Abstract
We aim to summarize data from studies of trastuzumab in patients with human epidermal growth factor receptor 2 (HER2)–positive metastatic breast cancer (MBC) and brain metastasis and to describe novel methods being developed to circumvent the blood–brain barrier (BBB). A literature search was conducted to obtain data on the clinical efficacy of trastuzumab and lapatinib in patients with HER2-positive MBC and brain metastasis, as well as the transport of therapeutic molecules across the BBB. Trastuzumab-based therapy is the standard of care for patients with HER2-positive MBC. Post hoc and retrospective analyses show that trastuzumab significantly prolongs overall survival when given after the diagnosis of central nervous system (CNS) metastasis; this is probably attributable to its control of extracranial disease, although trastuzumab may have a direct effect on CNS disease in patients with local or general perturbation of the BBB. In patients without a compromised BBB, trastuzumab is thought to have limited access to the brain, because of its relatively large molecular size. Several approaches are being developed to enhance the delivery of therapeutic agents to the brain. These include physical or pharmacologic disruption of the BBB, direct intracerebral drug delivery, drug manipulation, and coupling drugs to transport vectors. Available data suggest that trastuzumab extends survival in patients with HER2-positive MBC and brain metastasis. Novel methods for delivery of therapeutic agents into the brain could be used in the future to enhance access to the CNS by trastuzumab, thereby improving its efficacy in this setting.
Keywords: Blood–brain barrier, Trastuzumab, HER2-positive, Metastatic breast cancer, Central nervous system, Brain metastases
Introduction
The number of patients found to have central nervous system (CNS) metastasis is increasing,1 with an estimated incidence of up to 170,000 cases per year in the United States alone.2,3 This increase is due to several factors that include earlier and more accurate detection of CNS disease4 and the increasing availability of improved treatments for systemic disease.1
Brain metastasis is documented in 10–16% of patients with metastatic breast cancer (MBC) during the course of their disease. 5,6 However, a study of data from autopsies suggests that up to 30% of patients with MBC have CNS involvement.7 Furthermore, since brain metastases generally occur late in the course of MBC, a result of the improving overall survival (OS) rate in these patients is that the incidence of brain metastasis will likely increase.8 Breast cancer brain metastasis is associated with a poor prognosis with 1- and 2-year survival rates of 20% and 2%, respectively.9–11
The overexpression of human epidermal growth factor receptor 2 (HER2) on the surface of breast cancer cells is known to be a risk factor for brain metastasis.12–15 The HER2-directed monoclonal antibody (mAb) trastuzumab (Herceptin®, Genentech, Inc.; South San Francisco, CA) in combination with chemotherapy is the standard of care for patients with HER2-positive MBC. However no HER2-directed agent, including trastuzumab, is approved for the treatment of brain metastasis. Like many chemotherapeutic and other large biologic agents, the ability of trastuzumab to cross the blood–brain barrier (BBB) when administered as an intravenous infusion is limited. This review discusses the clinical utility of trastuzumab and describes how novel approaches to enhancing drug delivery to the CNS could be applied to trastuzumab for the treatment of HER2-positive breast cancer brain metastasis.
Current standard of care for CNS metastases
Treatment strategies for CNS metastases aim to improve locoregional CNS disease control. Those treatments currently in use include various combinations of surgery, stereotactic radiosurgery (SRS), whole brain radiotherapy (WBRT), and chemotherapy. Strategies designed to control symptoms, such as corticosteroids to reduce peritumoral edema and anticonvulsants to prevent recurrent seizures, are combined with these therapeutic approaches.
Surgery and radiosurgery can be effective treatment options for patients with limited CNS disease burden.16–19 Randomized prospective studies have demonstrated that for a solitary CNS metastasis, an improved median survival rate of 10 months is achieved after surgical resection and WBRT compared with 4–6 months following WBRT alone.20,21 However, with only a minority of CNS brain metastases demonstrating solitary disease,6,22 surgery is not an option for a majority of patients. Notably, the results from two recent studies have demonstrated that SRS alone may provide a comparable survival advantage compared with SRS in combination with WBRT in patients with limited CNS disease.23,24 Conflicting reports do exist in terms of the neurocognitive effects of WBRT, and its risks and benefits are currently the subject of much debate and study.
Prophylactic WBRT is routinely used in small cell lung cancer as it clearly reduces the incidence of brain metastases and improves survival with a relatively low frequency of neurotoxicity.25,26 The limited data currently available, however, suggest that prophylactic WBRT may not be appropriate for advanced breast cancer because the risks of this therapy may outweigh the benefits.27 Neuroimaging can be used to detect occult brain metastases in patients with HER2-positive breast cancer.5,28,29 Limited data suggest that while overall survival remains the same whether WBRT is given to patients with symptomatic or nonsymptomatic brain metastases identified with neuroimaging techniques,5,29 the rate of death due to progression within the brain is threefold lower when WBRT is administered to patients with asymptomatic brain metastases.29 Given the potential improvement in cognitive function that may be gained by controlling the growth of these metastases, further investigation of the impact of routine screening for occult brain metastases on patient care is warranted.
Because CNS metastasis is a stipulated exclusion criterion in most clinical trials of systemic chemotherapy in patients with breast cancer, there are limited clinical data reporting the efficacy of systemic therapy for breast cancer brain metastases; data that are available have, for the most part, been disappointing.15
Evidence for HER2 expression in CNS metastases
There is a substantial body of clinical trial data demonstrating that patients with HER2-positive MBC are two to four times more likely to develop brain metastases than patients with HER2-negative disease13,30 and that HER2-positive status is a significant risk factor for CNS relapse.14,31–33
Studies performed before the use of adjuvant trastuzumab therapy became commonplace offer valuable insight into the association between breast cancer brain metastasis and HER2 status. In a large retrospective study of 9524 women with early-stage breast cancer (EBC) who were enrolled in 10 clinical trials between 1978 and 1999, the 10-year cumulative incidences of CNS as first relapse site were 2.7% and 1.0% in patients with HER2-positive and HER2- negative tumors, respectively (P < 0.01).33 Similarly, the cumulative incidences of CNS metastases as either a first or subsequent event were greater in patients with HER2-positive compared with HER2-negative disease (6.8% vs 3.5%; P < 0.01). These data are in accordance with the findings of an earlier study in 319 patients that showed a significantly increased risk of lung, liver, and brain metastases in patients with HER2-positive compared with HER2- negative disease (P = 0.0002).34
The etiology of the higher incidence of brain metastasis in HER2-positive breast cancer has not yet been clearly defined and is most likely multifactorial. Contributing factors include the more aggressive nature of HER2-positive compared with HER2-negative disease34,35 and the ability of HER2 to increase brain colonization via downstream targets, such as the protumorigenic and prometastatic enzyme heparanase.36
Although the major, prospective clinical trials of adjuvant trastuzumab did not reveal an increased risk of brain metastasis,37,38 retrospective analyses have reported an apparent increase in CNS disease in patients with HER2-positive disease who received trastuzumab compared with historical controls.39–41 In a recent population- based registry study in 1458 patients with EBC, CNS as first recurrence was documented for 0.6% of patients with HER2-negative disease and for 4% and 1.2% of patients with HER2-positive disease who had and had not received adjuvant trastuzumab, respectively.32 Time to CNS as first recurrence, however, was significantly prolonged in patients with HER2-positive disease who received adjuvant trastuzumab (20.3 months) compared with patients with HER2-negative disease (19.8 months) and those with HER2-positive disease who did not receive trastuzumab (10.3 months) (P = 0.018).
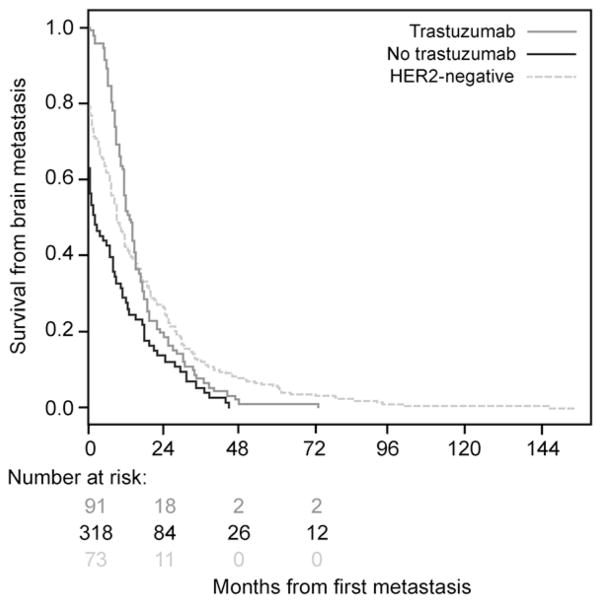
Another study in 598 patients with invasive breast cancer and CNS metastases showed that time to CNS recurrence was significantly prolonged after trastuzumab (13.1 vs 2.1 months in trastuzumab-naive disease (P = 0.0008) and 8.9 months in HER2- negative disease (Fig. 1)).42 Together, these data indicate that trastuzumab does not itself increase the risk of brain metastasis but that the CNS may represent a potential sanctuary site in patients with HER2-positive disease who are treated with trastuzumab; in effect, the prolonged survival afforded by trastuzumab allows the so-called unmasking of CNS recurrence that would otherwise remain clinically silent before death.
Fig. 1.
Time to central nervous system recurrence in patients with HER2-negative, or HER2-positive metastatic breast cancer who had and had not received treatment with trastuzumab. HER2 indicates human epidermal growth factor receptor 2. Reproduced with permission from Dawood et al.42
The blood–brain barrier
Structure and function
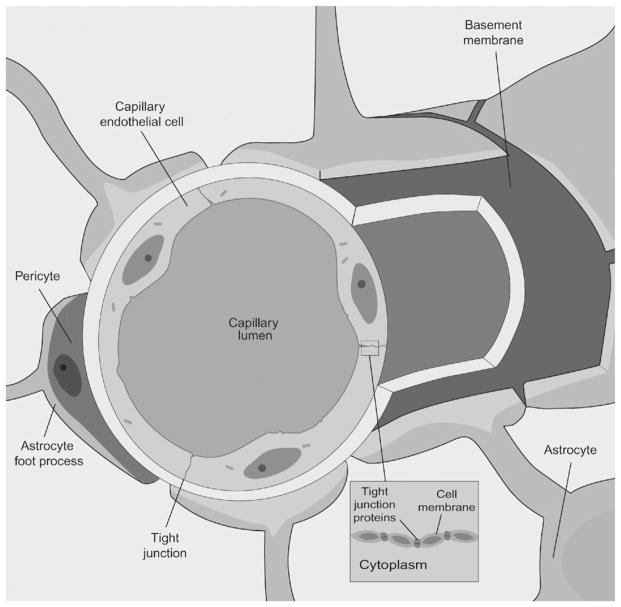
The BBB is composed of capillary endothelial cells with tight intercellular junctions, pericytes, a basement membrane, and astrocyte foot processes (Fig. 2). The BBB regulates bidirectional control over the passage of a large diversity of regulatory proteins, nutrients, and electrolytes, as well as potential neurotoxins, into the brain.43 The passage of molecules across the BBB is regulated by complex transport systems that can prevent many drugs from entering the CNS, including a number of chemotherapeutic and molecular-targeted agents. In addition, efflux pumps, such as P-glycoprotein (PgP), impede some chemotherapeutic agents from penetrating the brain by actively removing them.44
Fig. 2.
Structure of the blood–brain barrier (BBB). The cerebral capillaries are composed of endothelial cells that are sealed by tight junctions. The capillaries are in close contact with pericytes and are ensheathed by astrocyte foot processes and the basal lamina.43 The passage of molecules across the BBB is tightly regulated; some hydrophilic molecules enter the brain via specific transporters and carrier-mediated endocytosis, and a limited number cross the barrier via diffusion through tight junctions.44
Tumor cell penetration of the BBB
The metastatic process into the CNS requires that the tumor cell traverse the BBB.45 It is thought that tumor cells shed from the primary tumor enter the circulation and become trapped in brain capillary beds at vascular branch points. Subsequent changes in the local environment, including the secretion of factors by brain vascular endothelial cells, astrocytes, and tumor cells, then promote tumor cell proliferation and invasion into the CNS.
The integrity of the BBB can be compromised either as a result of neuropathology or because of a precipitating event.43 Perturbation of the BBB leads to an increase in permeability and an edematous response. When metastatic tumors grow beyond 1 mm to 2 mm within the brain parenchyma, the BBB becomes structurally and functionally compromised, as evidenced by homogeneous contrast enhancement on magnetic resonance imaging (MRI). In an experimental brain metastasis model, lesions ≥0.2 mm2 were associated with perturbation of the BBB.46 Moreover, CNS metastases from lung adenocarcinomas and melanomas can induce a significant reduction in PgP expression.44 Recently published evidence also suggests that the integrity of the BBB may be influenced by the phenotype of the metastatic tumor cell. In a small study in 29 patients with breast cancer brain metastases, BBB disruption was observed more frequently with triple-negative (i.e., estrogen receptor–negative, progesterone receptor–negative, HER2-negative) tumors than with HER2-positive tumors.47
Antibody penetration of the BBB
Since their introduction, therapeutic mAbs have become widely used in all fields of medicine, including oncology. However, the usefulness of systemic chemotherapies and targeted agents for the treatment of neurologic disease has been called into question as many of these agents are thought to be too large (i.e., molecular mass >450 kDa) or hydrophilic to routinely cross the BBB at relevant levels.15,48 This is supported by a study reporting cerebrospinal fluid (CSF) concentrations of trastuzumab 420-fold lower than in serum in patients treated with trastuzumab prior to radiotherapy. 49 In addition, preclinical studies assessing tissue distribution of zirconium 89–labeled trastuzumab (89Zr-trastuzumab) in animals bearing HER2-positive tumor xenografts showed minimal penetration into the brain.50 Penetration of the tumor by mAbs and other agents may also be hindered by increased intratumoral interstitial pressure, which can both limit the uptake of the agent into the tumor and lead to nonuniform distribution of the agent within it,51 or by the binding of mAbs to tumor antigens near the blood vessel egress site, thereby blocking access of therapeutic mAbs to tumor antigens deeper within the tumor—the so-called binding site barrier.52,53
Recently, however, a study using positron electron tomography imaging demonstrated CNS penetration by 89Zr-trastuzumab in patients with MBC with an 18-fold higher uptake in brain tumors than in normal brain tissue (Fig. 3).54 This increased penetration is likely because of a BBB that is compromised because of MBC (i.e., local or general perturbation). It is interesting to note that tumor- induced perturbation of the BBB would, in effect, aid targeted delivery of trastuzumab, as sites of BBB leakiness would be correlated with the presence of a tumor, at least at a first level of approximation. The increased penetration could also be explained by the effect of antigen-rich sites within the tumors (e.g., necrotic areas), which may serve as so-called antigen sinks for trastuzumab. 55 Another potential mechanism for transporting trastuzumab across the BBB is the Fc receptor for immunoglobulin G (FcRn), which is highly expressed on vessels in the brain.56
Fig. 3.
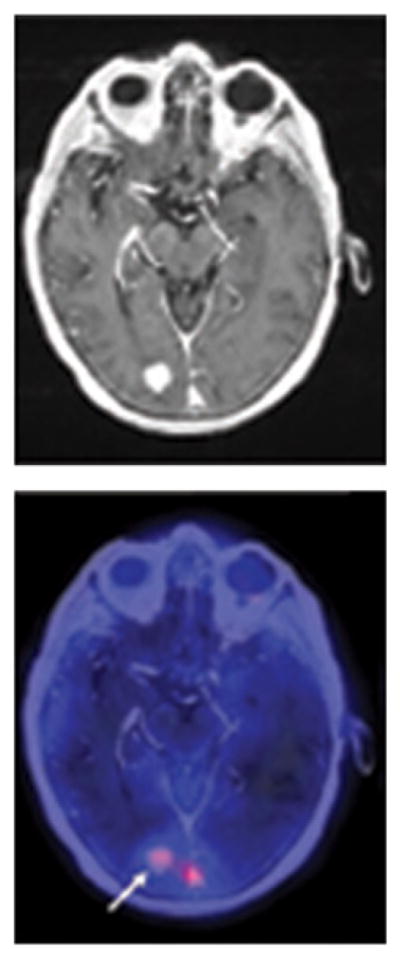
PET image showing penetration of the brain in a patient with HER2-positive metastatic breast cancer. Arrow indicates a HER2-positive brain lesion. HER2 indicates human epidermal growth factor receptor 2; PET, positron emission tomography; 89Zr-trastuzumab, zirconium 89–labeled trastuzumab. Reproduced with permission from Dijkers et al.54
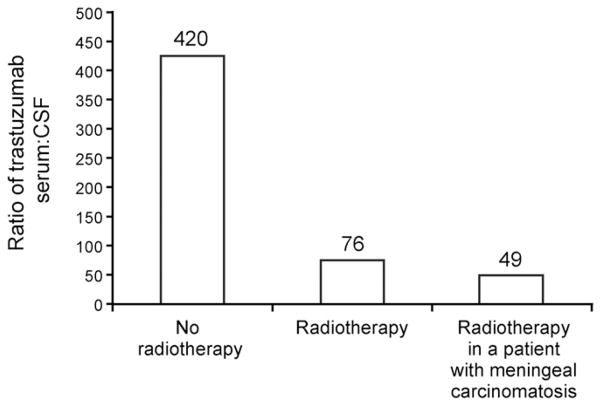
Radiotherapy may also affect the integrity of the BBB. Changes in BBB permeability have been reported after WBRT in preclinical and clinical evaluations.57 The influence of WBRT on mAb uptake in the brain was examined in a pilot study in patients with HER2-positive breast cancer brain metastases. This study demonstrated increased trastuzumab uptake after WBRT; the median serum CSF trastuzumab concentration ratios were 420:1 and 76:1 pre- and post-WBRT (Fig. 4).49 Notably, a low pre-WBRT ratio was observed in two patients with concomitant meningeal carcinomatosis (ratio, 49:1). However, a case study in a patient with meningeal carcinomatosis reported serum trastuzumab levels 300-fold greater than CSF levels.58
Fig. 4.
Concentrations of functional, reactive trastuzumab in serum and cerebrospinal fluid (CSF) of patients with metastatic breast cancer in relation to clinical parameters. Reproduced with permission from Stemmler.49
Taken together, these data suggest that trastuzumab access to the brain may be markedly increased in patients with cancer because of the perturbations in the BBB caused by the cancer itself or by cancer therapy (e.g., WBRT).
Trastuzumab in the management of HER2-positive MBC
Mechanism of action
Trastuzumab is a humanized mAb directed against HER2, which is overexpressed on the surface of 20% to 25% of breast tumors.59–61 The mechanism of the effect of trastuzumab on tumor cell growth and survival is not fully understood,62 but it is thought to incorporate the inhibition of intracellular proliferative signal transduction, 63–65 the inhibition of HER2 extracellular domain shedding,66 the activation of antibody-dependent cellular cytotoxicity,67 and the inhibition of tumor-induced angiogenesis.68,69 It is likely that the balance of the different effector functions of trastuzumab differs depending on the tumor phenotypes and microenvironments. For example, whether antibody-dependent cellular cytotoxicity occurs in the intact brain is unknown.
Clinical trials of trastuzumab in patients with MBC and CNS metastases
Trastuzumab-based therapy is firmly established as the standard of care for patients with HER2-positive breast cancer, owing to demonstrated survival benefits in large, randomized clinical trials in both EBC and MBC with survival end points.37,38,70–72 Evidence obtained from clinical studies also suggests that trastuzumab- based therapy can significantly prolong survival in patients with HER2-positive brain metastases. Median OS in patients with HER2-positive breast cancer who received trastuzumab- based therapy following the diagnosis of brain metastasis ranged from 9.0 to approximately 26 months compared with median OS ranging from 2.0 months to approximately 9 months in patients who did not receive trastuzumab (Table 1).42,73–82 However it must be noted that the available data derive from post hoc and/or retrospective analyses (see Table 1).
Table 1.
Median survival from diagnosis of brain metastasis in patients who did/did not continue to receive trastuzumab-based treatment.a.
| Study/design | N | Median overall survival, months
|
P value | |
|---|---|---|---|---|
| Received trastuzumab after diagnosis of brain metastasis | No trastuzumab following diagnosis of brain metastasis | |||
| Bartsch et al73/retrospective analysis | 53 | 21 (range: 3–38) | 9 (range: 1–14) | <0.001 |
| Bartsch et al74/retrospective analysis | 37 | 13 (95% CI, 8.9–17.2) | 9 (95% CI, 0.0–20.7) | Not reported |
| Brufsky et al75/post hoc analysis of a large registry | 377 | 17.5 | 3.7 | <0.001 |
| Church et al76/retrospective analysis | 26 | 11.9 | 3.0 | 0.05 |
| Dawood et al42/retrospective analysis | 280 | 11.6 | 6.1 | 0.03 |
| Kirsch et al77/retrospective analysis | 47 | ~26 | ~9 | <0.0001 |
| Le Scodan et al78/retrospective analysis | 52 | 19.5 | 5.6 | <0.004 |
| Nam et al79/retrospective analysis | 56 | 12.8 | 4.0 | 0.0011 |
| Park et al80/retrospective analysis | 78 | 13.6 (95% CI, 9.0–18.2) | 5.5 (95% CI, 0.0–13.6) | <0.001 |
| Park et al81/retrospective analysis | 77 | 14.9 (95% CI, 11.6–18.2) | 4.0 (95% CI, 2.1–5.9) | 0.0005 |
| Witzel et al82/restrospective analysis | 29 | 9.0 (95% CI, 7–11) | 2.0 (95% CI, 0.7–3) | 0.006 |
Abbreviations: CI, confidence interval.
English language articles were identified for inclusion by searching PubMed [search terms: trastuzumab AND metastatic breast cancer AND (brain metastases OR central nervous system metastases] for articles published within the last 10 years [01012002 to 01012012] that reported overall survival in patients who had received trastuzumab after diagnosis of brain metastases and those who had not.
Multiple clinical studies have also reported prolonged OS in patients with HER2-positive disease (4.0–22.4 months) compared with those who had HER2-negative disease (3.4–9.4 months), which is largely attributable to the favorable outcomes observed in patients with HER2-positive breast cancer treated with trastuzumab after diagnosis of brain metastases.42,76,77,79 Two of these studies reported median OS durations of 4.0 and 5.5 months in patients with HER2-positive breast cancer that was not treated with trastuzumab.79,80
In patients with invasive breast cancer who went on to develop brain metastases (n = 598), time to development of brain metastasis and survival were extended in patients with HER2-positive disease treated with trastuzumab compared with patients with trastuzumab-naive HER2-positive disease and those who had HER2-negative disease.42 Median time to CNS recurrence was 8.9 months in patients with HER2-negative disease and 13.1 and 2.1 months in patients with HER2-positive disease who had and had not received trastuzumab, respectively. The difference in median time to CNS recurrence between patients with trastuzumab-treated and trastuzumab-naive HER2-positive disease was statistically significant (P = 0.0008; see Fig. 1).
Further evidence of a benefit with trastuzumab after the development of CNS metastases emerged from a retrospective study in 78 patients with HER2-positive breast cancer brain metastases.80 This study showed prolonged median survival in patients who had received trastuzumab after diagnosis of brain metastases compared with patients who received trastuzumab before brain metastasis diagnosis and patients who were trastuzumab-naive (13.6 vs 4.0 and 5.5 months, respectively; P < 0.001). Notably, median time to brain metastasis was more than doubled in patients who received trastuzumab before diagnosis (19 months) compared with patients in the other two groups (7.0 and 8.0 months in the other two groups, respectively).
Breast cancer leptomeningeal metastasis (LM) is comparatively rare, but it is associated with a particularly poor prognosis.75 Promising activity has been observed in case reports with trastuzumab-based therapy in patients with LM. The combination of trastuzumab and capecitabine was effective in a case of MBC and lepto-meningeal/bone metastasis. Combination trastuzumab and capecitabine given as sixth-line treatment resulted in a clinically notable reduction of LM and tumor markers.83 Further case reports also documented the efficacy of intrathecal (IT) trastuzumab in LM, when used either as a high-dose (20 mg weekly) single-agent84 or in combination with chemotherapy, such as thiotepa85 or methotrexate.86
Taken together, the results from these studies provide a substantial body of evidence to support the continued use of trastuzumab therapy in patients with HER2-positive breast cancer and CNS metastases. It is likely that at least some of the benefit afforded by trastuzumab in this setting is attributable to systemic disease control, but radiologically documented regression or prolonged stabilization of CNS metastases achieved in patients treated with trastuzumab-based therapy highlights the potential for a direct effect on CNS disease.87,88 Trastuzumab is likely gaining access to the CNS in these patients via a compromised BBB.
Lapatinib in the management of HER2-positive MBC
Lapatinib is another HER2-targeted agent used in the treatment of breast cancer. Lapatinib differs from trastuzumab in that it is a small molecule reversible tyrosine kinase inhibitor that is designed to target both HER1 (epidermal growth factor receptor) and HER2. Because it is a small molecule (molecular weight <1 kDa), it could, theoretically, cross the BBB and access metastases in the CNS. A preclinical study by Smith and colleagues showed that 14C-lapatinib is able to penetrate breast cancer brain metastases in immunocompromised mice.89 In healthy animals, however, the ability of lapatinib to cross the BBB may be limited. A later preclinical study found that concentrations of lapatinib in the brains of these animals were low, which may have been the result of the effect of efflux transporters in the BBB, such as PgP.90
In the clinical setting, there is little evidence to show that single- agent lapatinib is an effective treatment for patients with HER2-positive breast cancer brain metastases. In a multicenter phase II study (NCI-6969), 39 patients with HER2-positive breast cancer and metastases to the brain received single-agent lapatinib. 91 Because the primary end point of this study (four patients with a CNS response) was not achieved, a volumetric analysis of CNS lesions was carried out. Of the 34 patients assessed, three had a volumetric reduction of 30% and seven had reductions of 10–30%. The clinical significance of these results is, however, unknown.
In a larger study (EGF105084) of single-agent lapatinib in 237 patients with progressive CNS metastases after radiotherapy, CNS objective response (i.e., complete response or ≥50% reduction in the volumetric sum of all measurable CNS lesions) was the primary end point. The CNS response rate was 6% (15/237; all partial responses). 92 At disease progression, 50 patients received further treatment with lapatinib plus capecitabine. Ten of these patients (20%) had an objective CNS response (all partial responses). While these data are encouraging and suggest that lapatinib plus capecitabine may be active in this setting, the omission of a control arm makes the size of the effect difficult to interpret.
A control arm was included in the pivotal phase III trial of lapatinib, which evaluated the efficacy of lapatinib plus capecitabine compared with capecitabine alone.93 An exploratory analysis of CNS involvement at first disease progression determined that the addition of lapatinib to capecitabine decreased the risk of CNS metastasis. In the combination treatment group, only four patients (2%) had developed CNS involvement at first progression compared with 13 patients (6%) in the single-agent capecitabine group. However, it is important to consider that the number of patients who participated in this analysis was small and that the statistical methodology employed to calculate the difference between the two treatment arms was not described.
Metro and colleagues conducted a retrospective analysis of data for patients who received lapatinib plus capecitabine for treatment of their brain metastases.94 They selected patients who had previously received treatment with a taxane, an anthracycline, and trastuzumab, but who were also naive for lapatinib and capecitabine. Of the 22 patients available for response, seven had a partial response (32%) and six had stable disease (27.3%). The duration of response was 6 months, and the median progression-free survival was 5.6 months. Median OS was 27.9 months in the lapatinib-plus-capecitabine group (n = 22) compared with 16.7 months in patients who received trastuzumab-based treatment (n = 23) beyond brain progression (P = 0.01).
More recently, the LANDSCAPE phase II study was conducted to evaluate the use of lapatinib and capecitabine combination therapy to avoid or delay WBRT as first-line treatment for patients with HER2-positive breast cancer brain metastases.95 In this study, an objective response was defined as a ≥50% volumetric reduction of CNS lesions. After a median follow-up of 10 months (range, 2.9–16.5 months), the objective response in the CNS in 43 evaluable patients was an impressive 67% (95% confidence interval, 51–81). A recent prospective phase II study comparing lapatinib plus capecitabine with lapatinib plus topotecan in this patient population found a 38% (95% confidence interval, 14–68) CNS objective response rate (i.e., ≥50% volumetric reduction of CNS lesions) in the lapatinib-plus-capecitabine arm. No responses were seen in the lapatinib-plus-topotecan arm.96
The clinical utility of lapatinib continues to be studied in this setting. Currently, lapatinib is being evaluated in combination with temozolomide in a phase I study97 and in the CEREBREL study,98 which uses 11C lapatinib to assess the penetration of this agent into the brains of patients with and without brain metastases.
Enhancing drug delivery to brain tumors
Intraventricular (IVent) administration of mAb therapy represents an opportunity for circumventing the BBB and delivering drugs targeted to leptomeningeal disease. A pilot study of IVent or IT rituximab in six patients with relapsed CNS B-cell lymphoma identified IVent rituximab as a promising strategy.99 Meningeal tumor cells were completely cleared from the CSF of three patients following treatment, and complete remission of leptomeningeal involvement was reported in a fourth patient. A subsequent phase I study in 10 patients showed that IVent rituximab achieved ventricular concentrations that were similar to peak levels of rituximab in serum obtained following administration via intravenous injection. Furthermore, IVent rituximab demonstrated promising activity: meningeal responses in six patients, intraocular responses in two patients, and resolution of brain parenchymal lymphoma in one patient.100
Intracerebral drug delivery via impregnated polymer discs or bolus injection can be used to deliver high concentrations of drugs directly into brain tumors, but its reliance on physical diffusion limits its utility for delivery of mAbs. Alternatively, convection-enhanced delivery has the potential to distribute even large molecules consistently throughout the brain.55,101–105 However, all of these techniques are invasive and have associated administration risks. Noninvasive strategies to penetrate the BBB remain a focus of research in brain disease.
Drug manipulation techniques, such as lipid encapsulation to exploit transcellular lipopholic pathways (Fig. 5), have been explored, but their use is limited for large molecules such as mAbs. The observed activity of therapeutic mAbs in association with disrupted BBB caused by WBRT or meningeal carcinomatosis has provided a sound rationale for the evaluation of strategies for deliberate perturbation of the BBB to augment mAb penetration in the CNS. Pharmacologic approaches to disrupting the BBB include the use of vasoactive and hyperosmotic agents (Fig. 5a). For example, the use of the phosphodiesterase inhibitor vardenafil resulted in a twofold increase in trastuzumab uptake in brain metastases in mouse models.106
Fig. 5.
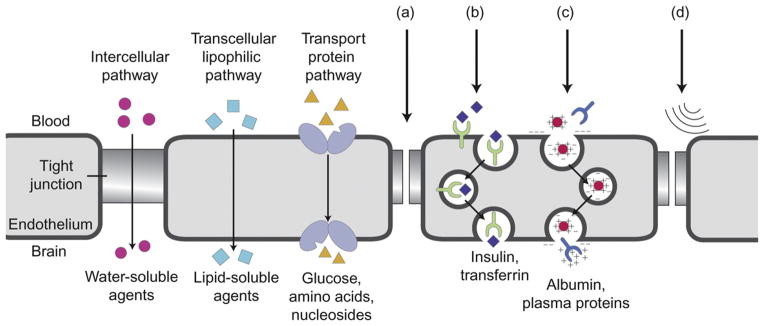
Approaches to enhance drug delivery across the blood–brain barrier (a) bradykinin analogs, (b) receptor-mediated endocytosis, (c) absorptive transcytosis, (d) ultrasound. Adapted with permission from Eichler et al.45.
Drugs can be coupled to transport vectors to assist in drug penetration; proteins such as cationized albumin and mAbs to the transferrin receptor undergo receptor-mediated transcytosis (Fig. 5b) and absorptive-mediated transport (Fig. 5c), respectively, through the BBB. These strategies have been investigated as methods of improving penetration of the CNS by mAbs.
Physical approaches to BBB disruption have also been explored. One of the simplest techniques is to schedule radiotherapy and chemotherapy to increase uptake of cytotoxic and targeted agents. Another method that has been evaluated is the use of ultrasound radiation (Fig. 5d). In a study in mice, Kinoshita et al.107 used MRI-guided focused ultrasound to disrupt the BBB and deliver trastuzumab directly to the CNS. Notably, there was a direct correlation between the amount of trastuzumab delivered and the extent to which the BBB opened. As a result, the amount of trastuzumab delivered to the target tissue could be estimated indirectly.
Conclusions
CNS metastasis affects up to 30% of patients with MBC and is associated with a poor prognosis and substantial morbidity. Current treatment strategies utilize surgery, SRS, WBRT, and chemotherapy in various combinations. Several risk factors have been identified for the development of breast cancer brain metastases, including HER2-positive tumor status. The standard of care for patients with HER2-positive breast cancer is treatment with the HER2-targeted mAb trastuzumab, which is used in combination with chemotherapy. There is a substantial body of clinical evidence that trastuzumab may extend survival in patients with breast cancer brain metastases; these data support the continuation of trastuzumab therapy in patients with breast cancer brain metastases. There are concerns about the usefulness of systemic trastuzumab in patients with breast cancer brain metastases, based on its theoretic inability to cross the BBB. There is evidence, however, that prior WBRT, or even the tumor itself, may compromise the integrity of the BBB, thereby allowing antibody penetration. Current and future research areas for improving the management of HER2-positive CNS metastases include direct delivery of trastuzumab through IVent or IT administration and optimized BBB disruption to achieve enhanced penetration of trastuzumab into the CNS.
Acknowledgments
Role of the funding source
Study sponsors had no involvement in the writing of or decision to submit this manuscript for publication.
Support for third-party writing assistance was provided by Genentech, Inc.
Footnotes
Conflicts of interest statement
Drs. Brufsky, Mehta and Sampson have no financial disclosures impacting this manuscript. This research was supported in part by National Institutes of Health grants 5R25-NS065731-02 (AIM, JHS), RO1-CA97222-05 (JHS), 5P50 NS20023 (DDB, JHS), and 5P50 CA108786 (DDB, JHS), and by grants from the American Brain Tumor Association (JHS), the Accelerate Brain Cancer Cure (JHS), and the Brain Tumor Society (JHS).
Contributor Information
Ankit I. Mehta, Email: ankit.mehta@duke.edu.
Adam M. Brufsky, Email: brufskyam@upmc.edu.
John H. Sampson, Email: john.sampson@duke.edu.
References
- 1.Patel RR, Mehta MP. Targeted therapy for brain metastases: improving the therapeutic ratio. Clin Cancer Res. 2007;13:1675–83. doi: 10.1158/1078-0432.CCR-06-2489. [DOI] [PubMed] [Google Scholar]
- 2.Vogelbaum MA, Suh JH. Resectable brain metastases. J Clin Oncol. 2006;24:1289–94. doi: 10.1200/JCO.2005.04.6235. [DOI] [PubMed] [Google Scholar]
- 3.Chang JE, Robins HI, Mehta MP. Therapeutic advances in the treatment of brain metastases. Clin Adv Hematol Oncol. 2007;5:54–64. [PubMed] [Google Scholar]
- 4.Palmieri D, Chambers AF, Felding-Habermann B, Huang S, Steeg PS. The biology of metastasis to a sanctuary site. Clin Cancer Res. 2007;13:1656–62. doi: 10.1158/1078-0432.CCR-06-2659. [DOI] [PubMed] [Google Scholar]
- 5.Miller KD, Weathers T, Haney LG, et al. Occult central nervous system involvement in patients with metastatic breast cancer: prevalence, predictive factors and impact on overall survival. Ann Oncol. 2003;14:1072–7. doi: 10.1093/annonc/mdg300. [DOI] [PubMed] [Google Scholar]
- 6.Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22:3608–17. doi: 10.1200/JCO.2004.01.175. [DOI] [PubMed] [Google Scholar]
- 7.Tsukada Y, Fouad A, Pickren JW, Lane WW. Central nervous system metastasis from breast carcinoma. Autopsy study Cancer. 1983;52:2349–54. doi: 10.1002/1097-0142(19831215)52:12<2349::aid-cncr2820521231>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 8.Leyland-Jones B. Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. J Clin Oncol. 2009;27:5278–86. doi: 10.1200/JCO.2008.19.8481. [DOI] [PubMed] [Google Scholar]
- 9.DiStefano A, Yong Yap Y, Hortobagyi GN, Blumenschein GR. The natural history of breast cancer patients with brain metastases. Cancer. 1979;44:1913–8. doi: 10.1002/1097-0142(197911)44:5<1913::aid-cncr2820440554>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 10.Patanaphan V, Salazar OM, Risco R. Breast cancer: metastatic pattern and their prognosis. South Med J. 1988;81:1109–12. [PubMed] [Google Scholar]
- 11.Engel J, Eckel R, Aydemir U, et al. Determinants and prognoses of locoregional and distant progression in breast cancer. Int J Radiat Oncol Biol Phys. 2003;55:1186–95. doi: 10.1016/s0360-3016(02)04476-0. [DOI] [PubMed] [Google Scholar]
- 12.Arslan C, Dizdar O, Altundag K. Systemic treatment in breast-cancer patients with brain metastasis. Expert Opin Pharmacother. 2010;11:1089–100. doi: 10.1517/14656561003702412. [DOI] [PubMed] [Google Scholar]
- 13.Gabos Z, Sinha R, Hanson J, et al. Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol. 2006;24:5658–63. doi: 10.1200/JCO.2006.07.0250. [DOI] [PubMed] [Google Scholar]
- 14.Hicks DG, Short SM, Prescott NL, et al. Breast cancers with brain metastases are more likely to be estrogen receptor negative, express the basal cytokeratin CK5/6, and overexpress HER2 or EGFR. Am J Surg Pathol. 2006;30:1097–104. doi: 10.1097/01.pas.0000213306.05811.b9. [DOI] [PubMed] [Google Scholar]
- 15.Steeg PS, Camphausen KA, Smith QR. Brain metastases as preventive and therapeutic targets. Nat Rev Cancer. 2011;11:352–63. doi: 10.1038/nrc3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Black PM, Johnson MD. Surgical resection for patients with solid brain metastases: current status. J Neurooncol. 2004;69:119–24. doi: 10.1023/b:neon.0000041875.09048.e7. [DOI] [PubMed] [Google Scholar]
- 17.Melisko ME, Glantz M, Rugo HS. New challenges and opportunities in the management of brain metastases in patients with ErbB2-positive metastatic breast cancer. Nat Clin Pract Oncol. 2009;6:25–33. doi: 10.1038/ncponc1243. [DOI] [PubMed] [Google Scholar]
- 18.Mahmoud-Ahmed AS, Suh JH, Lee SY, Crownover RL, Barnett GH. Results of whole brain radiotherapy in patients with brain metastases from breast cancer: a retrospective study. Int J Radiat Oncol Biol Phys. 2002;54:810–7. doi: 10.1016/s0360-3016(02)02967-x. [DOI] [PubMed] [Google Scholar]
- 19.Chang EL, Lo S. Diagnosis and management of central nervous system metastases from breast cancer. Oncologist. 2003;8:398–410. doi: 10.1634/theoncologist.8-5-398. [DOI] [PubMed] [Google Scholar]
- 20.Noordijk EM, Vecht CJ, Haaxma-Reiche H, et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys. 1994;29:711–7. doi: 10.1016/0360-3016(94)90558-4. [DOI] [PubMed] [Google Scholar]
- 21.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 22.Lee SS, Ahn JH, Kim MK, et al. Brain metastases in breast cancer: prognostic factors and management. Breast Cancer Res Treat. 2008;111:523–30. doi: 10.1007/s10549-007-9806-2. [DOI] [PubMed] [Google Scholar]
- 23.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–44. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 24.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–91. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 25.Slotman BJ, Senan S. Radiotherapy in small-cell lung cancer: lessons learned and future directions. Int J Radiat Oncol Biol Phys. 2011;79:998–1003. doi: 10.1016/j.ijrobp.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 26.Paumier A, Cuenca X, Le Péchoux C. Prophylactic cranial irradiation in lung cancer. Cancer Treat Rev. 2011;37:261–5. doi: 10.1016/j.ctrv.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Huang F, Alrefae M, Langleben A, Roberge D. Prophylactic cranial irradiation in advanced breast cancer: a case for caution. Int J Radiat Oncol Biol Phys. 2009;73:752–8. doi: 10.1016/j.ijrobp.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 28.Lai R, Dang CT, Malkin MG, Abrey LE. The risk of central nervous system metastases after trastuzumab therapy in patients with breast carcinoma. Cancer. 2004;101:810–6. doi: 10.1002/cncr.20418. [DOI] [PubMed] [Google Scholar]
- 29.Niwińska A, Tacikowska M, Murawska M. The effect of early detection of occult brain metastases in HER2-positive breast cancer patients on survival and cause of death. Int J Radiat Oncol Biol Phys. 2010;77:1134–9. doi: 10.1016/j.ijrobp.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 30.Kaal EC, Vecht CJ. CNS complications of breast cancer: current and emerging treatment options. CNS Drugs. 2007;21:559–79. doi: 10.2165/00023210-200721070-00003. [DOI] [PubMed] [Google Scholar]
- 31.Sanna G, Franceschelli L, Rotmensz N, et al. Brain metastases in patients with advanced breast cancer. Anticancer Res. 2007;27:2865–9. [PubMed] [Google Scholar]
- 32.Musolino A, Ciccolallo L, Panebianco M, et al. Multifactorial central nervous system recurrence susceptibility in patients with HER2-positive breast cancer: epidemiological and clinical data from a population-based cancer registry study. Cancer. 2011;117:1837–46. doi: 10.1002/cncr.25771. [DOI] [PubMed] [Google Scholar]
- 33.Pestalozzi BC, Zahrieh D, Price KN, et al. Identifying breast cancer patients at risk for central nervous system (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG) Ann Oncol. 2006;17:935–44. doi: 10.1093/annonc/mdl064. [DOI] [PubMed] [Google Scholar]
- 34.Kallioniemi OP, Holli K, Visakorpi T, Koivula T, Helin HH, Isola JJ. Association of c-erbB-2 protein over-expression with high rate of cell proliferation, increased risk of visceral metastasis and poor long-term survival in breast cancer. Int J Cancer. 1991;49:650–5. doi: 10.1002/ijc.2910490504. [DOI] [PubMed] [Google Scholar]
- 35.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, Sullivan PS, Goodman JC, Gunaratne PH, Marchetti D. MicroRNA-1258 suppresses breast cancer brain metastasis by targeting heparanase. Cancer Res. 2011;71:645–54. doi: 10.1158/0008-5472.CAN-10-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith I, Procter M, Gelber RD, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 38.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 39.Altaha R, Crowell E, Hobbs G, Higa G, Abraham J. Increased risk of brain metastases in patients with HER-2/neu-positive breast carcinoma. Cancer. 2005;103:442–3. doi: 10.1002/cncr.20813. [DOI] [PubMed] [Google Scholar]
- 40.Stemmler HJ, Kahlert S, Siekiera W, Untch M, Heinrich B, Heinemann V. Characteristics of patients with brain metastases receiving trastuzumab for HER2 overexpressing metastatic breast cancer. Breast. 2006;15:219–25. doi: 10.1016/j.breast.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 41.Yau T, Swanton C, Chua S, et al. Incidence, pattern and timing of brain metastases among patients with advanced breast cancer treated with trastuzumab. Acta Oncol. 2006;45:196–201. doi: 10.1080/02841860500486630. [DOI] [PubMed] [Google Scholar]
- 42.Dawood S, Broglio K, Esteva FJ, et al. Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol. 2008;19:1242–8. doi: 10.1093/annonc/mdn036. [DOI] [PubMed] [Google Scholar]
- 43.Hawkins BT, Davis TP. The blood–brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–85. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 44.Régina A, Demeule M, Laplante A, et al. Multidrug resistance in brain tumors: roles of the blood–brain barrier. Cancer Metastasis Rev. 2001;20:13–25. doi: 10.1023/a:1013104423154. [DOI] [PubMed] [Google Scholar]
- 45.Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK. The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol. 2011;8:344–56. doi: 10.1038/nrclinonc.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang RD, Price JE, Fujimaki T, Bucana CD, Fidler IJ. Differential permeability of the blood–brain barrier in experimental brain metastases produced by human neoplasms implanted into nude mice. Am J Pathol. 1992;141:1115–24. [PMC free article] [PubMed] [Google Scholar]
- 47.Yonemori K, Tsuta K, Ono M, et al. Disruption of the blood brain barrier by brain metastases of triple-negative and basal-type breast cancer but not HER2/neu-positive breast cancer. Cancer. 2010;116:302–8. doi: 10.1002/cncr.24735. [DOI] [PubMed] [Google Scholar]
- 48.Eichler AF, Loeffler JS. Multidisciplinary management of brain metastases. Oncologist. 2007;12:884–98. doi: 10.1634/theoncologist.12-7-884. [DOI] [PubMed] [Google Scholar]
- 49.Stemmler HJ, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood–brain barrier. Anticancer Drugs. 2007;18:23–8. doi: 10.1097/01.cad.0000236313.50833.ee. [DOI] [PubMed] [Google Scholar]
- 50.Dijkers EC, Kosterink JG, Rademaker AP, et al. Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neu immunoPET imaging. J Nucl Med. 2009;50:974–81. doi: 10.2967/jnumed.108.060392. [DOI] [PubMed] [Google Scholar]
- 51.Jain RK. Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Res. 1990;50(Suppl 3):814s–9s. [PubMed] [Google Scholar]
- 52.Weinstein JN, Eger RR, Covell DG, et al. The pharmacology of monoclonal antibodies. Ann NY Acad Sci. 1987;507:199–210. doi: 10.1111/j.1749-6632.1987.tb45802.x. [DOI] [PubMed] [Google Scholar]
- 53.Fujimori K, Covell DG, Fletcher JE, Weinstein JN. A modeling analysis of monoclonal antibody percolation through tumors: a binding-site barrier. J Nucl Med. 1990;31:1191–8. [PubMed] [Google Scholar]
- 54.Dijkers EC, Oude Munnink TH, Kosterink JG, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther. 2010;87:586–92. doi: 10.1038/clpt.2010.12. [DOI] [PubMed] [Google Scholar]
- 55.Grossi PM, Ochiai H, Archer GE, et al. Efficacy of intracerebral microinfusion of trastuzumab in an athymic rat model of intracerebral metastatic breast cancer. Clin Cancer Res. 2003;9:5514–20. [PubMed] [Google Scholar]
- 56.Lampson LA. Monoclonal antibodies in neuro-oncology: getting past the blood–brain barrier. MAbs. 2011;3:153–60. doi: 10.4161/mabs.3.2.14239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Vulpen M, Kal HB, Taphoorn MJ, El-Sharouni SY. Changes in blood–brain barrier permeability induced by radiotherapy: implications for timing of chemotherapy? Oncol Rep. 2002;9:683–8. [PubMed] [Google Scholar]
- 58.Pestalozzi BC, Brignoli S. Trastuzumab in CSF. J Clin Oncol. 2000;18:2349–51. doi: 10.1200/JCO.2000.18.11.2349. [DOI] [PubMed] [Google Scholar]
- 59.Sjögren S, Inganäs M, Lindgren A, Holmberg L, Bergh J. Prognostic and predictive value of c-erbB-2 overexpression in primary breast cancer, alone and in combination with other prognostic markers. J Clin Oncol. 1998;16:462–9. doi: 10.1200/JCO.1998.16.2.462. [DOI] [PubMed] [Google Scholar]
- 60.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 61.Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004;5:63–9. doi: 10.3816/cbc.2004.n.011. [DOI] [PubMed] [Google Scholar]
- 62.Spector NL, Blackwell KL. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2009;27:5838–47. doi: 10.1200/JCO.2009.22.1507. [DOI] [PubMed] [Google Scholar]
- 63.Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62:4132–41. [PubMed] [Google Scholar]
- 64.Le XF, Pruefer F, Bast RC., Jr HER2-targeting antibodies modulate the cyclin-dependent kinase inhibitor p27Kip1 via multiple signaling pathways. Cell Cycle. 2005;4:87–95. doi: 10.4161/cc.4.1.1360. [DOI] [PubMed] [Google Scholar]
- 65.Asanuma H, Torigoe T, Kamiguchi K, et al. Survivin expression is regulated by coexpression of human epidermal growth factor receptor 2 and epidermal growth factor receptor via phosphatidylinositol 3-kinase/AKT signaling pathway in breast cancer cells. Cancer Res. 2005;65:11018–25. doi: 10.1158/0008-5472.CAN-05-0491. [DOI] [PubMed] [Google Scholar]
- 66.Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (herceptin), a humanized anti-HER2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61:4744–9. [PubMed] [Google Scholar]
- 67.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–6. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 68.Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK. Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature. 2002;416:279–80. doi: 10.1038/416279b. [DOI] [PubMed] [Google Scholar]
- 69.Niu G, Carter WB. Human epidermal growth factor receptor 2 regulates angiopoietin-2 expression in breast cancer via AKT and mitogen-activated protein kinase pathways. Cancer Res. 2007;67:1487–93. doi: 10.1158/0008-5472.CAN-06-3155. [DOI] [PubMed] [Google Scholar]
- 70.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 71.Perez EA, Romond EH, Suman VJ, et al. Updated results of the combined analysis of NCCTG N9831 and NSABP B-31 adjuvant chemotherapy with/without trastuzumab in patients with HER2-positive breast cancer. J Clin Oncol. 2007;25(Suppl 18S):6s, Abstract 512. [Google Scholar]
- 72.Slamon D, Eiermann W, Robert N, et al. Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC→T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in Her2neu positive early breast cancer patients: BCIRG 006 Study. Cancer Res. 2009;69(Suppl 3):500s, Abstract 62. [Google Scholar]
- 73.Bartsch R, Rottenfusser A, Wenzel C, et al. Trastuzumab prolongs overall survival in patients with brain metastases from Her2 positive breast cancer. J Neurooncol. 2007;85:311–7. doi: 10.1007/s11060-007-9420-5. [DOI] [PubMed] [Google Scholar]
- 74.Bartsch R, Berghoff A, Pluschnig U, et al. Impact of anti-HER2 therapy on overall survival in HER2-overexpressing breast cancer patients with brain metastases. Br J Cancer. 2012;106:25–31. doi: 10.1038/bjc.2011.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brufsky AM, Mayer M, Rugo HS, et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17:4834–43. doi: 10.1158/1078-0432.CCR-10-2962. [DOI] [PubMed] [Google Scholar]
- 76.Church DN, Modgil R, Guglani S, et al. Extended survival in women with brain metastases from HER2-overexpressing breast cancer. Am J Clin Oncol. 2008;31:250–4. doi: 10.1097/COC.0b013e31815a43c4. [DOI] [PubMed] [Google Scholar]
- 77.Kirsch DG, Ledezma CJ, Mathews CS, et al. Survival after brain metastases from breast cancer in the trastuzumab era. J Clin Oncol. 2005;23:2114–6. doi: 10.1200/JCO.2005.05.249. [DOI] [PubMed] [Google Scholar]
- 78.Le Scodan R, Jouanneau L, Massard C, et al. Brain metastases from breast cancer: prognostic significance of HER-2 overexpression, effect of trastuzumab and cause of death. BMC Cancer. 2011;11:395. doi: 10.1186/1471-2407-11-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nam BH, Kim SY, Han HS, et al. Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res. 2008;10:R20. doi: 10.1186/bcr1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park IH, Ro J, Lee KS, Nam BH, Kwon Y, Shin KH. Trastuzumab treatment beyond brain progression in HER2-positive metastatic breast cancer. Ann Oncol. 2009;20:56–62. doi: 10.1093/annonc/mdn539. [DOI] [PubMed] [Google Scholar]
- 81.Park YH, Park MJ, Ji SH, et al. Trastuzumab treatment improves brain metastasis outcomes through control and durable prolongation of systemic extracranial disease in HER2-overexpressing breast cancer patients. Br J Cancer. 2009;100:894–900. doi: 10.1038/sj.bjc.6604941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Witzel I, Kantelhardt EJ, Milde-Langosch K, et al. Management of patients with brain metastases receiving trastuzumab treatment for metastatic breast cancer. Onkologie. 2011;34:304–8. doi: 10.1159/000328679. [DOI] [PubMed] [Google Scholar]
- 83.Shigekawa T, Takeuchi H, Misumi M, et al. Successful treatment of leptomeningeal metastases from breast cancer using the combination of trastuzumab and capecitabine: a case report. Breast cancer. 2009;16:88–92. doi: 10.1007/s12282-008-0056-x. [DOI] [PubMed] [Google Scholar]
- 84.Mir O, Ropert S, Alexandre J, Lemare F, Goldwasser F. High-dose intrathecal trastuzumab for leptomeningeal metastases secondary to HER-2 overexpressing breast cancer. Ann Oncol. 2008;19:1978–80. doi: 10.1093/annonc/mdn654. [DOI] [PubMed] [Google Scholar]
- 85.Ferrario C, Davidson A, Bouganim N, Aloyz R, Panasci LC. Intrathecal trastuzumab and thiotepa for leptomeningeal spread of breast cancer. Ann Oncol. 2009;20:792–5. doi: 10.1093/annonc/mdp019. [DOI] [PubMed] [Google Scholar]
- 86.Stemmler HJ, Mengele K, Schmitt M, et al. Intrathecal trastuzumab (Herceptin) and methotrexate for meningeal carcinomatosis in HER2-overexpressing metastatic breast cancer: a case report. Anticancer Drugs. 2008;19:832–6. doi: 10.1097/CAD.0b013e32830b58b0. [DOI] [PubMed] [Google Scholar]
- 87.Baculi RH, Suki S, Nisbett J, Leeds N, Groves M. Meningeal carcinomatosis from breast carcinoma responsive to trastuzumab. J Clin Oncol. 2001;19:3297–8. doi: 10.1200/JCO.2001.19.13.3297. [DOI] [PubMed] [Google Scholar]
- 88.Church DN, Bahl A, Jones A, Price CG. HER2-positive breast cancer brain metastases: multiple responses to systemic chemotherapy and trastuzumab—a case report. J Neurooncol. 2006;79:289–92. doi: 10.1007/s11060-006-9139-8. [DOI] [PubMed] [Google Scholar]
- 89.Smith QR, Rudraraju V, Taskar K, et al. Lapatinib distribution in brain metastases of breast cancer following oral dosing in mice. Presented at the Breast Cancer Symposium; Washington, DC. September 5–7, 2008; p. Abstract 158. [Google Scholar]
- 90.Polli JW, Humphreys JE, Harmon KA, et al. The role of efflux and uptake transporters in N-{3-chloro-4-[(3-fluorobenzyl)oxyphenyl}-6-[5-({[2- methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine (GW572016, lapatinib) disposition and drug interactions. Drug Metab Dispos. 2008;36:695–701. doi: 10.1124/dmd.107.018374. [DOI] [PubMed] [Google Scholar]
- 91.Lin NU, Carey L, Liu MC, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2008;26:1993–9. doi: 10.1200/JCO.2007.12.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin NU, Diéras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15:1452–9. doi: 10.1158/1078-0432.CCR-08-1080. [DOI] [PubMed] [Google Scholar]
- 93.Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112:533–43. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- 94.Metro G, Foglietta L, Russillo M, et al. Clinical outcome of patients with brain metastases from HER2-positive breast cancer treated with lapatinib and capecitabine. Ann Oncol. 2011;22:625–30. doi: 10.1093/annonc/mdq434. [DOI] [PubMed] [Google Scholar]
- 95.Bachelot TD, Romieu G, Campone M, et al. LANDSCAPE: an FNCLCC phase II study with lapatinib and capecitabine in patients with brain metastases from HER2-positive (+) metastatic breast cancer (MBC) before whole-brain radiotherapy (WBR) J Clin Oncol. 2011;29(Suppl):47s, Abstract 509. [Google Scholar]
- 96.Lin NU, Eierman W, Greil R, et al. Randomized phase II study of lapatinib plus capecitabine or lapatinib plus topotecan for patients with HER2-positive breast cancer brain metastases. J Neurooncol. 2011;105:613–20. doi: 10.1007/s11060-011-0629-y. [DOI] [PubMed] [Google Scholar]
- 97.De Azambuja E, Lemort M, Rossari JR, et al. Phase I study of lapatinib (L) and temozolomide (T) combination for the treatment of progressive brain metastases (BM) in HER2-positive metastatic breast cancer patients (Pts) (LAPTEM, LAP 111172) J Clin Oncol. 2011;29(Suppl):62s, Abstract 570. [Google Scholar]
- 98.Coombes RC, Reise JA, Lau M, et al. An open-label positron emission tomography (PET) study to investigate and quantify brain and tumor penetration of carbon-11 labeled lapatinib in patients with HER2-overexpressing (HER2+) advanced or metastatic breast cancer (MBC) J Clin Oncol. 2011;29(Suppl):8S, Abstract TPS107. [Google Scholar]
- 99.Schulz H, Pels H, Schmidt-Wolf I, Zeelen U, Germing U, Engert A. Intraventricular treatment of relapsed central nervous system lymphoma with the anti-CD20 antibody rituximab. Haematologica. 2004;89:753–4. [PubMed] [Google Scholar]
- 100.Rubenstein JL, Fridlyand J, Abrey L, et al. Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol. 2007;25:1350–6. doi: 10.1200/JCO.2006.09.7311. [DOI] [PubMed] [Google Scholar]
- 101.Ding D, Kanaly CW, Bigner DD, et al. Convection-enhanced delivery of free gadolinium with the recombinant immunotoxin MR1-1. J Neurooncol. 2010;98:1–7. doi: 10.1007/s11060-009-0046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kunwar S, Chang S, Westphal M, et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010;12:871–81. doi: 10.1093/neuonc/nop054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Raghavan R, Brady ML, Rodríguez-Ponce MI, Hartlep A, Pedain C, Sampson JH. Convection-enhanced delivery of therapeutics for brain disease, and its optimization. Neurosurg Focus. 2006;20:E12. doi: 10.3171/foc.2006.20.4.7. [DOI] [PubMed] [Google Scholar]
- 104.Sampson JH, Brady M, Raghavan R, et al. Co-localization of gadolinium-DTPA with high molecular weight molecules after intracerebral convection-enhanced delivery in man. Neurosurgery. 2011;69:668–76. doi: 10.1227/NEU.0b013e3182181ba8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vogelbaum MA, Sampson JH, Kunwar S, et al. Convection-enhanced delivery of cintredekin besudotox (interleukin-13-PE38QQR) followed by radiation therapy with and without temozolomide in newly diagnosed malignant gliomas: phase 1 study of final safety results. Neurosurgery. 2007;61:1031–7. doi: 10.1227/01.neu.0000303199.77370.9e. [DOI] [PubMed] [Google Scholar]
- 106.Hu J, Ljubimova JY, Inoue S, et al. Phosphodiesterase type 5 inhibitors increase Herceptin transport and treatment efficacy in mouse metastatic brain tumor models. PLoS One. 2010;5:e10108. doi: 10.1371/journal.pone.0010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood–brain barrier disruption. Proc Natl Acad Sci U S A. 2006;103:11719–23. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]