Abstract
Objective
To determine the utility of soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) levels in bronchoalveolar lavage fluid (BALF) and exhaled breath condensate (EBC) samples from patients who underwent bronchoscopy for a clinical suspicion of ventilator-associated pneumonia (VAP), to categorize patients as VAP positive and VAP negative, when compared to quantitative culture results of BALF.
Methods
Observational study conducted on admitted patients in the trauma-surgical, medical-cardiac, burn, and neurosurgical ICUs of Harborview Medical Center between March 2009 and May 2010. BALF and EBC samples were obtained from 45 patients with clinically suspected VAP. Bronchoscopy was performed on the day of clinically suspected VAP. sTREM-1 levels in EBC and BAL fluid were measured using quantikine human TREM-1 immunoassay. VAP was diagnosed by quantitative cultures of BALF.
Results
The concentrations of sTREM-1 in BALF and EBC did not correlate with VAP status. sTREM-1 levels did not discriminate VAP positive from VAP negative patients, when compared to quantitative cultures of BALF as the gold standard. Using a cutoff value of 204 pg/mL for BALF sTREM-1 levels resulted in a sensitivity of 79% and a specificity of 23%. A cutoff value of 10 pg/mL for EBC sTREM-1 levels resulted in a sensitivity of 42% and a specificity of 50%.
Conclusions
EBC and BALF sTREM-1 levels did not effectively categorize patients as VAP positive or VAP negative when using direct bronchoscopic quantitative culture samples as the comparison standard.
Keywords: soluble triggering receptor expressed on myeloid cells, sTREM-1 protein, ventilator-associated pneumonia, VAP, biomarkers, bronchoalveolar lavage fluid, acute respiratory distress syndrome, ARDS, human
Introduction
Ventilator associated pneumonia (VAP) is defined as inflammation of the lung parenchyma attributed to bacterial infection and occurring 48 hours after endotracheal intubation and mechanical ventilation.1 VAP is the most common nosocomial infection in the ICU, occurring in 27% of all intubated patients.1,2 VAP is associated with increased mortality, morbidity, costs, hospital stay, and duration of mechanical ventilation.1,2 Almost 50% of all antibiotic prescriptions in the ICU are attributed to VAP.3 A clinical suspicion of VAP is overly sensitive, and may result in the unnecessary use of antibiotics in patients presenting with noninfectious processes.4 Indiscriminate use of antibiotics is associated with increased mortality and the emergence of antibiotic resistant strains.4,5
The clinical pulmonary infection score (CPIS), developed to enhance the specificity of clinical diagnosis, initially demonstrated improved diagnostic accuracy for VAP, compared to clinical criteria.6–8 However, the usefulness of CPIS to discriminate for VAP remains controversial.9,10 Several invasive or semi-invasive methods can be used to diagnose VAP. Cultures from endotracheal aspirates have been used to diagnose VAP. However, endotracheal aspirates do not sample deeply into the lung, and cultures may represent colonization of the endotracheal tube rather than true infection.11,12 Nondirected bronchoalveolar lavage utilized to retrieve fluid from the lung without direct visualization could miss the area of infection, resulting in a false negative test. Quantitative culture of bronchoalveolar lavage fluid (BALF) retrieved via direct bronchoscopic methods has the most consistently high sensitivity and specificity to diagnose VAP and differentiate true infection from colonization or inflammation.4,13 However, bronchoscopy is invasive and requires specialized skills. All culture-based methods may require up to 48 hours for microbial identification.
Triggering receptor expressed on myeloid cells-1 (TREM) is a glycoprotein member of the immunoglobulin superfamily, whose expression is up-regulated in the presence of extracellular bacteria and fungi and some inflammatory conditions.14–22 TREM-1 triggers the secretion of pro-inflammatory mediators through a signaling pathway (DAP12) and amplifies the inflammatory response.14 In response to infection, soluble TREM-1 (sTREM-1) is either secreted or shed.23–25 sTREM-1 levels can then be measured in body fluids, including BALF and exhaled breath condensate (EBC).8,26–28 sTREM-1 levels are not detectable at baseline in normal individuals.
Because sTREM-1 levels increase with active infection, sTREM-1 has been suggested as a biomarker to differentiate VAP positive from VAP negative patients.8,28,29 An early study by Gibot et al8 demonstrated high specificity and sensitivity of sTREM-1 levels in the diagnosis of VAP. However, subsequent studies have shown much lower sensitivity and specificity for sTREM-1, despite standardized methods to detect sTREM.28,30,31
We measured sTREM-1 levels in BALF and EBC samples from patients who underwent bronchoscopy for a clinical suspicion of VAP, to determine the ability to measure a potential biomarker in EBC and to determine the usefulness of sTREM-1 to properly categorize patients as VAP positive and VAP negative, when compared to quantitative culture results of BALF.
Methods
Study Subjects
This was a prospective observational study that included BALF and EBC samples from eligible patients > 18 years of age admitted to the trauma-surgical, medical-cardiac, burn, and neurosurgical ICUs of Harborview Medical Center, Seattle, Washington, between March 2009 and May 2010, who underwent bronchoscopy while mechanically ventilated for clinically suspected VAP. Exclusion criteria included age < 18 years, human immunodeficiency virus infection, and pregnancy. For patients in whom more than one sample of BALF and EBC were collected during the course of their hospitalization, only the first samples were included in this study. The study was approved by the University of Washington's institutional review board. Informed consent to access data for this study was obtained from patients or their legal next of kin, in person or via a mailed and returned consent form.
VAP Diagnosis
Patients with a clinical suspicion of VAP and who had not had a change or addition in antibiotics in the preceding 72 hours underwent same-day bronchoscopy. Clinical criteria included: ≥ 48 hours of mechanical ventilation; new or progressive bilateral pulmonary infiltrates on radiograph; and one or more of: fever, leukocytosis, or leukopenia, and an increase in purulent endotracheal secretions.4 VAP was confirmed by BALF culture of ≥ 10,000 colony forming units per mL or protected specimen brush culture of ≥ 1,000 colony forming units/mL.4
Bronchoalveolar Lavage Fluid
We have a standard protocol for obtaining, transporting, analyzing, and storing samples at our institution, as previously described.32,33 An initial 25 mL wash was discarded, and lavage was performed from the most affected region identified by radiograph, using four 30-mL washes of normal saline.1,4 An aliquot was removed and underwent standard quantitative cultures in the clinical microbiology laboratory. BALF was centrifuged, and cell-free supernatants were aliquoted into polypropylene tubes and stored at − 80°C. Samples did not undergo repeated freeze/ thaw.
Exhaled Breath Condensate
The EBC fluid was collected immediately preceding bronchoscopy. The fluid was collected in a plastic container located in the center of the exhaled portion of the ventilator tubing (mid-way between the ventilator and the patient's endotracheal tube). EBC was centrifuged and cell-free supernatants were aliquoted into 2 polypropylene tubes and stored at − 80°C.
Soluble Triggering Receptor Expressed on Myeloid Cells Type-1 Assay
sTREM-1 levels in EBC and BALF were measured using second generation (DTRM10B) quantikine human TREM-1 immunoassay (R&D Systems, Minneapolis, Minnesota) per manufacturer's directions. Concentrations were extrapolated from a standard curve. All samples were run in duplicate.
Statistical Analysis
Individual sTREM-1 levels in BALF and EBC were grouped according to VAP positive and VAP negative and expressed as mean ± SD. Continuous variables (sTREM-1 levels, PaO2/FIO2, day of bronchoscopy, hospital stay, age, and alive at discharge) were expressed as mean ± SD, and comparisons between groups were evaluated using the Mann-Whitney U test for non-normally distributed variables (sTREM-1 levels). The strength of association between variables was analyzed using a Spearman rank-order correlation. A 2-tailed P value < .05 was considered statistically significant. A receiver operating characteristic curve was produced to determine the diagnostic value of the sTREM-1 assay. Analysis was performed with statistics software (Stata 11.0, StataCorp, College Station, Texas).
Results
Patient Characteristics
We enrolled 45 patients who underwent bronchoscopic BALF for clinically suspected VAP. The baseline characteristics of the 45 patients are shown in Table 1. Of note, we had more males enrolled in the study, which reflects the demographics of our ICU population, due to the prevalence of male trauma victims. Among the 45 patients, 19 (42%) were confirmed as VAP by quantitative culture results. Over half of all patients (58%) were receiving antibiotics at the time of bronchoscopy: 77% of VAP negative patients and 32% of VAP positive patients were receiving antibiotics at the time of bronchoscopy, but none of the patients had a change in antibiotic therapy in the preceding 72 hours from bronchoscopy.
Table 1. Patient Characteristics.
| All Patients (n = 45) | VAP Positive (n = 19) | VAP Negative (n = 26) | P | |
|---|---|---|---|---|
| Male, no. (%) | 36 (80) | 18 (95) | 18 (69) | .03 |
| Age, y | 52 ± 17 | 50 ± 16 | 54 ± 19 | .54 |
| White, no. (%) | 40 (85) | 16 (84) | 24 (86) | |
| PaO2/FIO2, mm Hg* | 226 ± 84 | 217 ± 69 | 233 ± 94 | .85 |
| Day of bronchoscopy, d† | 12 ± 15 | 8 ± 4 | 15 ± 19 | .35 |
| Antibiotics, no. (%) | 26 (58) | 6 (32) | 20 (77) | .002 |
| Hospital stay, d | 41 ± 38 | 36 ± 34 | 44 ± 41 | .37 |
| Alive at discharge, no. (%) | 37 (82) | 16 (84) | 21 (81) | .77 |
| Admit Diagnosis, no. | ||||
| Burns | 2 | 1 | 1 | |
| Congestive heart failure | 2 | 1 | 1 | |
| Ventricular fibrillation/arrest | 3 | 2 | 1 | |
| ARDS | 2 | 0 | 2 | |
| Pneumonia | 3 | 0 | 3 | |
| Sepsis | 4 | 3 | 1 | |
| Cerebral vascular accident/subarachnoid hemorrhage | 5 | 2 | 3 | |
| Trauma (blunt and penetrating) | 21 | 9 | 12 | |
| Other | 3 | 1 | 2 |
Values are mean ± SD.
PaO2/FIO2 = at closest time to bronchoscopy.
Days since hospital admit.
sTREM-1 Detection
sTREM-1 was detected in all 45 (100%) BALF samples, and in 40 (89%) EBC samples. sTREM-1 levels were consistently higher in BALF than EBC in all patients. While the BALF sTREM-1 concentrations were higher in VAP positive patients than VAP negative patients, this was not statistically significant (P = .45) (Table 2, Fig. 1). EBC sTREM-1 levels were not statistically different between VAP positive patients and VAP negative patients (P = .27) (see Table 2 and Fig. 1). Receiver-operating characteristic curves are shown in Figure 2. The area under the receiver-operating characteristic curve for BALF sTREM-1 and EBC sTREM-1 were not statistically significant (BALF sTREM-1 95% CI 0.38–0.73, EBC sTREM-1 95% CI 0.22–0.59). BALF sTREM-1 levels were similar in patients receiving antibiotics at the time of bronchoscopy (490 pg/mL), compared to patients not receiving antibiotics (500 pg/mL). Although EBC sTREM-1 levels were lower in patients receiving antibiotics (29 pg/ mL), compared to patients not receiving antibiotics (88 pg/ mL), the difference was not statistically significant.
Table 2. sTREM-1 Levels.
| All Patients, pg/mL (n = 45) | VAP, pg/mL (n = 19) | No VAP, pg/mL (n = 26) | P | |
|---|---|---|---|---|
| Bronchoalveolar lavage fluid | 494 ± 510 | 591 ± 668 | 443 ± 353 | .45 |
| Exhaled-breath condensate | 54 ± 139 | 38 ± 63 | 66 ± 175 | .27 |
Values are mean ± SD.
Fig 1.
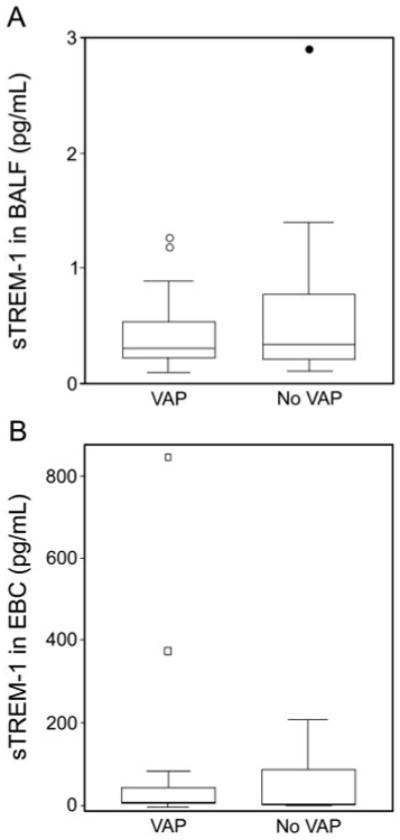
Median soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) levels in bronchoalveolar lavage fluid (BALF) and exhaled breath condensate (EBC) in 19 patients with and 26 patients without ventilator-associated pneumonia (VAP).In the data bars: the mid-lines represent medians; the tops and bottoms of the bars represent the 25th and 75th percentiles; the whiskers represent the range of the non-outlying data points; and the dots represent outliers.
Fig 2.
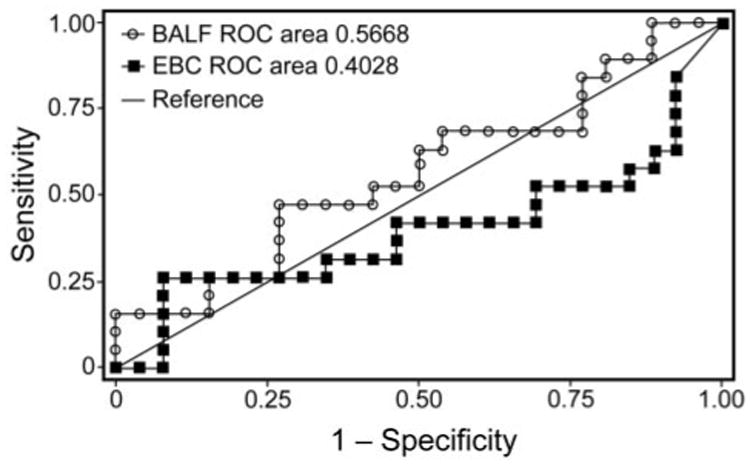
Receiver operating characteristic curves of soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) in bronchoalveolar lavage fluid (BALF) and exhaled breath condensate (EBC) samples.
We constructed a scatter plot to determine whether there was a correlation between BALF and EBC sTREM-1. No statistically significant relationship between the ranks of BALF and EBC sTREM-1 values was detected (Fig. 3). The Spearman rank correlation coefficient (rs) was −0.09.
Fig 3.
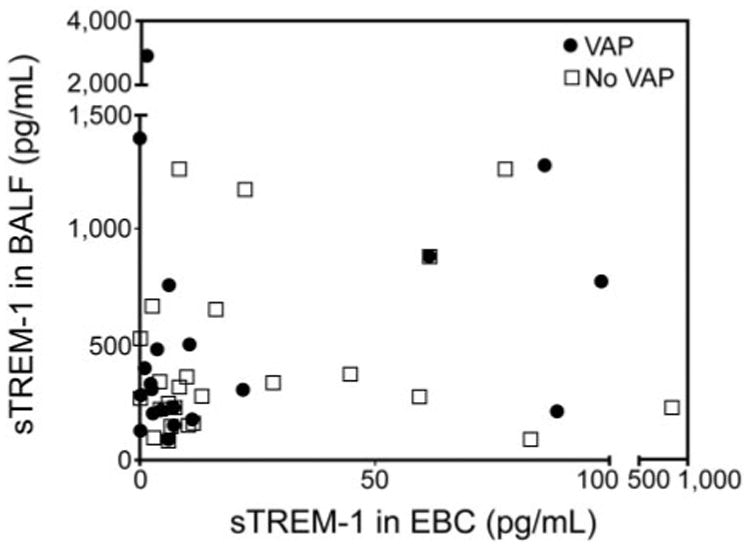
Scatter plot of sTREM-1 levels in bronchoalveolar lavage fluid (BALF) and exhaled breath condensate (EBC) of patients with and without ventilator-associated pneumonia. Spearman Rank correlation coefficient (rs) = −0.09.
sTREM-1 Sensitivity and Specificity and Predictive Values for VAP
A cutoff value of 204 pg/mL for sTREM-1 in BALF resulted in a sensitivity of 79% and specificity of 23%, correctly classifying 47% of the patients with VAP. The positive predictive value was 43%, and the negative predictive value of BALF sTREM-1 levels was 60%. A cutoff value of 10 pg/mL for sTREM-1 in EBC resulted in a sensitivity of 42% and a specificity of 50%, correctly classifying 47% of the patients with VAP. The positive predictive value of EBC sTREM-1 levels was 38%, and the negative predictive value of EBC sTREM-1 levels was 54%.
Discussion
In our study, sTREM-1 in BALF was not a good predictor of VAP among critically ill patients undergoing direct bronchoscopy. Our findings are consistent with more recent studies that report the lower sensitivity and specificity of sTREM-1 to detect patients with pneumonia.28,30,31 An important explanation for the differences between earlier study results may be in the collection technique used for BALF retrieval. Initial studies utilized nondirected bronchoalveolar lavage to retrieve fluid from the lung.8,29 This technique has a concordance rate of 80%, compared to direct bronchoscopic methods.1 Without direct visualization, alveolar segments may have been under sampled, accounting for less retrieval of sTREM-1. Additionally, an earlier study28 used CPIS to classify patients as VAP positive or VAP negative, which may have resulted in misclassification.
The difference in antibiotic use between groups was statistically significant (see Table 1), although none of the patients had an addition or change in antibiotic therapy in the preceding 72 hours, in accordance with American Thoracic Society guidelines on the diagnosis of VAP.34 Prior antibiotic use may have resulted in VAP positive patients being misclassified as VAP negative. Several studies have shown that appropriate antibiotic therapy decreases sTREM-1 levels.26,29 Indeed, monitoring of serial sTREM-1 levels has been proposed as a method to guide the duration of antibiotic therapy. Thus, both bacterial colony counts and sTREM-1 levels should decrease with prior appropriate antibiotic use. However, the kinetics of the decrease is unknown. Therefore, it is hard to predict the effect on sensitivity and specificity of sTREM-1 levels, compared to diagnosis of VAP by colony counts.
To date, our study and that of Horonenko et al28 are the only studies demonstrating detectable sTREM-1 levels in EBC collected directly from the expiratory lines of mechanically ventilated patients with a clinical suspicion of VAP. Horonenko et al detected sTREM-1 in the EBC s of 71% of VAP positive patients and 11% of VAP negative patients. Using a similar method of EBC collection, we detected sTREM-1 in 84% of VAP positive patients, but also in 92% of VAP negative patients. There are several explanations why we detected EBC sTREM-1 in most of our VAP negative patients. Horonenko et al used CPIS to classify patients as VAP positive or VAP negative, which may result in misclassification of VAP.1,9,10,27 Gibot et al8 measured sTREM-1 by immunoblot analysis, whereas we measured sTREM-1 levels by enzyme-linked immunosorbent assay, a more clinically useful technique.28,30,31 Our lower limit of detection of sTREM-1 was 2.5 pg/mL, compared to 5 pg/mL in the study by Gibot et al,8 and 7 pg/mL in the study by Horonenko et al.28 Of note, an earlier version of the sTREM-1 quantikine assay from R&D Systems was recalled in 2008, due to its inability to properly measure soluble TREM-1 concentrations. We used the second generation TREM-1 quantikine assay for all of our measurements.
Since the original study by Gibot, a number of studies have demonstrated up-regulation of sTREM-1 in an increasing number of non-infectious, inflammatory processes,19–21,35 thereby questioning the ability of sTREM-1 to discriminate between infectious and non-infectious causes in the ICU setting. Non-infectious causes of fever and pulmonary infiltrates in patients with clinically suspected VAP may include pancreatitis, chemical aspiration, ARDS, and drug fever.36 In addition, non-pulmonary causes of infection may account for some of the clinical presentations and may have led to elevated sTREM-1 levels. Our study did not address whether non-pulmonary source of infection or non-infectious source of fever were subsequently identified in the setting of negative quantitative cultures.
EBC sTREM-1 levels were lower than BALF sTREM-1 levels in both our study and that of Horonenko et al, but did not reach statistical significance in correlational analysis. The reduction of EBC sTREM-1, compared to BALF sTREM-1, may be a reflection of the dilutional effects of water vapor and the non-standardized method of collection in both studies. The EBC approach is limited by the variable dilution of the airway lining fluid by water vapor. Most inflammatory mediators are not volatile and can only be transferred from the lung by convective processes (droplet formation).37 This may be an important confounder in our study, as we did not correct for the variable distribution of water vapor collected in the tubing.
Conclusions
In conclusion, although sTREM-1 was detectable in BAL and EBC fluids, EBC and BALF sTREM-1 levels did not accurately categorize patients as VAP positive or VAP negative when using direct bronchoscopic quantitative culture samples as the comparison standard. Nonetheless, our study demonstrates that potential biomarkers of infection can be routinely detected in EBC and suggests that standardization of EBC collection may improve sensitivity and specificity of select biomarkers, and provide a noninvasive method of detection in future studies. At this time we cannot recommend the routine use of sTREM-1 levels in BALF or EBC to diagnose patients with VAP.
Quick Look.
Current knowledge
Ventilator-associated pneumonia is the most common nosocomial infection in the ICU and is associated with increased costs, morbidity, and mortality. The diagnosis of VAP using clinical criteria, the Clinical Pulmonary Infection Score, or quantitative culture from bronchoalveolar lavage each have shortcomings. Triggering receptor expressed on myeloid cells-1 (TREM-1) is up-regulated in the presence of inflammation and may be a marker for infection.
What this paper contributes to our knowledge
The presence of TREM-1 in bronchoalveolar lavage fluid was a poor predictor of ventilator-associated pneumonia in patients undergoing direct bronchoscopy.
Acknowledgments
This work was partly supported by National Institutes of Health grant K24 HL068796, Center for Intracellular Delivery of Biologics Life Sciences Discovery Fund grants 2496490 (to LMS) and F31 NR011390–01 (to SJP), and a Chisholm Foundation Achievement Rewards for College Scientists fellowship (to SJP).
Footnotes
The authors have disclosed no conflicts of interest.
Contributor Information
Dr Steven J Palazzo, Center for Lung Biology, Division of Pulmonary and Critical Care, Department of Medicine, University of Washington, Seattle, Washington; Department of Biobehavioral Nursing and Health Systems, School of Nursing, University of Washington, Seattle, Washington.
Dr Terri A Simpson, Department of Biobehavioral Nursing and Health Systems, School of Nursing, University of Washington, Seattle, Washington.
Ms Jillian M Simmons, Department of Respiratory Care, Harborview Medical Center, University of Washington, Seattle, Washington.
Dr Lynn M Schnapp, Center for Lung Biology, Division of Pulmonary and Critical Care, Department of Medicine, University of Washington, Seattle, Washington; Department of Biobehavioral Nursing and Health Systems, School of Nursing, University of Washington, Seattle, Washington.
References
- 1.Chastre J, Fagon J. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165(7):867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 2.Rello J, Ollendorf D, Oster G, Vera-Llonch M, Bellm L, Redman R, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122(6):2115–2121. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- 3.Sandiumenge A, Diaz E, Rodriguez A, Vidaur L, Canadell L, Olona M, et al. Impact of diversity of antibiotic use on the development of antimicrobial resistance. J Antimicrob Chemother. 2006;57(6):1197–1204. doi: 10.1093/jac/dkl097. [DOI] [PubMed] [Google Scholar]
- 4.Fagon J, Chastre J, Wolff M, Gervais C, Parer-Aubas S, Stéphan F, et al. Invasive and noninvasive strategies for management of suspected ventilator-associated pneumonia. A randomized trial. Ann Intern Med. 2000;132(8):621–630. doi: 10.7326/0003-4819-132-8-200004180-00004. [DOI] [PubMed] [Google Scholar]
- 5.Iregui M, Ward S, Sherman G, Fraser V, Kollef M. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest. 2002;122(1):262–268. doi: 10.1378/chest.122.1.262. [DOI] [PubMed] [Google Scholar]
- 6.Pugin J, Auckenthaler R, Mili N, Janssens J, Lew P, Suter P. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis. 1991;143(5 Pt 1):1121–1129. doi: 10.1164/ajrccm/143.5_Pt_1.1121. [DOI] [PubMed] [Google Scholar]
- 7.Fartoukh M, Maitre B, Honoré S, Cerf C, Zahar J, Brun-Buisson C. Diagnosing pneumonia during mechanical ventilation: the clinical pulmonary infection score revisited. Am J Respir Crit Care Med. 2003;168(2):173–179. doi: 10.1164/rccm.200212-1449OC. [DOI] [PubMed] [Google Scholar]
- 8.Gibot S, Cravoisy A, Levy B, Bene M, Faure G, Bollaert P. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med. 2004;350(5):451–458. doi: 10.1056/NEJMoa031544. [DOI] [PubMed] [Google Scholar]
- 9.Luyt C, Chastre J, Fagon J. Value of the clinical pulmonary infection score for the identification and management of ventilator-associated pneumonia. Intensive Care Med. 2004;30(5):844–852. doi: 10.1007/s00134-003-2125-0. [DOI] [PubMed] [Google Scholar]
- 10.Croce M, Swanson J, Magnotti L, Claridge J, Weinberg J, Wood G, et al. The futility of the clinical pulmonary infection score in trauma patients. J Trauma. 2006;60(3):523–527. doi: 10.1097/01.ta.0000204033.78125.1b. discussion 527-528. [DOI] [PubMed] [Google Scholar]
- 11.Inglis T, Millar M, Jones J, Robinson D. Tracheal tube biofilm as a source of bacterial colonization of the lung. J Clin Microbiol. 1989;27(9):2014–2018. doi: 10.1128/jcm.27.9.2014-2018.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adair C, Gorman S, Feron B, Byers L, Jones D, Goldsmith C, et al. Implications of endotracheal tube biofilm for ventilator-associated pneumonia. Intensive Care Med. 1999;25(10):1072–1076. doi: 10.1007/s001340051014. [DOI] [PubMed] [Google Scholar]
- 13.Mayhall C. Ventilator-associated pneumonia or not? Contemporary diagnosis. Emerg Infect Dis. 2001;7(2):200–204. doi: 10.3201/eid0702.010209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164(10):4991–4995. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- 15.Bouchon A, Hernández-Munain C, Cella M, Colonna M. A DAP12-mediated pathway regulates expression of CC chemokine receptor 7 and maturation of human dendritic cells. J Exp Med. 2001;194(8):1111–1122. doi: 10.1084/jem.194.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colonna M, Facchetti F. TREM-1 (triggering receptor expressed on myeloid cells): a new player in acute inflammatory responses. J Infect Dis. 2003;187(Suppl 2):S397–S401. doi: 10.1086/374754. [DOI] [PubMed] [Google Scholar]
- 17.Bleharski J, Kiessler V, Buonsanti C, Sieling P, Stenger S, Colonna M, et al. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J Immunol. 2003;170(7):3812–3818. doi: 10.4049/jimmunol.170.7.3812. [DOI] [PubMed] [Google Scholar]
- 18.Nathan C, Ding A. TREM-1: a new regulator of innate immunity in sepsis syndrome. Nat Med. 2001;7(5):530–532. doi: 10.1038/87846. [DOI] [PubMed] [Google Scholar]
- 19.Ferat-Osorio E, Wong-Baeza I, Esquivel-Callejas N, Figueroa-Figueroa S, Duarte-Rojo A, Guzmán-Valdivia-Gómez G, et al. Triggering receptor expressed on myeloid cells-1 expression on monocytes is associated with inflammation but not with infection in acute pancreatitis. Crit Care. 2009;13(3):R69. doi: 10.1186/cc7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tzivras M, Koussoulas V, Giamarellos-Bourboulis EJ, Tzivras D, Tsaganos T, Koutoukas P, et al. Role of soluble triggering receptor expressed on myeloid cells in inflammatory bowel disease. World J Gastroenterol. 2006;12(21):3416–3419. doi: 10.3748/wjg.v12.i21.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamei K, Yasuda T, Ueda T, Qiang F, Takeyama Y, Shiozaki H. Role of triggering receptor expressed on myeloid cells-1 in experimental severe acute pancreatitis. J Hepatobiliary Pancreat Sci. 2010;17(3):305–312. doi: 10.1007/s00534-009-0191-6. [DOI] [PubMed] [Google Scholar]
- 22.Bouchon A, Facchetti F, Weigand M, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410(6832):1103–1107. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 23.Knapp S, Gibot S, de Vos A, Versteeg H, Colonna M, van der Poll T. Cutting edge: expression patterns of surface and soluble triggering receptor expressed on myeloid cells-1 in human endotoxemia. J Immunol. 2004;173(12):7131–7134. doi: 10.4049/jimmunol.173.12.7131. [DOI] [PubMed] [Google Scholar]
- 24.Gibot S, Kolopp-Sarda M, Béné M, Bollaert P, Lozniewski A, Mory F, et al. A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J Exp Med. 2004;200(11):1419–1426. doi: 10.1084/jem.20040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahdy A, Lowes D, Galley H, Bruce J, Webster N. Production of soluble triggering receptor expressed on myeloid cells by lipopoly-saccharide-stimulated human neutrophils involves de novo protein synthesis. Clin Vaccine Immunol. 2006;13(4):492–495. doi: 10.1128/CVI.13.4.492-495.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibot S, Cravoisy A, Kolopp-Sarda MN, Béné MC, Faure G, Bollaert PE, et al. Time-course of sTREM (soluble triggering receptor expressed on myeloid cells)-1, procalcitonin, and C-reactive protein plasma concentrations during sepsis. Crit Care Med. 2005;33(4):792–796. doi: 10.1097/01.ccm.0000159089.16462.4a. [DOI] [PubMed] [Google Scholar]
- 27.Huh J, Lim C, Koh Y, Oh Y, Shim T, Lee S, et al. Diagnostic utility of the soluble triggering receptor expressed on myeloid cells-1 in bronchoalveolar lavage fluid from patients with bilateral lung infiltrates. Crit Care. 2008;12(1):R6. doi: 10.1186/cc6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horonenko G, Hoyt J, Robbins R, Singarajah C, Umar A, Pattengill J, et al. Soluble triggering receptor expressed on myeloid cell-1 is increased in patients with ventilator-associated pneumonia: a preliminary report. Chest. 2007;132(1):58–63. doi: 10.1378/chest.06-2731. [DOI] [PubMed] [Google Scholar]
- 29.Determann R, Millo J, Gibot S, Korevaar J, Vroom M, van der Poll T, et al. Serial changes in soluble triggering receptor expressed on myeloid cells in the lung during development of ventilator-associated pneumonia. Intensive Care Med. 2005;31(11):1495–1500. doi: 10.1007/s00134-005-2818-7. [DOI] [PubMed] [Google Scholar]
- 30.Anand N, Zuick S, Klesney-Tait J, Kollef M. Diagnostic implications of soluble triggering receptor expressed on myeloid cells-1 in BAL fluid of patients with pulmonary infiltrates in the ICU. Chest. 2009;135(3):641–647. doi: 10.1378/chest.08-1829. [DOI] [PubMed] [Google Scholar]
- 31.Oudhuis GJ, Beuving J, Bergmans D, Stobberingh EE, ten Velde G, Linssen CF, et al. Soluble Triggering Receptor Expressed on Myeloid cells-1 in bronchoalveolar lavage fluid is not predictive for ventilator-associated pneumonia. Intensive Care Med. 2009;35(7):1265–1270. doi: 10.1007/s00134-009-1463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J, Ryu S, Gharib SA, Goodlett DR, Schnapp LM. Exploration of the normal human bronchoalveolar lavage fluid proteome. Proteomics Clin Appl. 2008;2(4):585–595. doi: 10.1002/prca.200780006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinberg KP, Milberg JA, Martin TR, Maunder RJ, Cockrill BA, Hudson LD. Evolution of bronchoalveolar cell populations in the adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;150(1):113–122. doi: 10.1164/ajrccm.150.1.8025736. [DOI] [PubMed] [Google Scholar]
- 34.American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 35.Ferat-Osorio E, Esquivel-Callejas N, Wong-Baeza I, Aduna-Vicente R, Arriaga-Pizano L, Sánchez-Fernández P, et al. The increased expression of TREM-1 on monocytes is associated with infectious and noninfectious inflammatory processes. J Surg Res. 2008;150(1):110–117. doi: 10.1016/j.jss.2007.12.805. [DOI] [PubMed] [Google Scholar]
- 36.Meduri GU, Mauldin GL, Wunderink RG, Leeper KV, Jr, Jones CB, Tolley E, et al. Causes of fever and pulmonary densities in patients with clinical manifestations of ventilator-associated pneumonia. Chest. 1994;106(1):221–235. doi: 10.1378/chest.106.1.221. [DOI] [PubMed] [Google Scholar]
- 37.Effros RM, Biller J, Foss B, Hoagland K, Dunning MB, Castillo D, et al. A simple method for estimating respiratory solute dilution in exhaled breath condensates. Am J Respir Crit Care Med. 2003;168(12):1500–1505. doi: 10.1164/rccm.200307-920OC. [DOI] [PubMed] [Google Scholar]


