is important in quantifying the long-term associations of diabetes with cognitive decline.
Methods
Study Population
The Atherosclerosis Risk in Communities Study (ARIC) is a community-based prospective cohort of 15,792 middle aged adults from four U.S. communities: Washington County, Maryland; Forsyth County, North Carolina; suburbs of Minneapolis, Minnesota; and Jackson, Mississippi. The Jackson field center recruited only blacks and Forsyth recruited both blacks and whites. The other two field centers, like Jackson and Forsyth, selected participants by probability sampling; however the racial distribution in these locations at that time resulted in only a small percentage of non-white participants. Participants were seen at four visits approximately three years apart beginning in 1987-1989. A fifth ARIC visit took place in 2011-2013. Cognitive function was evaluated at visits 2 (1990-1992), 4 (1996-1998), and at visit 5 (2011-2013) as part of the ARIC Neurocognitive Study (ARIC-NCS). Detailed information about ARIC can be found elsewhere(16).
Baseline for the present analysis was visit 2, the first visit where cognitive data were collected. Of the 14,348 participants who attended visit 2, we excluded participants who were neither white nor black and the small number of blacks in the Minnesota and Washington county cohorts (n=91), those who were missing one or more cognitive function tests at baseline (n=217), and those missing variables of interest (n=689), giving a final sample size of 13,351 participants at baseline (93% of the visit 2 sample). A flow diagram of the study population and the pattern of visit attendance is included in the Appendix(eFigure1).
Assessment of Cognitive Function
Three cognitive tests were used to assess cognitive function: the Delayed Word Recall Test (DWRT)(17), the Digit Symbol Substitution Test (DSST) of the Wechsler Adult Intelligence Scale-Revised (WAIS-R)(18), and the Word Fluency Test (WFT)(19). Protocols for the neuropsychological tests were standardized, and trained examiners administered the tests in a fixed order during one session in a quiet room.
The DWRT is a test of verbal learning and recent memory. Participants were asked to learn 10 common nouns by using each in a sentence. Two exposures to each word were given. After a five-minute filled delay, participants had 60 seconds to recall the words. The score for the DWRT is the number of words recalled.
The DSST is a test of executive function and processing speed. In this 90-second test, participants were asked to translate numbers to symbols using a key. The score is the count of numbers correctly translated to symbols, with a range of possible scores of 0 to 93.
The WFT is a test of executive function and language. Participants were given 60 seconds for each of the letters F, A and S, and were asked to generate as many words as possible beginning with each letter, avoiding proper nouns. The WFT score is the total number of words generated for each of the letters.
To facilitate comparison across cognitive tests, Z scores standardized to visit 2 were calculated for each test by subtracting each participant's test score at each visit from the visit 2 mean and dividing by the visit 2 standard deviation. A composite global cognitive Z score was calculated by averaging the Z scores of the three tests, and was then standardized to visit 2 using the global Z mean and global Z standard deviation from visit 2. Thus, a Z score of -1 would describe cognitive performance that is 1 standard deviation below the mean score at visit 2. Composite global scores derived in this manner have been used in analyses of cognitive change in ARIC(20, 21) and elsewhere(22-24).
Assessment of Diabetes
Diabetes was defined based on self-reported physician diagnosis, diabetes medication use, or HbA1c ≥ 6.5%.
Measurement of Hemoglobin A1c
HbA1c was measured in stored whole blood samples using high-performance liquid chromatography methods standardized to the Diabetes Control and Complications Trial assay (Tosoh A1c 2.2 Plus and Tosoh G7 analyzers, Tosoh, Tokyo, Japan)(25). For analyses of the association between HbA1c category and cognitive decline, HbA1c was categorized using standard clinical cut-points: in persons without a history of diabetes, <5.7%,5.7-6.4%,≥6.5%; and in persons with a history of diabetes, <7.0% and ≥7.0%(10).
Covariates
All covariates used in the regression models were assessed during visit 2 except education, race, and sex, which were assessed during visit 1. The following covariates were evaluated as confounders: age, age-squared, sex, race-field center (Minnesota whites; Maryland whites; North Carolina whites; North Carolina blacks; Mississippi blacks), education (<high school; high school, high school equivalent, or vocational school; college, graduate, or professional school), cigarette smoking (current; former; never), alcohol consumption (current; former; never), body mass index (kg/m2), hypertension (yes; no – “yes” defined as blood pressure-lowering medication use, systolic blood pressure greater than 140 mmHg, or diastolic blood pressure greater than 90 mmHg), history of coronary heart disease(yes;no – persons who were unsure of their history of heart disease were classified as “no”), history of stroke(yes;no), and apolipoprotein E ε4 genotype(0;1;2 alleles). We also included interaction terms between these variables and time to allow for different rates of decline by these covariates. In sensitivity analyses we treated the following variables as time-varying, updating values at each study visit: cigarette smoking, alcohol consumption, body mass index, hypertension, history of coronary heart disease, and history of stroke. We also additionally adjusted for total cholesterol and lipid-lowering medication use.
Statistical Analysis
We used linear models to estimate associations between diabetes and cognitive decline, fit with generalized estimating equations to account for the within-person correlations of test scores arising from the repeated measures across time; unstructured correlation matrices and robust variance estimates were employed. Time since baseline was modeled using a linear spline with a knot at six years, the mean duration between visits 2 and 4. The spline term allows for a non-linear association between time and cognitive decline, more appropriately fits the study design than would a quadratic term, and was supported by diagnostic lowess smoothers. The primary coefficients of interest were the interactions between diabetes and the time spline terms, which address the hypothesis of greater decline among participants with diabetes adjusting for age and the other covariates. To examine the role of stroke in mediating the association between diabetes and cognitive decline, we censored participant values at the time of stroke, excluding any post-stroke cognitive information from our analyses. To test the robustness of our findings and to mitigate the differences in baseline characteristics between persons with and without diabetes, we reran analyses using propensity score matching. Propensity scores were developed using logistic regression and included sex, age, race-center, education, cigarette smoking, drinking status, hypertension status, prevalent CHD, prevalent stroke, and body mass index. All but 3 participants with diabetes were matched (details in Appendix).
We tested for effect modification between race and diabetes, and tested for linear trend across categories of HbA1c using a variable taking on values 1 through 5 for each category.
In a separate analysis we examined the association of diabetes duration on 14-year cognitive decline, using visit 4 as baseline, and information from all prior visits to categorize diabetes duration. We calculated duration as the difference between the date of the visit 4 exam and the date of the visit when diabetes was first identified (based on a diagnosis or elevated glucose at any prior visit) and categorized as follows: 1) no diabetes at visit 4 (reference), 2) diabetes duration <3 years, 3) diabetes duration 3-6 years, 4) diabetes duration 6-9 years, or 5) diabetes duration >9 years.
We used an inverse probability of attrition weighting (IPAW)(26, 27) approach to account for potential informative missingness effects (details in Appendix). Statistical analyses were performed with SAS 9.3 (SAS Institute, Cary, NC) and Stata 13.0 (StataCorp LP, College Station TX). PROC GENMOD was used for the generalized linear models, with a repeated statement to account for correlations between observations, and a weight statement to incorporate the IPAW weights.
Results
The mean age of participants at baseline was 57 years, 56% were female, 24% were black, and 13.3% had diabetes (Table 1). Participants with diabetes were older, had less education and lower cognitive scores, and had a more adverse cardiovascular risk factor profile at baseline than those without diabetes. Persons with diabetes at baseline were less likely to attend visit 5 (25% versus 48%), which was largely due to the cumulative incidence of mortality (46% versus 22%) rather than study dropout (29% versus 30%)(Table 1). Those with the lowest Z scores at visit 2 (<5th percentile) were also less likely to attend visit 5, with only 20% returning. Of the 13,351 participants who attended visit 2, 17% did not attend any follow-up visits. Of the remaining 83% of participants who had at least one follow-up visit (10,720 attended visit 4, 5,987 attended visit 5), the median follow-up was 19.3 years (25th,75th percentiles: 6.0, 20.9).
Table 1. ARIC population visit 2 baseline characteristics by diabetes status.
| Total (N=13,351) |
Diabetes (N=1,779) |
No Diabetes (N=11,572) |
|
|---|---|---|---|
| Age | 57.0 (5.7) | 58.2 (5.7) | 56.8 (5.7) |
| Female, % | 55.6 | 57.2 | 55.3 |
| Visit 5 Attendance, % | |||
| Died before visit 5 | 25.4 | 46.4 | 22.1 |
| Alive, but did not attend | 29.8 | 28.6 | 30.0 |
| Attended | 44.8 | 25.1 | 47.9 |
| Race-Center, % | |||
| Minneapolis - White | 26.9 | 13.9 | 28.8 |
| Washington County - White | 26.2 | 24.6 | 26.4 |
| Forsyth - White | 23.3 | 16.5 | 24.4 |
| Forsyth - Black | 2.7 | 4.9 | 2.4 |
| Jackson - Black | 21.0 | 40.1 | 18.0 |
| Cognitive scores | |||
| Global cognitive Z score | 0.00 (1.0) | -0.52 (1.0) | 0.08 (1.0) |
| Delayed word recall test, number of words Recalled | 6.6 (1.5) | 6.1 (1.6) | 6.7 (1.5) |
| Digit symbol substitution test, number of symbols translated | 44.7 (14.2) | 36.9 (14.4) | 45.9 (13.7) |
| Word fluency test, number of words generated | 33.2 (12.5) | 29.3 (12.4) | 33.8 (12.4) |
| Hemoglobin A1c | 5.8 (1.2) | 8.0 (2.1) | 5.4 (0.4) |
| Prevalent coronary heart disease, % | 5.7 | 11.1 | 4.8 |
| Prevalent stroke, % | 1.7 | 4.4 | 1.3 |
| Apolipoprotein E ε4 alleles, % | |||
| 0 | 69.2 | 69.4 | 69.2 |
| 1 | 28.1 | 27.8 | 28.2 |
| 2 | 2.6 | 2.9 | 2.6 |
| Hypertension, % | 35.6 | 59.0 | 32.0 |
| Body mass index, kg/m2 | 28.0 (5.4) | 31.4 (6.1) | 27.4 (5.1) |
| Total cholesterol level | |||
| mg/dL | 210 (39.5) | 216 (45.5) | 209 (38.4) |
| mmol/L | 5.43 (1.02) | 5.57 (1.18) | 5.41 (0.99) |
| HDL cholesterol level | |||
| mg/dL | 49.4 (16.7) | 43.1 (14.2) | 50.4 (16.8) |
| mmol/L | 1.28 (0.43) | 1.11 (0.37) | 1.30 (0.44) |
| Triglyceride level | |||
| mg/dL | 136 (90.3) | 178 (135.3) | 130 (79.4) |
| mmol/L | 1.54 (1.02) | 2.01 (1.53) | 1.46 (0.90) |
| Education, % | |||
| Less than high school | 21.2 | 34.9 | 19.1 |
| High school, graduate equivalence degree, or vocational school | 41.8 | 37.9 | 42.3 |
| College, graduate, or professional school | 37.0 | 27.2 | 38.6 |
| Cigarette smoking status, % | |||
| Current | 22.3 | 20.8 | 22.5 |
| Former | 37.9 | 37.0 | 38.1 |
| Never | 39.8 | 42.2 | 39.4 |
| Alcohol consumption, % | |||
| Current | 56.6 | 36.0 | 59.7 |
| Former | 20.8 | 33.2 | 18.9 |
| Never | 22.6 | 30.8 | 21.3 |
Age, cognitive scores, hemoglobin A1c, body mass index, total cholesterol, HDL cholesterol, and triglycerides are means (SD). All other values are percentages.
Table 2 shows the estimated 20-year decline from our linear models by diabetes status for global cognitive Z score, DWRT, DSST, and WFT. Diagnosed diabetes was associated with significantly greater decline in global cognitive Z score, the DSST, and the WFT although not in the DWRT. The average decline over 20 years in global cognitive Z score was 0.78 in persons without diabetes and 0.92 in persons with diabetes (difference: -0.15, 95% CI: (-0.22, -0.08)), i.e. a 19% greater decline among persons with diabetes (-0.15/-0.78=19%). The difference was similar in race-stratified analyses (p-for-interaction=0.4357, eTables 1-4). Adjusting for attrition using IPAW strengthened the magnitude of all associations by about 50%. To give these results some context, and because age-related decline in cognitive function is well-established, we used our linear model to estimate how much older a person without diabetes would need to be at baseline to have, on average, a 0.15 lower Z score. We estimated that a participant had to be 4.9 years older. In other words, a 0.15 lower Z score is equivalent to the difference in cognitive performance of a 60 year old versus to a 55 year old, who are otherwise similar (details in Appendix).
Table 2.
Average difference in 20-year decline in global cognitive Z score, delayed word recall, digit symbol substitution, and word fluency among persons with a history of diagnosed diabetes compared to persons without diabetes
| No attrition adjustment | ||||
|---|---|---|---|---|
| Test | 20 year decline – No diabetes Estimate (95% CI) |
20 year decline – Diabetes Estimate (95% CI) |
Difference* Estimate (95% CI) |
Percent† |
| Global Z | -0.78 (-0.80, -0.75) | -0.92 (-1.00, -0.85) | -0.15 (-0.22, -0.08) | 19% |
| Delayed Word Recall Test | -0.98 (-1.02, -0.94) | -1.04 (-1.15, -0.92) | -0.06 (-0.17, 0.06) | 6% |
| Digit Symbol Substitution Test | -0.69 (-0.71, -0.67) | -0.82 (-0.87, -0.77) | -0.13 (-0.18, -0.08) | 19% |
| Word Fluency Test | -0.17 (-0.19, -0.14) | -0.28 (-0.35, -0.22) | -0.12 (-0.18, -0.06) | 71% |
| Attrition-adjusted | ||||
| Test | 20 year decline – No diabetes | 20 year decline – Diabetes | Difference* | Percent† |
| Global Z | -0.79 (-0.82, -0.76) | -1.01 (-1.11, -0.92) | -0.23 (-0.32, -0.13) | 29% |
| Delayed Word Recall Test | -1.01 (-1.05, -0.96) | -1.09 (-1.22, -0.96) | -0.09 (-0.22, 0.04) | 9% |
| Digit Symbol Substitution Test | -0.70 (-0.72, -0.68) | -0.87 (-0.94, -0.81) | -0.18 (-0.24, -0.11) | 26% |
| Word Fluency Test | -0.17 (-0.20, -0.14) | -0.37 (-0.47, -0.28) | -0.21 (-0.31, -0.10) | 124% |
Calculated as the difference in 20-year decline between persons without and with diabetes (negative values indicate greater decline in persons with diabetes)
Calculated as the difference expressed as a percentage of the decline in those without diabetes. That is, (decline in participants without diabetes – decline in participants with diabetes)/(decline in participants without diabetes); thus a value of 19% indicates a 19% greater decline in those with diagnosed diabetes compared to those without. Note that the differences and percent declines are calculated before rounding of 20-year estimates.
Note: bold values indicate p-value < 0.05. Z scores can be interpreted as standard deviations above or below the mean. For example, a Z score difference of -0.15 means that, on average, persons with diabetes declined an additional 0.15 standard deviations compared to persons without diabetes. Time since baseline was the time metric, and cognitive function was modeled using generalized linear models fit using generalized estimating equations, with adjustment for age, age squared, race-center, sex, education, cigarette smoking, alcohol consumption, body mass index, hypertension, history of coronary heart disease, history of stroke, APOE ε4 genotype, and interactions between all of these covariates and time. N=30,058 total records, with N=13,351 participants at visit 2(N=1,779 with diabetes), N=10,720 at visit 4(N=1,209 with diabetes), and N=5,987 at visit 5(N=446 with diabetes).
Our results were robust to an alternative analytical approach using propensity score matching (eTable5-6, eFigure2). Results were also unchanged when we adjusted for total cholesterol, cholesterol-lowering medication use, or when using time-varying covariates. In our stroke mediation analysis, excluding post-stroke cognitive scores reduced the 20-year difference in cognitive decline between persons with and without diabetes by 13%, though results remained significant (eTable7).
Using visit 4 as baseline shows that duration of diabetes was associated with significantly greater subsequent 14-year cognitive decline (Table 3). The p-value for linear trend across categories was significant for all tests.
Table 3.
Average difference in 14-year decline in global cognitive Z score, delayed word recall test, digit symbol substitution test, and word fluency test comparing persons of varying diabetes duration to persons without diabetes
| No attrition adjustment | Attrition adjusted | ||||
|---|---|---|---|---|---|
|
| |||||
| Test | Diabetes duration (years) | Absolute 14-year decline Estimate (95% CI) |
Difference* Estimate (95% CI) |
Absolute 14-year decline Estimate (95% CI) |
Difference* Estimate (95% CI) |
| Global Z | No diabetes | -0.67 (-0.70, -0.64) | (reference) | -0.68 (-0.71, -0.65) | (reference) |
| < 3 | -0.81 (-0.90, -0.71) | -0.13 (-0.23, -0.04) | -0.85 (-0.97, -0.73) | -0.18 (-0.30, -0.05) | |
| 3 - 6 | -0.72 (-0.82, -0.62) | -0.05 (-0.14, 0.05) | -0.73 (-0.83, -0.63) | -0.05 (-0.15, 0.05) | |
| 6 - 9 | -0.81 (-0.93, -0.68) | -0.13 (-0.26, -0.01) | -0.86 (-1.01, -0.72) | -0.19 (-0.34, -0.04) | |
| > 9 | -0.85 (-0.95, -0.74) | -0.18 (-0.28, -0.07) | -0.91 (-1.02, -0.79) | -0.23 (-0.34, -0.12) | |
| p-value-for-trend | 0.001 | - | 0.002 | - | |
|
| |||||
| Delayed Word Recall Test | No diabetes | -0.89 (-0.94, -0.85) | (reference) | -0.90 (-0.95, -0.85) | (reference) |
| < 3 | -0.98 (-1.11, -0.84) | -0.08 (-0.22, 0.06) | -1.02 (-1.19, -0.86) | -0.12 (-0.29, 0.05) | |
| 3 - 6 | -0.96 (-1.10, -0.81) | -0.06 (-0.21, 0.08) | -0.97 (-1.13, -0.82) | -0.07 (-0.23, 0.08) | |
| 6 - 9 | -0.99 (-1.18, -0.81) | -0.10 (-0.28, 0.09) | -1.01 (-1.20, -0.82) | -0.11 (-0.30, 0.08) | |
| > 9 | -1.05 (-1.20, -0.89) | -0.16 (-0.31, 0.00) | -1.09 (-1.26, -0.91) | -0.19 (-0.36, -0.02) | |
| p-value-for-trend | 0.003 | - | 0.003 | - | |
|
| |||||
| Digit Symbol Substitution Test | No diabetes | -0.56 (-0.58, -0.53) | (reference) | -0.56 (-0.58, -0.54) | (reference) |
| < 3 | -0.68 (-0.75, -0.61) | -0.12 (-0.19, -0.05) | -0.70 (-0.78, -0.62) | -0.14 (-0.22, -0.06) | |
| 3 - 6 | -0.62 (-0.69, -0.55) | -0.07 (-0.14, 0.00) | -0.62 (-0.68, -0.55) | -0.05 (-0.12, 0.01) | |
| 6 - 9 | -0.65 (-0.74, -0.55) | -0.09 (-0.18, 0.00) | -0.68 (-0.77, -0.58) | -0.11 (-0.21, -0.02) | |
| > 9 | -0.73 (-0.81, -0.64) | -0.17 (-0.25, -0.09) | -0.77 (-0.87, -0.67) | -0.21 (-0.31, -0.11) | |
| p-value-for-trend | <0.001 | - | <0.001 | - | |
|
| |||||
| Word Fluency Test | No diabetes | -0.13 (-0.16, -0.11) | (reference) | -0.13 (-0.16, -0.10) | (reference) |
| < 3 | -0.20 (-0.29, -0.12) | -0.07 (-0.16, 0.01) | -0.23 (-0.33, -0.13) | -0.10 (-0.20, -0.00) | |
| 3 - 6 | -0.14 (-0.23, -0.05) | -0.01 (-0.10, 0.09) | -0.13 (-0.23, -0.03) | 0.00 (-0.10, 0.11) | |
| 6 - 9 | -0.27 (-0.39, -0.16) | -0.14 (-0.25, -0.03) | -0.36 (-0.53, -0.18) | -0.22 (-0.41, -0.04) | |
| > 9 | -0.24 (-0.33, -0.15) | -0.11 (-0.20, -0.02) | -0.29 (-0.39, -0.18) | -0.15 (-0.26, -0.05) | |
| p-value-for-trend | <0.001 | - | 0.001 | - | |
Calculated as the difference in 14-year decline between persons with no diabetes at either visit and persons who have prevalent diabetes at visit 2 or develop diabetes between visits 2 and 4 (negative values indicate greater decline in those with prevalent or incident diabetes)
Note: bold values indicate p-value < 0.05. Baseline for this analysis was visit 4, and visits 1,2, and 3 were used to calculate diabetes duration. Z scores can be interpreted as standard deviations above or below the mean. For example, a Z score difference of -0.15 means that, on average, persons with diabetes declined an additional 0.15 standard deviations compared to persons without diabetes. Time since baseline was the time metric, and cognitive function was modeled using generalized linear models fit using generalized estimating equations, with adjustment for age, age squared, race-center, sex, education, cigarette smoking, alcohol consumption, body mass index, hypertension, history of coronary heart disease, history of stroke, APOE ε4 genotype, and interactions between all of these covariates and time. N=16,707 total records, with N=10,720 at visit 4(N=1,209 with diabetes) and N=5,987 at visit 5(N=446 with diabetes).
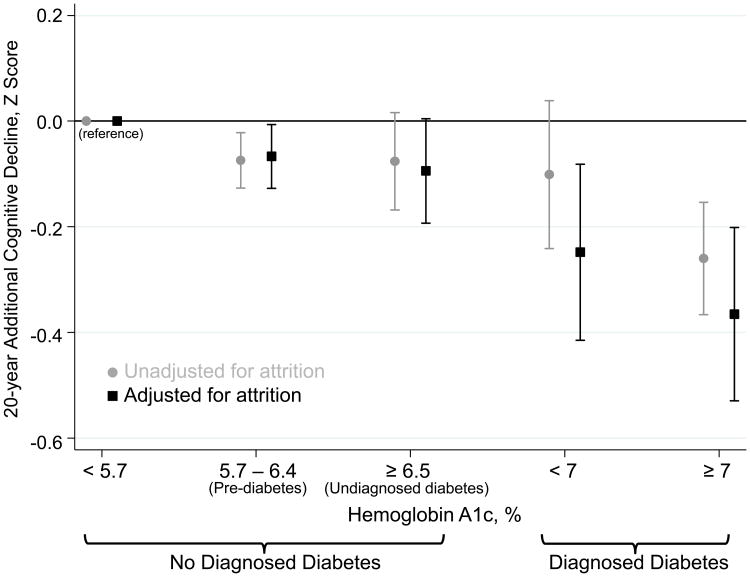
Figure 1 shows differences in 20-year decline in global cognitive Z score by clinical categories of HbA1c. The p-value for linear trend across all categories was significant (p=0.0367 without adjustment for attrition and p=0.006 for the attrition-adjusted values). Persons without diagnosed diabetes but HbA1c of 5.7-6.4% at baseline had significantly more cognitive decline over 20 years (adjusted difference in global cognitive Z score=-0.07, p-value=0.005) compared to persons without diabetes and HbA1c<5.7%. Persons without diagnosed diabetes but with HbA1c≥6.5% (undiagnosed diabetes) also had a greater decline in cognitive score compared to the reference group, however this difference was not statistically significant (p-value=0.105). The greatest decline was found in the group with diabetes and HbA1c≥7.0%. Participants in this group had a larger decline compared to persons with diabetes and HbA1c<7% (adjusted difference in global cognitive Z score=-0.16, p-value=0.071), which was borderline statistically significant. Adjusting for attrition strengthened the magnitude of all associations.
Figure 1.
Difference in global cognitive Z score decline by clinical categories of hemoglobin A1c compared to decline in persons without diabetes with hemoglobin A1c < 5.7%.
Legend: Adjusted for attrition refers to the inverse probability of attrition weighting used to account for participant death or dropout during follow-up. Estimates (95% confidence intervals) are from generalized linear models fit using generalized estimating equations for global cognitive Z score, with adjustment for age, age-squared, race-center, sex, education, cigarette smoking status, drinking status, hypertension, history of coronary heart disease, history of stroke, APOE ε4 genotype, body mass index, interactions between these variables and time (except for drinking status and history of coronary heart disease, which were not significant), and interactions between race-center and sex, hypertension, and education. Hemoglobin A1c was categorized using the standard clinical cut-points based on American Diabetes Association criteria (in participants without a diagnosis of diabetes (N=12,107): <5.7% (N=9,031), 5.7-6.4% (N=2,365), and ≥6.5% (N=711); in participants with diagnosed diabetes(N=1,244): <7% (N=415), ≥7% (N=829).
Discussion
In this community-based population, we found significantly greater cognitive decline among both black and white adults with diabetes compared to those without diabetes at baseline, with 20-year cognitive decline 19% larger in this group for the global score, or 30% larger after accounting for attrition. Duration of diabetes appeared to be a factor, with later life 14-year decline greater for participants with longer duration of diabetes. There were trends of increased cognitive decline across clinical categories of HbA1c, even among persons without a history of diabetes. Those with HbA1c in the 5.7-6.4% range (pre-diabetes) hhfda and those with HbA1c≥6.5% (undiagnosed diabetes) at baseline had larger declines over 20 years than those with HbA1c<5.7%. Excluding person with stroke post-baseline attenuated the results slightly, suggesting stroke partially mediates the association between diabetes and cognitive decline.
The observed association of diabetes with decline in global cognitive function was primarily driven by declines in the DSST and WFT, which reflect impairments in the processing speed and executive function domains(28, 29). These results suggest that the association of diabetes with cognitive function may involve the subcortical microvasculature that damages white matter pathways or subcortical grey matter in other ways(30-32). However, we also found associations with memory, but only in whites, after adjustment for attrition. This may be due to the fact that the DWRT, with only 10 words, is insensitive to small declines in memory.
Previous studies of diabetes and cognitive decline have mostly been short in duration: Cukierman's review included only one study with mean follow-up of more than 6 years' duration(6). In four recent reports, diabetes was associated with 12-year decline in several tests in the Maastricht Study(33), 10-year decline in a global test, memory, and reasoning in two Whitehall II studies(15, 34), and 8-year decline in one of 8 tests in the Framingham Offspring Study(35). However, only one of these reported associations with diabetes diagnosed before age 65.
ACCORD-MIND, a randomized clinical trial, showed that tight glucose control in elderly diabetics with high cardiovascular risk did not reduce cognitive decline measured by DSST(13, 14). Some have postulated that the lack of benefit in ACCORD-MIND may have been due to the older age of participants (mean age 63), the short treatment period (3.3 years), and a higher frequency of hypoglycemic episodes in the treatment compared to the control arm. However, our observations that higher HbA1c levels were associated with greater 20-year cognitive decline even in persons without a diagnosis of diabetes, and that longer duration of diabetes was associated with greater cognitive decline, suggests that a long-term trial, if one were feasible, could demonstrate the cognitive benefit of glycemic control. The potential benefit of early intervention deserves further study(36).
Some limitations of our study deserve consideration. We had only one test in each cognitive domain at each visit and only a single measurement of HbA1c at baseline. Blacks in ARIC come from just 2 study sites, limiting our ability to fully separate the effects of race from those of geography. Attrition is a likely concern for any long-term study. However, our attrition-adjustment likely provides less biased estimates of the effect of diabetes on cognition than when attrition is ignored, as in most prior reports. Although we adjusted for attrition using a broad set of available data, it is possible that our method of adjustment does not fully account for the effects of drop out, especially dropout directly related to low cognitive function, and our estimate of the association of diabetes with cognitive decline may remain conservative. As this is an observational study, we cannot conclude that the link between diabetes and cognitive decline is causal, and we cannot rule out the possibility of residual confounding.
Strengths of this study include the large community-based population of blacks and whites, rigorous assessment of variables that might affect the association between diabetes and cognitive function, and our methods to reduce the effects of dropout. The evaluation of cognitive change over time, with 20-year duration of follow-up with cognitive function assessed at several time points, is also a particular strength of this study. Rather than assessing dementia or cognitive performance at a single time point, examining scores over time reduces the influence of confounding variables(20).
Maintaining cognitive function is a critical aspect of successful aging and for ensuring a high quality of life. Diabetes and glucose control are potentially modifiable and may offer an important opportunity for the prevention of cognitive decline, thus delaying progression to dementia. At the population level, delaying the onset of dementia by even a couple of years could reduce the prevalence of dementia by more than 20% over the next 30 years(37).
This study documents that diabetes and pre-diabetes in middle age are associated with greater cognitive decline over the subsequent two decades. The association with cognitive decline was stronger for diabetes of longer duration, and our findings were similar in black and white adults. These data suggest that primary prevention of diabetes or glucose control in midlife may protect against later-life cognitive decline.
Supplementary Material
eTable 1: Average difference in 20-year decline in global Z score, delayed word recall, digit symbol substitution, and word fluency among persons with a history of diagnosed diabetes compared to persons without diabetes, white race
eTable 2: Average difference in 20-year decline in global Z score, delayed word recall, digit symbol substitution, and word fluency among persons with a history of diagnosed diabetes compared to persons without diabetes, black race
eTable 3: Average difference in 14-year decline in global Z score, delayed word recall, digit symbol substitution, and word fluency among persons with prevalent diagnosed diabetes at visit 2 or incident diagnosed diabetes or visit 4, compared to persons without diabetes at either visit, white race
eTable 4: Average difference in 14-year decline in global Z score, delayed word recall, digit symbol substitution, and word fluency among persons with prevalent diagnosed diabetes at visit 2 or incident diagnosed diabetes or visit 4, compared to persons without diabetes at either visit, black race
eTable 5: ARIC population visit 2 baseline characteristics by diabetes status, propensity score matched cohort
eTable 6: Average difference in 20-year decline in global cognitive Z score, delayed word recall, digit symbol substitution, and word fluency among persons with a history of diagnosed diabetes compared to persons without diabetes, for participants of the matched cohort
eTable 7: Average difference in 20-year decline in global cognitive Z score, delayed word recall, digit symbol substitution, and word fluency among persons with a history of diagnosed diabetes compared to persons without diabetes, censoring cognitive values of participants after they experience a stroke
eFigure 1: Flowchart of study visits and exclusions and pattern of attendance
eFigure 2: Propensity score distribution for persons with and without diabetes.
Legend: Panel A: propensity score distribution for all cohort participants (N=13,351). Panel B: propensity score distribution for matched participants(N=1,824 in each group). Propensity scores are calculated from logistic models that included sex, age, race-center, education, cigarette smoking, drinking status, hypertension status, prevalent CHD, prevalent stroke, and body mass index, starting with 13,766 participants at baseline, including those with “not reported” APOE status. All variables were significant. We used psmatch2 in Stata to select matches based on propensity score, with nearest neighbor selected without replacement, using a caliper set to .05. All but 3 persons with diabetes from the full cohort had a match.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
ARIC-NCS Steering Committee: Thomas Mosley, PhD (Chair), Josef Coresh, MD, PhD (Co-Chair), Marilyn Albert, PhD, Alvaro Alonso MD, PhD, Christie M. Ballantyne, MD, Eric Boerwinkle, PhD, David Couper, PhD, Gerardo Heiss, MD, PhD, Clifford Jack, MD, Barbara Klein, MD, MPH, Ronald Klein, MD, MPH, David Knopman, MD, Natalie Kurinij, PhD (NEI Project Officer), Claudia Moy, PhD (NINDS Project Officer), and Jacqueline Wright, PhD (NHLBI Project Officer). Ex Officio Members: Laura Coker, PhD, Aaron Folsom, MD, MPH, Rebecca F. Gottesman, MD, PhD, A. Richey Sharrett, MD, DrPH, Lynne E. Wagenknecht, DrPH, and Lisa Miller Wruck, PhD.
ARIC-NCS Data Analysis Committee (drafted and critically revised all analysis plans): A. Richey Sharrett, MD, DrPH (Chair), Karen Bandeen-Roche, PhD (Senior Statistician), Andrea L.C. Schneider, MD, PhD, Josef Coresh, MD, PhD, Jennifer A. Deal, PhD, Rebecca F. Gottesman, MD, PhD, Michael Griswold, PhD, Alden Gross, PhD, Thomas Mosley, PhD, Melinda Power PhD, Andreea M. Rawlings, MS, Lisa Miller Wruck, PhD, and Shoshana Ballew, PhD (Epidemiologist coordinator)
ARIC-NCS Neurocognitive Committee: Thomas Mosley, PhD (Chair), Rebecca F. Gottesman, MD, PhD (Co-Chair), Alvaro Alonso, MD, PhD, Laura Coker, PhD, David Couper, PhD, David Knopman, MD, Guy McKhann, MD, Ola Selnes, PhD, and A. Richey Sharrett, MD, DrPH.
A.M.R had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Preparation, review, or approval of the manuscript: A.M.R. and A.R.S. prepared the manuscript, all authors provided critical review and approved the manuscript
Analysis and interpretation of data: A.M.R, A.L.C.S, A.R.S
Critical revision of the manuscript for important intellectual content: All authors
Funding/Support: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C. Neurocognitive (ARIC-NCS) data is collected by U01HL096812, HL096814, HL096899, HL096902, HL096917 with previous brain MRI examinations funded by R01HL70825. The sponsor was not involved in the analysis of data, interpretation of findings, preparation, review, approval of the manuscript, nor in the decision to submit the manuscript for publication.
A.M.R. and A.L.C.S were supported by NIH/NHLBI training grant T32 HL007024. E.S. was supported by NIH/NIDDK grant R01DK089174. R.F.G was supported by R01AG040282.
Protocols and data for the parent ARIC-NCS Study may be obtained by approved individuals through written agreements with the ARIC Steering Committee and the research sponsor (NHLBI). Code is available to interested readers by contacting Dr. Elizabeth Selvin, eselvin@jhu.edu.
References
- 1.Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988-1994 and 1999-2010. Ann Intern Med. 2014;160(8):517–25. doi: 10.7326/M13-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emerging Risk Factors C. Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–41. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarwar N, Aspelund T, Eiriksdottir G, Gobin R, Seshasai SR, Forouhi NG, et al. Markers of dysglycaemia and risk of coronary heart disease in people without diabetes: Reykjavik prospective study and systematic review. PLoS Med. 2010;7(5):e1000278. doi: 10.1371/journal.pmed.1000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–36. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 5.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5(1):64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 6.Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes--systematic overview of prospective observational studies. Diabetologia. 2005;48(12):2460–9. doi: 10.1007/s00125-005-0023-4. [DOI] [PubMed] [Google Scholar]
- 7.Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J. 2012;42(5):484–91. doi: 10.1111/j.1445-5994.2012.02758.x. [DOI] [PubMed] [Google Scholar]
- 8.Power MC, Tchetgen EJ, Sparrow D, Schwartz J, Weisskopf MG. Blood pressure and cognition: factors that may account for their inconsistent association. Epidemiology. 2013;24(6):886–93. doi: 10.1097/EDE.0b013e3182a7121c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alonso A, Mosley TH, Jr, Gottesman RF, Catellier D, Sharrett AR, Coresh J. Risk of dementia hospitalisation associated with cardiovascular risk factors in midlife and older age: the Atherosclerosis Risk in Communities (ARIC) study. J Neurol Neurosurg Psychiatry. 2009;80(11):1194–201. doi: 10.1136/jnnp.2009.176818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Standards of medical care in diabetes--2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 11.Yaffe K, Falvey C, Hamilton N, Schwartz AV, Simonsick EM, Satterfield S, et al. Diabetes, glucose control, and 9-year cognitive decline among older adults without dementia. Arch Neurol. 2012;69(9):1170–5. doi: 10.1001/archneurol.2012.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanz CM, Ruidavets JB, Bongard V, Marquie JC, Hanaire H, Ferrieres J, et al. Relationship Between Markers of Insulin Resistance, Markers of Adiposity, HbA1c, and Cognitive Functions in a Middle-Aged Population-Based Sample: the MONA LISA Study. Diabetes Care. 2013;36(6):1512–21. doi: 10.2337/dc12-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Launer LJ, Miller ME, Williamson JD, Lazar RM, Gerstein HC, Murray AM, et al. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol. 2011;10(11):969–77. doi: 10.1016/S1474-4422(11)70188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herzog RI, Sherwin RS. Diabetes. Can tight glycemic control in diabetes benefit cognition? Nat Rev Neurol. 2011;8(3):124–6. doi: 10.1038/nrneurol.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuligenga RH, Dugravot A, Tabák AG, Elbaz A, Brunner EJ, Kivimäki M, et al. Midlife type 2 diabetes and poor glycaemic control as risk factors for cognitive decline in early old age: a post-hoc analysis of the Whitehall II cohort study. The Lancet Diabetes & Endocrinology. 2013 doi: 10.1016/S2213-8587(13)70192-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 17.Knopman DS, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol. 1989;46(2):141–5. doi: 10.1001/archneur.1989.00520380041011. [DOI] [PubMed] [Google Scholar]
- 18.W D. Wechsler Adult Intelligence Scale - Revised Manual. New York: Psychological Corp; 1981. [Google Scholar]
- 19.Benton AL, H K. Multilingual aphasia examination. 2nd. Iowa City: AJA Associates; 1989. [Google Scholar]
- 20.Gottesman RF, Rawlings AM, Sharrett AR, Albert M, Alonso A, Bandeen-Roche K, et al. Impact of Differential Attrition on the Association of Education with Cognitive Change Over 20 Years of Follow-up: The ARIC Neurocognitive Study. Am J Epidemiol. 2014 doi: 10.1093/aje/kwu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider AL, Sharrett AR, Gottesman RF, Coresh J, Coker L, Wruck L, et al. Normative Data for 8 Neuropsychological Tests in Older Blacks and Whites From the Atherosclerosis Risk in Communities (ARIC) Study. Alzheimer Dis Assoc Disord. 2014 doi: 10.1097/WAD.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elias PK, Elias MF, D'Agostino RB, Cupples LA, Wilson PW, Silbershatz H, et al. NIDDM and blood pressure as risk factors for poor cognitive performance. The Framingham Study. Diabetes Care. 1997;20(9):1388–95. doi: 10.2337/diacare.20.9.1388. [DOI] [PubMed] [Google Scholar]
- 23.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the rush Memory and Aging Project. Curr Alzheimer Res. 2012;9(6):646–63. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson RS, Segawa E, Boyle PA, Bennett DA. Influence of late-life cognitive activity on cognitive health. Neurology. 2012;78(15):1123–9. doi: 10.1212/WNL.0b013e31824f8c03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selvin E, Ning Y, Steffes MW, Bash LD, Klein R, Wong TY, et al. Glycated hemoglobin and the risk of kidney disease and retinopathy in adults with and without diabetes. Diabetes. 2011;60(1):298–305. doi: 10.2337/db10-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–64. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weuve J, Tchetgen Tchetgen EJ, Glymour MM, Beck TL, Aggarwal NT, Wilson RS, et al. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology. 2012;23(1):119–28. doi: 10.1097/EDE.0b013e318230e861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christman AL, Matsushita K, Gottesman RF, Mosley T, Alonso A, Coresh J, et al. Glycated haemoglobin and cognitive decline: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia. 2011;54(7):1645–52. doi: 10.1007/s00125-011-2095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knopman DS, Mosley TH, Catellier DJ, Coker LH. Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: the ARIC MRI Study. Alzheimers Dement. 2009;5(3):207–14. doi: 10.1016/j.jalz.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 30.Jokinen H, Kalska H, Mantyla R, Pohjasvaara T, Ylikoski R, Hietanen M, et al. Cognitive profile of subcortical ischaemic vascular disease. J Neurol Neurosurg Psychiatry. 2006;77(1):28–33. doi: 10.1136/jnnp.2005.069120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Brien JT, Wiseman R, Burton EJ, Barber B, Wesnes K, Saxby B, et al. Cognitive associations of subcortical white matter lesions in older people. Ann N Y Acad Sci. 2002;977:436–44. doi: 10.1111/j.1749-6632.2002.tb04849.x. [DOI] [PubMed] [Google Scholar]
- 32.Selnes OA, Vinters HV. Vascular cognitive impairment. Nat Clin Pract Neurol. 2006;2(10):538–47. doi: 10.1038/ncpneuro0294. [DOI] [PubMed] [Google Scholar]
- 33.Spauwen PJ, Kohler S, Verhey FR, Stehouwer CD, van Boxtel MP. Effects of Type 2 Diabetes on 12-Year Cognitive Change: Results from the Maastricht Aging Study. Diabetes Care. 2013 doi: 10.2337/dc12-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaffashian S, Dugravot A, Elbaz A, Shipley MJ, Sabia S, Kivimaki M, et al. Predicting cognitive decline: a dementia risk score vs. the Framingham vascular risk scores. Neurology. 2013;80(14):1300–6. doi: 10.1212/WNL.0b013e31828ab370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bangen KJ, Beiser A, Delano-Wood L, Nation DA, Lamar M, Libon DJ, et al. APOE Genotype Modifies the Relationship between Midlife Vascular Risk Factors and Later Cognitive Decline. J Stroke Cerebrovasc Dis. 2013 doi: 10.1016/j.jstrokecerebrovasdis.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerstein HC. Do lifestyle changes reduce serious outcomes in diabetes? N Engl J Med. 2013;369(2):189–90. doi: 10.1056/NEJMe1306987. [DOI] [PubMed] [Google Scholar]
- 37.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88(9):1337–42. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1: Average difference in 20-year decline in global Z score, delayed word recall, digit symbol substitution, and word fluency among persons with a history of diagnosed diabetes compared to persons without diabetes, white race
eTable 2: Average difference in 20-year decline in global Z score, delayed word recall, digit symbol substitution, and word fluency among persons with a history of diagnosed diabetes compared to persons without diabetes, black race
eTable 3: Average difference in 14-year decline in global Z score, delayed word recall, digit symbol substitution, and word fluency among persons with prevalent diagnosed diabetes at visit 2 or incident diagnosed diabetes or visit 4, compared to persons without diabetes at either visit, white race
eTable 4: Average difference in 14-year decline in global Z score, delayed word recall, digit symbol substitution, and word fluency among persons with prevalent diagnosed diabetes at visit 2 or incident diagnosed diabetes or visit 4, compared to persons without diabetes at either visit, black race
eTable 5: ARIC population visit 2 baseline characteristics by diabetes status, propensity score matched cohort
eTable 6: Average difference in 20-year decline in global cognitive Z score, delayed word recall, digit symbol substitution, and word fluency among persons with a history of diagnosed diabetes compared to persons without diabetes, for participants of the matched cohort
eTable 7: Average difference in 20-year decline in global cognitive Z score, delayed word recall, digit symbol substitution, and word fluency among persons with a history of diagnosed diabetes compared to persons without diabetes, censoring cognitive values of participants after they experience a stroke
eFigure 1: Flowchart of study visits and exclusions and pattern of attendance
eFigure 2: Propensity score distribution for persons with and without diabetes.
Legend: Panel A: propensity score distribution for all cohort participants (N=13,351). Panel B: propensity score distribution for matched participants(N=1,824 in each group). Propensity scores are calculated from logistic models that included sex, age, race-center, education, cigarette smoking, drinking status, hypertension status, prevalent CHD, prevalent stroke, and body mass index, starting with 13,766 participants at baseline, including those with “not reported” APOE status. All variables were significant. We used psmatch2 in Stata to select matches based on propensity score, with nearest neighbor selected without replacement, using a caliper set to .05. All but 3 persons with diabetes from the full cohort had a match.