Abstract
Objectives
Attenuation of adrenergic drive and cognitive enhancement, via stimulation of alpha2 pre- and post-synaptic receptors, may selectively enhance executive performance in early abstinent cocaine-dependent individuals. As these cognitive processes underpin important treatment-related behaviors, the alpha2 agonist, guanfacine HCl, may represent an effective pharmaco-therapeutic intervention.
Methods
Twenty-five early abstinent cocaine-dependent individuals were administered a battery of neurocognitive tasks on entry into treatment (baseline) and again following 3 weeks of either placebo or guanfacine treatment (up to 3 mg). Tasks included: Stop Signal, Stroop, 3-Dimentional Intra-dimensional/Extra-dimensional (IDED) task, Spatial Working Memory (SWM), Paired Associates Learning (PAL), Verbal Fluency and the Rey Auditory Verbal Learning Test (RAVLT).
Results
Compared with placebo, the guanfacine group demonstrated attenuated anxiety and negative affect as well as improved performance on selective executive tests. This included fewer directional errors on the stop signal task, fewer errors on the extra-dimensional shift component of the IDED task and better attentional switching during verbal fluency. Guanfacine did not improve strategic working memory or peripheral memory.
Conclusion
Guanfacine improves selective cognitive processes which may underlie salient treatment-related regulatory behaviors. Alpha2 agonists may therefore represent important agents for cocaine dependence.
Keywords: Guanfacine, cocaine dependence, inhibitory response, alpha2 agonists, executive function, verbal fluency, stop signal
Introduction
No effective FDA-approved medication currently exist which addresses the high rates of cocaine relapse during early recovery (Kang et al., 1991; O’Brien and Anthony, 2005; Sinha, 2001). “Agonist therapies”, which target the attenuation of reward and anxiolytics which focus on reducing negative reinforcement, have shown some success (Verrico et al., 2013); however, concerns have arisen with regard to abuse potential (Diana, 2011; Thanos et al., 2004) and sedation-induced cognitive decrement (Ijff and Aldenkamp, 2013), respectively. We propose that alpha-2 agonism may demonstrate low stimulant and sedative properties as well as enhance selective cognitive processes contributing to treatment-related behaviors and withdrawal severity. As such, the current study examines the feasibility of guanfacine HCl to enhance specific types of executive function during early recovery in dependent individuals.
The association between sympathetic overdrive and cocaine withdrawal severity indicates that adrenergic interventions may be of benefit in improving clinical outcome. For example, cocaine withdrawal reflects a bio-behavioral state characterized by tonic overactivity of locus coeruleus–norepinephrine (LC–NE) innervations within prefrontal regulatory brain regions including the anterior cingulate (ACC) and right inferior frontal gyrus (IFG). These systems are not only integral to anxiety pathophysiology and negative mood (Cecchi et al., 2002; Fox et al., 2007b; Sinha et al., 2009), but also key attentional mechanisms underlying the stopping and monitoring components of inhibitory control (Aston-Jones and Cohen, 2005; Aston-Jones and Gold, 2009). Behaviorally, this may be a central mechanism of distractibility, emotion dysregulation and impulsivity (Bari and Robbins, 2013; Fishbein et al., 2006; Kenemans et al., 2005) contributing to cocaine use despite negative consequences, thus representing critical targets for the development of effective addiction medications.
There are several lines of support for this hypothesis. First, although early withdrawal is often characterized by broad cognitive decrement across an array of functions (De Oliveira et al., 2009; Lorea et al., 2010), clinical course is typically predicted by more selective executive deteriorations in cognitive flexibility (Faraone et al., 2005), attention and inhibitory control (Brewer et al., 2008; Carroll et al., 2011; Streeter et al., 2008; Turner et al., 2009; Verdejo-Garcia et al., 2012). Second, Cognitive Behavioral Therapy (CBT), which promotes skills training to improve self-regulation, planning, organization, problem solving and concentration (Mischel, 2004), has been identified as an effective behavioral treatment for monitoring and changing substance use behavior (Blume and Marlatt, 2009; Sofuoglu et al., 2013). Third, beta adrenergic antagonism and alpha2 agonism have been shown to improve selective inhibitory processes underlying these behaviors (Bari et al., 2011; Eagle et al., 2008; Kelley et al., 2007), both in the absence of peripheral effects (Beversdorf et al., 2002) and during psychosocial stress (Alexander et al., 2007), supporting the idea that the modulation of central LC–NE stress systems may be a salient mechanism for medications intervention.
As an alpha2a adrenergic agonist, guanfacine is thought to diminish ascending adrenergic activity, via stimulation of pre-synaptic alpha2-adrenoceptors, and strengthen executive processes by stimulating post-synaptic alpha2-adrenoceptors in the dorsolateral regions (Arnsten, 1998; Arnsten and Goldman-Rakic, 1985; Arnsten and Jin, 2012). Furthermore, guanfacine is also 8–10 times more selective for alpha2a adrenoceptors than clonidine (Jarrott et al., 1982; Seedat, 1985; Summers et al., 1981), which has consistently shown greater side effects in terms of orthostatic hypotension and withdrawal syndrome (Gish et al., 2010; Sorkin and Heel, 1986). Guanfacine may also enhance regulatory cognitive-affective function by attenuating generalized sympathetic processes (Erb et al., 1998, 2000; Shaham et al., 2003) including anxiety and negative affective state (Ambrose-Lanci et al., 2010; Fox et al., 2012). In support, guanfacine has been shown to be particularly robust in treating conditions characterized by catecholaminergic psychopathology including symptoms of attention-deficit hyperactivity disorder (ADHD) (Chappell et al., 1995; Childress, 2012; Hunt et al., 1995; Sallee et al., 2012; Scahill et al., 2001), age-related decline of attention and working memory (Arnsten and Contant, 1992; Arnsten and Goldman-Rakic, 1990; Arnsten et al., 1988; Decamp et al., 2011) and stress sensitivity during early withdrawal in cocaine-dependent individuals (Fox et al., 2012, 2014).
We therefore present a double-blind, placebo-controlled preliminary study which examines the cognitive-enhancing effects of guanfacine in early abstinent cocaine-dependent individuals. A battery of tasks will be administered which examine inhibitory and strategic forms of executive function, as well as peripheral memory.
Methods and materials
Participants
All 25 cocaine-dependent individuals (22 males/three females) participating in the current study were recruited via advertisements placed either online or in local newspapers, as part of a larger “parent” study comprising both an inpatient or outpatient component. Objectives of the parent study were to assess the effects of guanfacine or placebo on stress system function in early abstinent inpatient or outpatient treatment-seeking individuals. All participants had to be completely abstinent in order to take part in both cognitive testing components (baseline and 3-weeks treatment) in the current study. Urine toxicology screens upon entry to the study, as well as The Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders IV (SCID IV (First et al., 1995) were used to verify current cocaine dependence. Participants were excluded if they met dependence criteria for any other illicit drug other than alcohol. They were also excluded for using prescription opiates and medications prescribed for any medical and/or psychiatric conditions. In addition, all participants had to meet stringent health requirements including healthy cardiovascular, renal, hepatic, pancreatic, hematopoietic and thyroid function. All participants had to be able to read and write in English and all gave both written and verbal informed consent. The study was approved by the Human Investigation Committee of the Yale University School of Medicine.
Procedures
After eligibility screening over the phone, all participants were scheduled for an intake appointment, where demographic and drug use assessments were completed as well as breath alcohol testing and urine toxicology screens. Physical examination and blood work was also conducted to ensure good health. Following completion of consent forms, all participants were scheduled for either a) inpatient admission to the Clinical Neuroscience Research Unit (CNRU) located at the Connecticut Mental Health Center (CMHC) for 4 weeks of inpatient treatment and study participation (week 1 and week 4), or b) outpatient admission to the Substance Abuse Center at the CMHC for 8 weeks of outpatient treatment and study participation (week 1 and week 4).
Inpatient admission procedures
All inpatient participants were admitted to the CNRU, which is a locked treatment research facility with no access to alcohol or drugs and very limited access to visitors. Drug testing was conducted regularly to ensure drug abstinence. All participants admitted to the study also participated in a specialized substance abuse treatment program initiated on admission. This comprised weekly individual therapy, provided by psychiatry residents on the CNRU, as well as twice weekly group drug counseling provided by addiction specialists.
All inpatient cocaine-dependent patients were randomly assigned to guanfacine 2 mg (n=6) or 3 mg (n=6) daily or placebo (n=13) in a randomized, double-blind manner. Guanfacine pills (marketed by Watson Pharmaceuticals) were purchased through the pharmacy located at the CMHC and the research pharmacist ensured that both active and placebo capsules used for medication administration appeared identical. All placebo pills contained lactose. Subjects were randomly assigned to either guanfacine (2 or 3 mg) or placebo. As guanfacine had not been previously used in addicted samples, we started the study with placebo versus 2 mg dosing in a double-blind manner. After observing no major or serious side effects (Fox et al., 2012), we increased the guanfacine dosing to 3 mg for subsequent subjects who were randomized to the active condition (See Table 1 for all dosing schedules). For the purpose of the primary analysis, both doses were collapsed and compared with placebo.
Table 1.
Inpatient and outpatient dosing schedule.
| Medication Groups | Days | |||||
|---|---|---|---|---|---|---|
| Inpatient dosing (2 mg) | Days 1–3 | Days 4–13 | Days 14→ | |||
| 0.5 mg (bedtime) | 0.5 mg–1.0 mg | 1.0 mg–1.0 mg | ||||
| Inpatient dosing (3 mg) | Days 1–2 | Days 3–5 | Days 6–8 | Days 9–11 | Days 12→ | |
| 0.5 mg (bedtime) | 0.5 mg–1.0 mg | 1.0 mg–1.0 mg | 1.0 mg–1.5 mg | 1.5 mg–1.5 mg | ||
| Outpatient dosing (3 mg) | Days 1–2 | Days 3–8 | Days 9–11 | Days 12–18 | Days 19–24 | Days 25→ |
| 0.5 mg (bedtime) | 0.5 mg–1.0 mg | 1.0 mg–1.0 mg | 1.0 mg–1.5 mg | 1.5 mg–1.5 mg | 1.5 mg–1.5 mg | |
Outpatient admission procedures
All outpatient participants were required to attend the clinic at the CMHC three times per week (Monday, Wednesday and Friday) for 8 weeks. At each visit they were required to provide a urine specimen, a breathalyzer screen and vital signs; taken every 15 minutes for 1 hour. Participants were randomly assigned to either guanfacine (3 mg) or placebo, and received the exact amount of medication until their next visit, which was provided in unit/dose blister packs and labeled with the time and date in order to facilitate appropriate dosing (Table 1 for all dosing schedules). In addition, riboflavin was added to the medications to ensure compliance (Ramanujam et al., 2011).
All outpatient participants also received standard individual drug counseling sessions twice per week, as outlined in the Individual Drug Counseling Manual (Mercer and Woody, 1999) as well as contingency management, where redeemable vouchers were provided for clean urines (Higgins et al., 2002). Any participants unable to achieve abstinence within 4 weeks were offered inpatient treatment or referred to a higher level of care at another facility. Participants achieving abstinence but experiencing occasional lapses were continued in outpatient treatment; however, they were excluded from the current cognitive study. Only fully abstinent outpatient participants were included in the cognitive testing sessions. This was assessed via urine toxicology and breathalyzer screening prior to testing.
Following completion of either inpatient or outpatient treatment, all participants underwent a standard 4-day taper similar that used with lofexidine in opiate withdrawal protocols (Bearn et al., 1996).
Neuro-cognitive test assessment battery
All inpatient and outpatient participants completed the battery twice, i) pre-medication (Day 4 of either inpatient or outpatient treatment) and ii) during medication (approximately Day 24 of either inpatient or outpatient treatment). All tasks were administered in the following order and counterbalanced. However, the Rey Auditory Verbal Learning Test (RAVLT) was administered first in all cases.
Cognitive testing
Rey Auditory Verbal Learning Test
The RAVLT (Rey, 1964) involves the repeated administration of a word list (List A) across five learning trials. Free recall immediately follows each presentation (List A, trials 1–5). After the last learning trial a second “interference” list (List B) is presented prior to the free recall of List B. Immediately following recall of List B, participants are again required to recall List A, without additional presentation (List A, trial 6). After a 30-minute delay, they are again asked to recall List A without presentation (List A, trial 7). A subsequent recognition list is then presented comprising 45 words, (15 target words from List A; 15 from List B; 15 phonologically or semantically similar to those in List A).
No time limit is applied to the recall trials and participants are prompted to recall words in any order. Both stimulus word lists (A and B) are presented to the participants on tape, at a rate of one word per second. Recall, recognition and error scores are recorded by adding the number for each trial.
Verbal fluency
The verbal fluency task (Benton, 1968) comprises a phonemic and semantic category. The phonemic category requires participants to generate as many words as possible starting with a given letter in 1 minute. The letters (F A S) or (P R W) were used for the letter fluency section. The semantic component requires participants to produce as many words as possible belonging to a given semantic category. The category “animals” or “fruit” were used for the semantic component of the task. The two different conditions were counterbalanced between the first (pre-medication) and second (during-medication) testing sessions. The only limitations were that participants could not use proper nouns or the same word with different grammatical endings (e.g. show, showed, showing). Standard administration provides three letters; the most common being the letters F, A, and S (Barry et al., 2008). Other letters are commonly employed, and in the current study we additionally used PRW for counterbalancing (Bolla et al., 1990). The dependent variable was the number of words produced in each letter category (FAS or PRW) and the number of words produced in the semantic category.
As verbal fluency is a multi-factorial task with potentially many underlying processes reflected in the final score, two clinically relevant and independent components of the fluency task were also included that are thought to optimize successful generation or initiation of verbal responses (Troyer et al., 1997; Turner, 1999). The first comprises “clustering”, which is the generation of words within a meaningful phonemic or semantic subcategory, and the second is “switching”, which is the ability to shift efficiently to a new sub-category of words when the old sub-category is exhausted (Begeer et al., 2014; Troyer et al., 1997, 1998).
Clustering scores
In the phonemic component of the fluency task, clusters were defined as contiguous words that fell into one of the following categories: a) two or more words that began with at least the same first two letters (e.g. stone, stack, stand), b) two or more words that differed only by a vowel sound (e.g. rise, rose, rouse), c) words that were homophones (e.g. see, sea), and d) two or more words that fell into the same semantic category (e.g. sand sea sun shell). In the semantic component of the fluency task, clusters were defined as groups of contiguous words belonging to the same semantic sub-category. These included farmyard animals, pets, marsupials, birds, insects and aquatic animals. All words belonging to a semantic or phonemic cluster are referred to as related words (RW).
Switching scores
Switches were calculated as the number of transitions between clusters, including single words, in the phonemic and semantic tests. Switching score was calculated as the number of words produced minus the number of related words, plus the number of clusters. Errors and repetitions were excluded in cluster and switch scoring, in order to provide information about the underlying cognitive processes regardless of whether or not they were included in the total number of words generated.
Stroop Color/Word Test
The Stroop Test (Golden, 1975) is a test of selective attention where the scores represent the number of items completed in 45 s during three trials. The first trial consists of reading the name of as many colors as possible in 45 s (e.g. “red”, “blue”, “green”). The second trial comprises naming the color of a series of Xs (XXXX) printed in either red or blue or green ink. Again, participants have to name as many colors as possible in 45 s. The final trial consists of the 100 words presented in the initial trial in the colors presented in the second trial. In all cases, the word (e.g. red) is different from the color it is printed in (e.g. blue. (i.e. “red”)). Subjects are given 45 s to name the color of the ink and suppress the pre-potent inclination to name the word. Participants are immediately informed of any errors made and must correct all errors before continuing. Hence, all scores for each component include error time.
The number of raw items read for each trial is recorded and converted to standardized T scores.
The following tasks were selected from the Cambridge Autonomic Test Assessment Battery (CANTAB: www.camcog.com).
The Visuo-Spatial Paired Associates Learning Task
The Visuo-Spatial Paired Associates Learning (PAL) Task (www.camcog.com) assesses simple pattern and visuo-spatial associative learning, as well as delayed aspects of working memory (Jakala et al., 1999b). Six white boxes are presented in a circle around the screen. Each of the boxes opens in a random sequence revealing an abstract pattern “inside”. The patterns are then displayed individually in the center of the screen and participants are requested to touch the box in which they had seen each of the patterns. Participants perform two trials of one pattern–location association, two trials of two pattern–locations and two trials of three pattern–locations. One trial of six pattern–locations and one trial of eight pattern–locations is then presented. If an error is made, a “reminder” phase is shown. Participants are allowed nine “reminder” phases for each trial, making a total of 10 attempts prior to the task being terminated.
The dependent variables were the total number of presentations required on each trial (Attempts); the total number of patterns successfully located on initial presentation (memory score; maximum score being 26); and total number of errors made on each trial (error score). With regard to the number of errors made, the score obtained by the worst participant attempting a set was given to those participants not reaching that set.
Spatial Working Memory Task
The Spatial Working Memory Task (www.camcog.com) requires the mental manipulation and monitoring of spatial memory, and planning a self-ordered search strategy. Participants must search through a display of boxes to find “hidden” blue tokens. Only one token is hidden at any one time, and once a token is found in a particular box, that box is not used again to conceal other counters.
The dependent variables reflected two types of errors: a) returning to a box in which a blue token has already been found (“Between Search Error”) and b) returning to a box (in the same search sequence) that is empty (“Within Search Error”). Each participant is required to attempt four 3-box trials, four 4-box trials, four 6-box trials and four 8-box trials.
Three Dimensional Intra-Dimensional/Extra-Dimensional Shift Task
The Three Dimensional Intra-Dimensional/Extra-Dimensional Shift Task (3D-IDED) task (www.camcog.com) is focused primarily on attentional shifting and reversal learning. The ability to form, maintain and shift attentional set is assessed through learning a series of two alternative forced choice discriminations and their reversals. Stimuli vary along three possible dimensions (color, shape, number). In the “intra-dimensional shift” stage, the relevant dimension (color) remains unchanged despite the introduction of two completely novel stimuli. In the final “extra-dimensional shift” stage participants are required to “shift” response set to a previously irrelevant dimension (shape). Participants must achieve six correct successive discriminations. If these discriminations are not achieved following 50 attempts, the task is terminated.
Performance indices of the 3D-IDED task include: Attrition rate, Number of Trials (required to reach criterion), Number of Errors and Response Latency at each of the nine stages. The 3-IDED is not an appropriate task for a within-subjects design, and was therefore only administered once, following 3 weeks of guanfacine treatment.
Stop/Signal Task
The Stop/Signal task (www.camcog.com) assesses an individual’s ability to inhibit an ongoing motor response. In the first part of this test, participants are required to press the left hand button when they see a left-pointing arrow, and the right hand button when they see a right-pointing arrow. Sixteen practice trials are presented. In the second part, participants are told to continue pressing the buttons when they see the arrows, as before, but, if they hear an auditory signal (a beep), they should withhold their response. Direction errors, proportion of successful stops, and relevant reaction times are recorded. The task incorporates a “dual race model” algorithm (Logan et al., 1984) which uses interleaved staircase functions where the timing of the auditory stop signal changes throughout the test, depending on the subject’s past performance, so that stopping occurs approximately 50% of the time for each subject. This allows for the estimation of a reaction time based on an individual’s ability to inhibit a pre-potent motor response. As such, outcome variables include Directional Errors (i.e. pressing the incorrect arrow in both the “go” and “stop and go” trials), and Mean Stop Signal Reaction Time.
Subjective measures
The Profile of Mood States Bi-Polar Form POMS-B
The Profile of Mood States Bi-Polar Form POMS-B (McNair et al., 1971) was collected at three time-points during each cognitive testing session (at the beginning, during and at completion). The scales comprises a list of 72 mood-related words (e.g. happy, angry, tense, shaky) and participants are requested to rate the extent to which they felt each emotion on a scale of 0 (not at all) to 4 (extremely). Positive items are reverse coded. The 72 items are then collapsed into eight subscales: anger, anxiety, arousal, confusion, depression, elation, fatigue, and vigor.
Statistical analysis
All data analyses were performed using SPSS Inc., version 19 (Chicago, IL, USA). T-tests and chi-square analyses were used to examine group variation in demographic and drug use variables.
Linear mixed-effect models were implemented to analyze all cognitive data where Medication group (guanfacine vs. placebo), Session (pre-medication vs. during-medication) and Task Trials (varying levels) represented the fixed effects, and Subjects represented the random effect. Bonferroni tests were used as adjustments for all multiple comparisons.
As the guanfacine group comprised individuals administered both 2 mg (n=6) and 3 mg (n=6) of guanfacine as well as placebo (n=13), a preliminary secondary analyses was additionally conducted on all significant dependent variables using guanfacine dose (placebo, 2 mg, 3 mg) as the independent variable.
Results
Participants
No significant group differences were observed in terms of either demographic variables, cocaine withdrawal severity on admission (as measured by the Cocaine Selective Severity Assessment; CSSA, (Kampman et al., 2002) or drug use prior to inpatient admission (see Table 2). None of the participants required a medicated detoxification.
Table 2.
Demographics and drug use.
| N=25 | Placebo n=13 | Guanfacine n=12 |
|---|---|---|
| % Inpatient | 8 (61.5%) | 9 (75%) |
| Gender – no. of females | 2 (15.4%) | 1 (8.3%) |
| Race | ||
| Caucasian | 2 (15.4%) | 2 (16.7%) |
| African American | 8 (61.5%) | 10 (83.3%) |
| Hispanic | 2 (15.4%) | 0 (0) |
| Other | 1 (7.7%) | 0 (0) |
| Age | 40.6 ± 8.3 | 44.1 ± 5.9 |
| IQ (Shipley) | 100.4 ± 10.9 | 103.8 ± 10.9 |
| Years spent in education | 12.3 ± 3.2 | 12.1 ± 1.6 |
| BMI | 28.6 ± 5.1 | 26.0 ± 6.1 |
| Cocaine use: no. of years | 9.9 ± 4.6 | 14.5 ± 6.8 |
| Cocaine use: no. of days in past month | 15.8 ± 8.0 | 14.6 ± 9.1 |
| Cocaine use: amt. in past month (grams) | 38.7 ± 35.7 | 17.5 ± 23.7 |
| Alcohol use: no. of years | 21.3 ± 8.4 | 16.2 ± 11.7 |
| Alcohol use: no. of days in past month | 12.6 ± 10.0 | 12.1 ± 6.7 |
| Alcohol use: amt. in past month (drinks) | 215.9 ± 239.1 | 135.4 ± 146.5 |
| CSSA Scores on admission (0–126) | 32.1 ± 11.4 | 25.9 ± 7.8 |
| Current mood disorder | 0 | 0 |
| Lifetime mood disorder | 0 | 2 (16.7%) |
| Current anxiety disorder (incl PTSD) | 1 (7.7%) | 0 |
| Lifetime anxiety disorder (incl PTSD) | 1 (7.7%) | 2 (16.7%) |
Data indicate means and standard deviations. p>.05 for all measures.
BMI: body mass index; CSSA: Cocaine Selective Severity Assessment.
Subjective measures
POMS (anxiety)
A significant Medication Group × Session interaction [F 1,89=4.5, p<.04] indicated a significant attenuation of anxiety from baseline in the guanfacine group (Baseline > Post-Medication, p<.0001) which was not observed in the placebos.
POMS (anger)
Similarly, a significant Medication Group × Session interaction [F 1,89=4.5, p<.04] showed that a significant attenuation of anger was observed in the guanfacine group only (Baseline > Post-Medication, p<.0001).
POMS (depression)
A significant Medication Group × Session interaction [F 1,91=6.3, p=.01] showed a significant attenuation of depressive symptoms from baseline in both the guanfacine (Baseline > Post-Medication, p<.0001) and placebo (Baseline > Post-Medication, p=.002) groups. However, at the baseline time-point, the guanfacine group demonstrated a trend for reporting higher depressive symptoms compared with the placebos (p=.1) possibly indicating a more robust overall reduction in depression. No significant Group × Session differences were observed on any other subscales of the POMS (range: p=.12 to p=.88).
Cognitive measures
Verbal fluency
Phonemic fluency
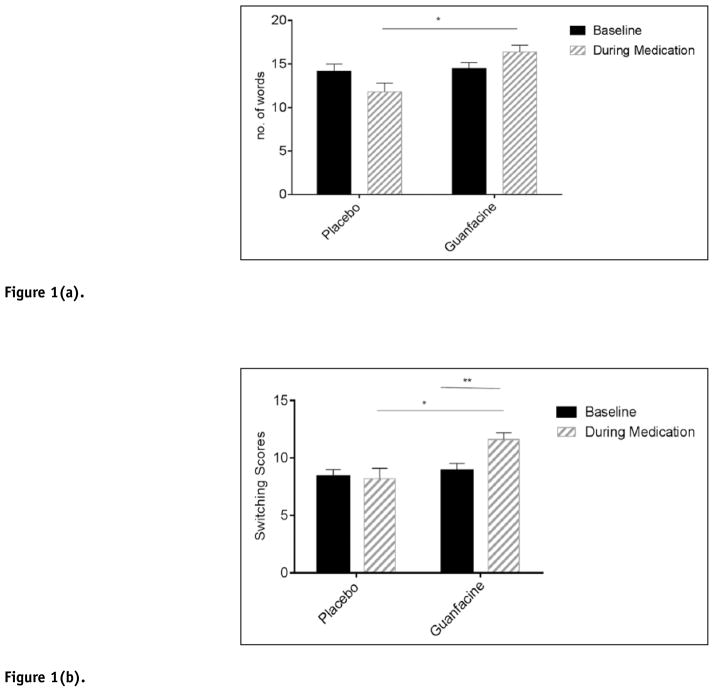
A significant Medication Group × Session interaction [F 1,96=4.9, p<.03] showed that the guanfacine group demonstrated improved phonemic fluency compared with the placebo group following 3 weeks of treatment (p<.05). (Figure 1(a)).
Figure 1.
Figure 1(a). Verbal fluency: number of words produced.
*p≤.05. Bars represent mean ± SEM.
Figure 1(b). Verbal fluency: number of switches made.
*p≤.05. **p≤.01. Bars represent mean ± SEM.
Semantic fluency
A main effect of Medication Group [F 1,21=6.0, p=.02] indicated that the guanfacine group produced a significantly higher number of words compared with the placebo group across both testing sessions.
During the phonemic component of the verbal fluency test, a significant Group × Session interaction was also observed for the number of transitions or “switches” performed between clusters [F 1,95=3.8, p=.05]. The interaction indicated that following three weeks of guanfacine treatment, the guanfacine group produced a significantly higher number of switches compared with the placebos (p=.03) (Figure 1(b)). A significant increase in switches was also observed following treatment compared with baseline in the guanfacine group (p=.003). This time-point effect was not seen in the placebo group. No group differences were seen with regard to the number of clusters produced across the three conditions (FAS/PRW). No significant group variation was observed with regard to either number of switches or clusters produced in the semantic component of the task.
Stop Signal Task
Directional errors
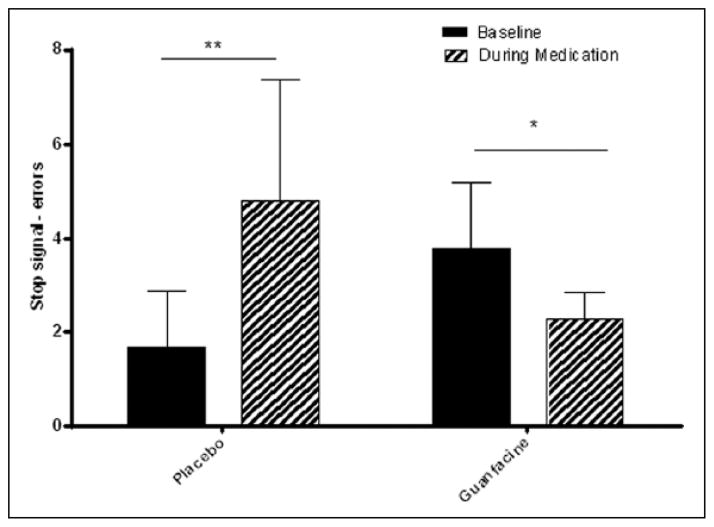
A significant Medication Group × Session interaction [F 1,148= 2.4, p=.001] indicated that following 3 weeks of treatment the guanfacine group made significantly fewer errors on the stop signal task compared with their baseline (p<.05). Conversely, the placebo group made a greater number of errors during the second testing session compared with their baseline (p=.003). No significant group variation was observed with regard to latency (Figure 2).
Figure 2.
Stop Signal Task: Number of errors made.
*p≤.05 **p≤.01. Bars represent mean ± SEM.
3D-IDED
Attrition rates
Table 3 shows the percentage of participants in each of the medication groups to fail at each of the nine stages. All nine stages were completed by 60% of the placebo group and 89% of the guanfacine group. In addition, chi-square analyses showed that group differences in attrition rates were significantly better in the guanfacine compared with the placebo group during the extra-dimension shift (p<.0001) and the reversal stage of the extra-dimensional shift (p<.0001).
Table 3.
Percentage of participants failing to complete IDED task stages.
| N=25 | Attrition Rate (% failing stage) | ||
|---|---|---|---|
|
| |||
| IDED stages | Placebo | Guanfacine | Chi-square test |
| SD- simple discrimination | 0% | 0% | – |
| SDR - reversal | 0% | 0% | – |
| CD- compound discrimination | 0% | 0% | – |
| CD_2 - compound discrimination 2 | 0% | 0% | – |
| CDR - reversal | 10% | 0% | – |
| ID - intra-dimensional shift | 10% | 0% | – |
| IDR - reversal | 10% | 0% | – |
| ED - extra-dimensional shift | 40% | 11% | X2=16.5; df =1; p<.0001 |
| EDR - reversal | 50% | 11% | X2=24.9; df =1; p<.0001 |
With regard to no. of errors made, a significant Medication Group × Stage interaction [F 8,136=2.2, p =.03] indicated that the placebo group also made a significantly greater number of errors compared with the guanfacine group during the extra-dimensional shift (p=.04) and the extra-dimensional reversal shift (p<.0001) (Figure 3). In terms of latency, no significant group variation was observed with regards to response latency.
Figure 3.
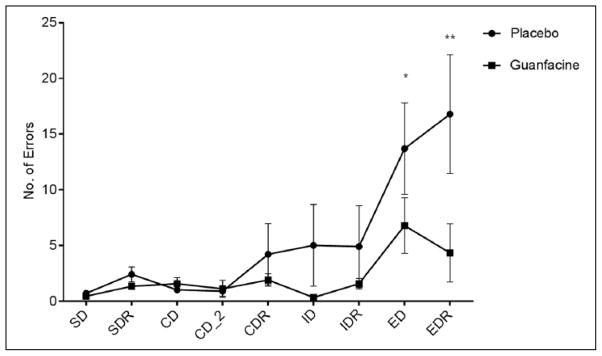
3D-IDED Task: Number of errors made at each stage.
SD: simple discrimination; SDR: reversal of simple discrimination.
CD: compound discrimination; CD_2: compound discrimination 2; CDR: reversal of compound discrimination ID: intra-dimensional shift; IDR: reversal of intra-dimensional shift. ED: extra-dimensional shift; EDR: reversal of extra-dimensional shift. *p≤.05, **p≤.0001.M
Lines represent mean ± SEM.
Stroop
A Significant effect of Testing Session F [1,20=30.7, p<.0001] indicated that both the guanfacine and the placebo groups demonstrated improved Color/Word performance at the second testing session compared with baseline performance. No main effects of Group or Group × Session interactions were observed for Color/Word performance.
Spatial Working Memory
Main effects of Trial for Between Search Errors [F 3,134=83.9, p<.0001] and Within Search Errors [F 3,120=8.7, p<.0001] indicated that both the guanfacine and placebo groups made a progressively greater number of errors as the trials increased in difficulty. All participants made more Between Search Errors during the 6-box and 8-box trials compared with the easier 3-box and 4-box trials (p<.0001, in all cases). They also made more Within Search Errors at the 8-box stage compared with the 6-box (p=.03), 4-box (p<.0001) and 3-box stage (p<.0001). No main effects of Group or Group × Session interactions were observed with regard to the number of Between or Within Search Errors made.
Visuo-Spatial PAL
No significant interactions between Group × Session were observed for the number of attempts required to complete each trial (Attempts), the number of errors made on each trial (Errors), or the memory score (Memory). As expected, a significant main effect of Trial for Attempts [F 4,175=56.7, p<.0001], Errors [F 4,175=39.2, p<.0001] and Memory [F 4,175=10.7, p<.0001] indicated that a greater number of attempts were required, errors made and patterns recalled in the 8- and 6-box conditions compared with the 3- and 2-box conditions, both at baseline and following guanfacine treatment.
RAVLT
No significant Group × Session differences were observed in terms of immediate recall or verbal learning ability. A significant main effect of Trial [F 4,165=54.0, p<.0001] indicated that both groups demonstrated a similar incremental learning curve both at baseline and following guanfacine treatment. For example, the number of words recalled in List A, trial 1 was significantly less than those recalled in all four other trials (p<.0001, in all cases). Similarly, the highest number of words recalled was observed in List A, trial 5 compared with trial 1 (p<.0001), trial 2 (p<.0001) and trial 3 (p<.04).
No significant Group × Session effects were seen for either delayed verbal memory (Trial 7), verbal recognition, proactive interference (Trial 1 – List B), retroactive interference (Trial 5 – Trial 6) or number of repetitions and errors made during both recall and recognition trials.
Although no significant Group × Session interactions were seen for delayed verbal recall (trial 7) and recognition scores, main effects of Session for both [delayed recall: F 1,16=15.6, p=.001] [recognition: F 1,17=4.9, p=.04] indicated that these scores were improved compared with baseline in both the placebo and guanfacine medication groups.
Secondary dose analyses
No significant main effects of Dose or Dose × Session interactions were observed for Stop Signal or the 3D-IDED task. Trends only were seen with regard to the total number of words produced on the Verbal Fluency test [F 2,96=2.39, p=.09] and the total number of transitions or “switches” performed between clusters [F 2,95=2.53, p=.08]. Participants administered 2 mg of guanfacine demonstrated higher scores following treatment compared with their baseline (p=.09) and participants administered both 2 mg (p=.03) and 3 mg (p=.05) of guanfacine performed a greater number of “switches” following treatment compared with their baseline. This improvement was not observed in the placebo group.
Discussion
Findings from the current study show that 3–4 weeks of guanfacine treatment was highly selective in improving aspects of response inhibition. While guanfacine enhanced motor inhibition (Stop/Signal) and attentional switching (3D-IDED/Verbal Fluency), no improvements were observed on tasks that included strategic planning demands, such as spatial working memory and paired associated learning. Neither did guanfacine enhance peripheral memory on tests with high non-executive learning and mnemonic components.
Directional errors on the stop signal task were improved in the guanfacine compared with the placebo group, and have typically been interpreted as a prototypical indicator of impulsivity in a range of neuropsychiatry conditions including ADHD (Nigg, 2001; Senderecka et al., 2012), pathological gambling (Billieux et al., 2012; Brevers et al., 2012; Rodriguez-Jimenez et al., 2006), borderline personality disorder (Nigg et al., 2005), and most notably, cocaine dependence (Fillmore and Rush, 2002; Hester et al., 2007; Li et al., 2006). Improvement in extra-dimensional shifting and verbal fluency “switching” scores are also thought to reflect enhanced cognitive flexibility, rule acquisition and inhibitory function (Cools et al., 2001; Troyer et al., 1997). Not only are these selective executive processes compromised during early recovery from cocaine dependence (Colzato et al., 2007, 2009; Fernandez-Serrano et al., 2010; Garavan and Hester, 2007; Li et al., 2006; Soar et al., 2012; van Holst and Schilt, 2011; Verdejo-Garcia and Perez-Garcia, 2007), they are critical to impulse-related behaviors effecting outcome, such as planning, organizational ability, and decision-making (Blume and Marlatt, 2009; Fishbein et al., 2006). As such, the enhancement of selective inhibitory processes via the facilitation of attentional flexibility may provide an important behavioral, therapeutic mechanism associated with guanfacine’s central adrenergic effects.
Findings in the current study also corroborate other pharmacological studies showing that modulating noradrenergic drive via administration of agents such as guanfacine and atomoxetine enhances inhibitory control in a range of clinical populations. Both medications have been shown to improve inhibitory-related processes in non-human primates as well as humans with ADHD, tic disorders and neurodegenerative disorders (Arnsten and Li, 2005; Gau and Shang, 2010; Hunt et al., 1995; Jakala et al., 1999a; Scahill et al., 2001; Shang and Gau, 2012; Swartz et al., 2008; Ye et al., 2014). While guanfacine was able to enhance motor inhibition in the Stop Signal task, it surprisingly did not improve Stroop performance, often considered a global measure of inhibitory control. One parsimonious explanation for this may be that the Stroop task invokes very different prefrontal systems potentially due to the lack of motor component and a greater focus on interference rather than inhibition, per se (Vitkovitch et al., 2002).
While alpha2 agonists cause diffuse prefrontal activation of adrenergic systems (Arnsten et al., 1988; Ji et al., 2008; Le et al., 2011; Rama et al., 1996; Shields et al., 2009), the enhanced attentional shifting observed in the current guanfacine sample may be compatible with the drug’s targeting of post-synaptic alpha2 receptors located on the pyramidal dendritic spines of the dorsolateral prefrontal cortex (dlPFC) (Arnsten, 2011; Arnsten and Contant, 1992; Arnsten and Jin, 2012; Clerkin et al., 2009). For example, guanfacine’s stimulation of post-synaptic alpha2 receptors in the dlPFC has been shown to strengthen the local network connectivity associated with response set maintenance (Clerkin et al., 2009; MacDonald et al., 1997; Wang et al., 2007). Furthermore, studies examining the cortical mechanisms of response inhibition and extra-dimensional shifting have highlighted the role of both dorsolateral and ventrolateral prefrontal function (Bissonette et al., 2008; Dias et al., 1996; Fassbender et al., 2004; Garavan and Hester, 2007; Hampshire and Owen, 2006; Hester et al., 2004; Rogers et al., 2000; Swann et al., 2013). Findings also support the modulation of broad LC–NE systems by alpha2 agonists (Engberg and Eriksson, 1991) known to underlie various attentional levels of inhibitory function including stimulus detection, behavioral orienting, attentional shifting and conflict detection (Bari and Robbins, 2013).
Guanfacine’s more pervasive effects on sympathetic output, via the central reduction of LC firing and stimulation of pre-synaptic a2 receptors (Mosqueda-Garcia, 1990; Sorkin and Heel, 1986), may also account for both its effects on mood and cognition. Specifically, guanfacine ameliorated anxiety, anger and negative affect alongside improvements in inhibitory function. This is clinically important as anxiety and mood-related symptoms during early abstinence may contribute to the course, treatment outcome, and prognosis of substance abuse disorders (Ahmadi et al., 2006; O’Leary et al., 2000; Naifeh et al., 2012; Sanchez-Hervas and Llorente del Pozo, 2012; Sinha et al., 2011), possibly due to impinging upon vulnerable self-regulatory pathways (Fox et al., 2007a; Tice et al., 2001). Notably, while anxiolytic GABA-ergic agents including tiagabine, baclofen and topiramate have all demonstrated some promise in terms of reducing cocaine administration (Gonzalez et al., 2003; Kampman et al., 2004; Roberts, 2005; Roberts and Brebner, 2000), they have also been associated with cognitive impairment and sedation (Ijff and Aldenkamp, 2013; Haney et al., 2010; Sommer et al., 2013), not observed in guanfacine (Fox et al., 2012).
Although guanfacine enhanced aspects of attentional control overall, its effects were shown to be highly selective. For example, Paired Associates Learning which includes visual and spatial peripheral memory as well as delayed aspects of working memory commonly associated with planning and organizational skills (Champod and Petrides, 2007; Sahakian et al., 1988) remained unchanged. This is consistent with the lack of improvement observed in the RAVLT task which measures peripheral verbal recall (immediate and delayed), verbal recognition, and verbal learning. It also corroborates a lack of enhanced performance seen on the spatial working memory task, which again employs both a peripheral spatial memory component and a planning and self-ordered search strategy. Combined, these findings may broadly indicate dissociations in the effects of guanfacine between verbal and visuo-spatial episodic memory and executive function. In addition, its effects on working memory may be further disseminated into observed improvement in motor inhibition and attentional switching, but not strategic processes.
In terms of study limitations, the current participant sample is small and as such findings are preliminary. Second, the combination of two doses in the guanfacine group makes it harder to ascertain the exact therapeutic dose in terms of cognitive enhancement. In view of this, however, secondary analysis does show some indication that 2 mg of guanfacine may be adequate to enhance verbal fluency and attentional switching during early abstinence from cocaine. Third, the current sample is also composed predominantly of males, and future research is encouraged to assess the moderating effects of sex on guanfacine-induced cognitive changes. For example, a recent study from our own laboratory has shown that while guanfacine attenuates peripheral sympathetic arousal in both early abstinent cocaine-dependent men and women, anxiety, negative affect and craving decreases are significantly more robust in women (Fox et al., 2014).
Despite these limitations, preliminary findings indicate that attenuation of adrenergic drive as well as mood and anxiety symptoms via stimulation of alpha2 receptors may enhance selective inhibitory processes that are integral to behavioral change in substance abusers.
Acknowledgments
We would like to thank the staff at The Clinical Neuroscience Research Unit and The Substance Abuse Center located at The Connecticut Mental Health Center, as well as the staff at The Yale Stress Center for all their help in completing this study.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institutes of Health awards: K01 DA029040 (Fox) and R01 DA027130 (Sinha). All authors declare that they have no competing financial interests pertaining to the aims and results of this study.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Ahmadi J, Kampman K, Dackis C. Outcome predictors in cocaine dependence treatment trials. Am J Addict. 2006;15:434–439. doi: 10.1080/10550490600998476. [DOI] [PubMed] [Google Scholar]
- Alexander JK, Hillier A, Smith RM, et al. Beta-adrenergic modulation of cognitive flexibility during stress. J Cogn Neurosci. 2007;19:468–478. doi: 10.1162/jocn.2007.19.3.468. [DOI] [PubMed] [Google Scholar]
- Ambrose-Lanci LM, Sterling RC, Van Bockstaele EJ. Cocaine withdrawal-induced anxiety in females: Impact of circulating estrogen and potential use of delta-opioid receptor agonists for treatment. J Neurosci Res. 2010;88:816–824. doi: 10.1002/jnr.22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine modulation of prefrontal cortical cognitive function. Trends Cogn Sci. 1998;2:436–447. doi: 10.1016/s1364-6613(98)01240-6. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine influences on dorsolateral prefrontal cortical networks. Biol Psychiatry. 2011;69:e89–e99. doi: 10.1016/j.biopsych.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Contant TA. Alpha-2 adrenergic agonists decrease distractibility in aged monkeys performing the delayed response task. Psychopharmacology (Berl) 1992;108:159–169. doi: 10.1007/BF02245302. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Alpha 2-adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science. 1985;230:1273–1276. doi: 10.1126/science.2999977. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Analysis of alpha-2 adrenergic agonist effects on the delayed nonmatch-to-sample performance of aged rhesus monkeys. Neurobiol Aging. 1990;11:583–590. doi: 10.1016/0197-4580(90)90021-q. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Jin LE. Guanfacine for the treatment of cognitive disorders: A century of discoveries at Yale. Yale J Biol Med. 2012;85:45–58. [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Li BM. Neurobiology of executive functions: Catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Goldman-Rakic PS. The alpha-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: Evidence for alpha-2 receptor subtypes. J Neurosci. 1988;8:4287–4298. doi: 10.1523/JNEUROSCI.08-11-04287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J Comp Neurol. 2005;493:99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Gold JI. How we say no: Norepinephrine, inferior frontal gyrus, and response inhibition. Biol Psychiatry. 2009;65:548–549. doi: 10.1016/j.biopsych.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Mar AC, Theobald DE, et al. Prefrontal and monoaminergic contributions to stop-signal task performance in rats. J Neurosci. 2011;31:9254–9263. doi: 10.1523/JNEUROSCI.1543-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Inhibition and impulsivity: Behavioral and neural basis of response control. Prog Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Barry D, Bates ME, Labouvie E. FAS and CFL forms of verbal fluency differ in difficulty: A meta-analytic study. Appl Neuropsychol. 2008;15:97–106. doi: 10.1080/09084280802083863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearn J, Gossop M, Strang J. Randomised double-blind comparison of lofexidine and methadone in the in-patient treatment of opiate withdrawal. Drug Alcohol Depend. 1996;43:87–91. doi: 10.1016/s0376-8716(96)01289-6. [DOI] [PubMed] [Google Scholar]
- Begeer S, Wierda M, Scheeren AM, et al. Verbal fluency in children with autism spectrum disorders: Clustering and switching strategies. Autism. 2014;18:1014–1018. doi: 10.1177/1362361313500381. [DOI] [PubMed] [Google Scholar]
- Benton AL. Differential behavioural effects in frontal lobe disease. Neuropsychologia. 1968:455–461. [Google Scholar]
- Beversdorf DQ, White DM, Chever DC, et al. Central beta-adrenergic modulation of cognitive flexibility. Neuroreport. 2002;13:2505–2507. doi: 10.1097/00001756-200212200-00025. [DOI] [PubMed] [Google Scholar]
- Billieux J, Lagrange G, Van der Linden M, et al. Investigation of impulsivity in a sample of treatment-seeking pathological gamblers: A multidimensional perspective. Psychiatry Res. 2012;198:291–296. doi: 10.1016/j.psychres.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Bissonette GB, Martins GJ, Franz TM, et al. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J Neurosci. 2008;28:11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume AW, Marlatt GA. The role of executive cognitive functions in changing substance use: What we know and what we need to know. Ann Behav Med. 2009;37:117–125. doi: 10.1007/s12160-009-9093-8. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Lindgren KN, Bonaccorsy C, et al. Predictors of verbal fluency (FAS) in the healthy elderly. J Clin Psychol. 1990;46:623–628. doi: 10.1002/1097-4679(199009)46:5<623::aid-jclp2270460513>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Brevers D, Cleeremans A, Verbruggen F, et al. Impulsive action but not impulsive choice determines problem gambling severity. PLoS One. 2012;7:e50647. doi: 10.1371/journal.pone.0050647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, et al. Pretreatment brain activation during Stroop task is associated with outcomes in cocaine-dependent patients. Biol Psychiatry. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Kiluk BD, Nich C, et al. Cognitive function and treatment response in a randomized clinical trial of computer-based training in cognitive-behavioral therapy. Subst Use Misuse. 2011;46:23–34. doi: 10.3109/10826084.2011.521069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Morilak DA. Modulatory effects of norepinephrine, acting on alpha 1 receptors in the central nucleus of the amygdala, on behavioral and neuroendocrine responses to acute immobilization stress. Neuropharmacology. 2002;43:1139–1147. doi: 10.1016/s0028-3908(02)00292-7. [DOI] [PubMed] [Google Scholar]
- Champod AS, Petrides M. Dissociable roles of the posterior parietal and the prefrontal cortex in manipulation and monitoring processes. Proc Natl Acad Sci U S A. 2007;104:14837–14842. doi: 10.1073/pnas.0607101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell PB, Riddle MA, Scahill L, et al. Guanfacine treatment of comorbid attention-deficit hyperactivity disorder and Tourette’s syndrome: Preliminary clinical experience. J Am Acad Child Adolesc Psychiatry. 1995;34:1140–1146. doi: 10.1097/00004583-199509000-00010. [DOI] [PubMed] [Google Scholar]
- Childress AC. Guanfacine extended release as adjunctive therapy to psychostimulants in children and adolescents with attention-deficit/hyperactivity disorder. Adv Ther. 2012;29:385–400. doi: 10.1007/s12325-012-0020-1. [DOI] [PubMed] [Google Scholar]
- Clerkin SM, Schulz KP, Halperin JM, et al. Guanfacine potentiates the activation of prefrontal cortex evoked by warning signals. Biol Psychiatry. 2009;66:307–312. doi: 10.1016/j.biopsych.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato LS, Huizinga M, Hommel B. Recreational cocaine polydrug use impairs cognitive flexibility but not working memory. Psychopharmacology (Berl) 2009;207:225–234. doi: 10.1007/s00213-009-1650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato LS, van den Wildenberg WP, Hommel B. Impaired inhibitory control in recreational cocaine users. PLoS One. 2007;2:e1143. doi: 10.1371/journal.pone.0001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, et al. Mechanisms of cognitive set flexibility in Parkinson’s disease. Brain. 2001;124:2503–2512. doi: 10.1093/brain/124.12.2503. [DOI] [PubMed] [Google Scholar]
- De Oliveira LG, Barroso LP, Silveira CM, et al. Neuropsychological assessment of current and past crack cocaine users. Subst Use Misuse. 2009;44:1941–1957. doi: 10.3109/10826080902848897. [DOI] [PubMed] [Google Scholar]
- Decamp E, Clark K, Schneider JS. Effects of the alpha-2 adrenoceptor agonist guanfacine on attention and working memory in aged non-human primates. Eur J Neurosci. 2011;34:1018–1022. doi: 10.1111/j.1460-9568.2011.07815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M. The dopamine hypothesis of drug addiction and its potential therapeutic value. Front Psychiatry. 2011;2:64. doi: 10.3389/fpsyt.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: Effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Bari A, Robbins TW. The neuropsychopharmacology of action inhibition: Cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology (Berl) 2008;199:439–456. doi: 10.1007/s00213-008-1127-6. [DOI] [PubMed] [Google Scholar]
- Engberg G, Eriksson E. Effects of alpha2-adrenoceptor agonists on locus coeruleus firing rate and brain noradrenaline turnover in N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ)-treated rats. Naunyn Schmiedebergs Arch Pharmacol. 1991;343:472–7. doi: 10.1007/BF00169548. [DOI] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, et al. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18:5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Spencer T, et al. Efficacy of atomoxetine in adult attention-deficit/hyperactivity disorder: A drug-placebo response curve analysis. Behav Brain Funct. 2005;1:16. doi: 10.1186/1744-9081-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C, Murphy K, Foxe JJ, et al. A topography of executive functions and their interactions revealed by functional magnetic resonance imaging. Brain Res Cogn Brain Res. 2004;20:132–143. doi: 10.1016/j.cogbrainres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Fernandez-Serrano MJ, Perez-Garcia M, Perales JC, et al. Prevalence of executive dysfunction in cocaine, heroin and alcohol users enrolled in therapeutic communities. Eur J Pharmacol. 2010;626:104–112. doi: 10.1016/j.ejphar.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 2002;66:265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, et al. Structured Clinical Interview for DSM-IV. Patient Edition. Washington DC: American Psychiatric Press Inc; 1995. [Google Scholar]
- Fishbein DH, Herman-Stahl M, Eldreth D, et al. Mediators of the stress-substance-use relationship in urban male adolescents. Prev Sci. 2006;7:113–126. doi: 10.1007/s11121-006-0027-4. [DOI] [PubMed] [Google Scholar]
- Fox HC, Axelrod SR, Paliwal P, et al. Difficulties in emotion regulation and impulse control during cocaine abstinence. Drug Alcohol Depend. 2007a;89:298–301. doi: 10.1016/j.drugalcdep.2006.12.026. [DOI] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Hong KI, et al. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol Clin Exp Res. 2007b;31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Fox HC, Morgan PT, Sinha R. Sex differences in guanfacine effects on drug craving and stress arousal in cocaine-dependent individuals. Neuropsychopharmacology. 2014;39:1527–1537. doi: 10.1038/npp.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Seo D, Tuit K, et al. Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: preliminary findings. J Psychopharmacol. 2012;26:958–972. doi: 10.1177/0269881111430746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychol Rev. 2007;17:337–345. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- Gau SS, Shang CY. Improvement of executive functions in boys with attention deficit hyperactivity disorder: An open-label follow-up study with once-daily atomoxetine. Int J Neuropsycho-pharmacol. 2010;13:243–256. doi: 10.1017/S1461145709990836. [DOI] [PubMed] [Google Scholar]
- Gish EC, Miller JL, Honey BL, et al. Lofexidine, an {alpha}2-receptor agonist for opioid detoxification. Ann Pharmacother. 2010;44:343–351. doi: 10.1345/aph.1M347. [DOI] [PubMed] [Google Scholar]
- Golden CJ. A group version of the Stroop Color and Word Test. J Pers Assess. 1975;39:386–388. doi: 10.1207/s15327752jpa3904_10. [DOI] [PubMed] [Google Scholar]
- Gonzalez G, Sevarino K, Sofuoglu M, et al. Tiagabine increases cocaine-free urines in cocaine-dependent methadone-treated patients: Results of a randomized pilot study. Addiction. 2003;98:1625–1632. doi: 10.1046/j.1360-0443.2003.00544.x. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Owen AM. Fractionating attentional control using event-related fMRI. Cereb Cortex. 2006;16:1679–1689. doi: 10.1093/cercor/bhj116. [DOI] [PubMed] [Google Scholar]
- Haney M, Gunderson EW, Jiang H, et al. Cocaine-specific antibodies blunt the subjective effects of smoked cocaine in humans. Biol Psychiatry. 2010;67:59–65. doi: 10.1016/j.biopsych.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, D’Esposito M, Cole MW, et al. Neural mechanisms for response selection: Comparing selection of responses and items from working memory. Neuroimage. 2007;34:446–454. doi: 10.1016/j.neuroimage.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Hester RL, Murphy K, Foxe JJ, et al. Predicting success: Patterns of cortical activation and deactivation prior to response inhibition. J Cogn Neurosci. 2004;16:776–785. doi: 10.1162/089892904970726. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Alessi SM, Dantona RL. Voucher-based incentives. A substance abuse treatment innovation. Addict Behav. 2002;27:887–910. doi: 10.1016/s0306-4603(02)00297-6. [DOI] [PubMed] [Google Scholar]
- Hunt RD, Arnsten AF, Asbell MD. An open trial of guanfacine in the treatment of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1995;34:50–54. doi: 10.1097/00004583-199501000-00013. [DOI] [PubMed] [Google Scholar]
- Ijff DM, Aldenkamp AP. Cognitive side-effects of antiepileptic drugs in children. Handb Clin Neurol. 2013;111:707–718. doi: 10.1016/B978-0-444-52891-9.00073-7. [DOI] [PubMed] [Google Scholar]
- Jakala P, Riekkinen M, Sirvio J, et al. Guanfacine, but not clonidine, improves planning and working memory performance in humans. Neuropsychopharmacology. 1999a;20:460–470. doi: 10.1016/S0893-133X(98)00127-4. [DOI] [PubMed] [Google Scholar]
- Jakala P, Riekkinen M, Sirvio J, et al. Clonidine, but not guanfacine, impairs choice reaction time performance in young healthy volunteers. Neuropsychopharmacology. 1999b;21:495–502. doi: 10.1016/S0893-133X(99)00048-2. [DOI] [PubMed] [Google Scholar]
- Jarrott B, Louis WJ, Summers RJ. [3H]-guanfacine: A radioligand that selectively labels high affinity alpha2-adrenoceptor sites in homogenates of rat brain. Br J Pharmacol. 1982;75:401–408. doi: 10.1111/j.1476-5381.1982.tb08801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji XH, Ji JZ, Zhang H, et al. Stimulation of alpha2-adrenoceptors suppresses excitatory synaptic transmission in the medial prefrontal cortex of rat. Neuropsychopharmacology. 2008;33:2263–2271. doi: 10.1038/sj.npp.1301603. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Pettinati HM, Volpicelli JR, et al. Cocaine dependence severity predicts outcome in outpatient detoxification from cocaine and alcohol. Am J Addict. 2004;13:74–82. doi: 10.1080/10550490490265389. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, Mulvaney F, et al. Cocaine withdrawal severity and urine toxicology results from treatment entry predict outcome in medication trials for cocaine dependence. Addict Behav. 2002;27:251–260. doi: 10.1016/s0306-4603(01)00171-x. [DOI] [PubMed] [Google Scholar]
- Kang SY, Kleinman PH, Woody GE, et al. Outcomes for cocaine abusers after once-a-week psychosocial therapy. Am J Psychiatry. 1991;148:630–635. doi: 10.1176/ajp.148.5.630. [DOI] [PubMed] [Google Scholar]
- Kelley BJ, Yeager KR, Pepper TH, et al. The effect of propranolol on cognitive flexibility and memory in acute cocaine withdrawal. Neurocase. 2007;13:320–327. doi: 10.1080/13554790701846148. [DOI] [PubMed] [Google Scholar]
- Kenemans JL, Bekker EM, Lijffijt M, et al. Attention deficit and impulsivity: Selecting, shifting, and stopping. Int J Psychophysiol. 2005;58:59–70. doi: 10.1016/j.ijpsycho.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Le AD, Funk D, Juzytsch W, et al. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology (Berl) 2011;218:89–99. doi: 10.1007/s00213-011-2178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Milivojevic V, Kemp K, et al. Performance monitoring and stop signal inhibition in abstinent patients with cocaine dependence. Drug Alcohol Depend. 2006;85:205–212. doi: 10.1016/j.drugalcdep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: A model and a method. J Exp Psychol Hum Percept Perform. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Lorea I, Fernandez-Montalvo J, Tirapu-Ustarroz J, et al. Neuro-psychological performance in cocaine addiction: A critical review. Rev Neurol. 2010;51:412–426. [PubMed] [Google Scholar]
- MacDonald E, Kobilka BK, Scheinin M. Gene targeting – homing in on alpha 2-adrenoceptor-subtype function. Trends Pharmacol Sci. 1997;18:211–219. doi: 10.1016/s0165-6147(97)01063-8. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppelman LF. Manual for the Profile of Mood States. San Diego: Educational and industrial Testing Service; 1971. [Google Scholar]
- Mercer DE, Woody GE. Therapy Manuals for Drug Addiction Series: Individual Drug Counseling. Maryland: US Department of Health and Human Services; 1999. NIH Pub. No. 99–4380. [Google Scholar]
- Mischel W. Toward an integrative science of the person. Annu Rev Psychol. 2004;55:1–22. doi: 10.1146/annurev.psych.55.042902.130709. [DOI] [PubMed] [Google Scholar]
- Mosqueda-Garcia R. Guanfacine: A second generation alpha 2-adrenergic blocker. Am J Med Sci. 1990;299:73–76. doi: 10.1097/00000441-199001000-00016. [DOI] [PubMed] [Google Scholar]
- Naifeh JA, Tull MT, Gratz KL. Anxiety sensitivity, emotional avoidance, and PTSD symptom severity among crack/cocaine dependent patients in residential treatment. Cognit Ther Res. 2012;36:247–257. doi: 10.1007/s10608-010-9337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT. Is ADHD a disinhibitory disorder? Psychol Bull. 2001;127:571–598. doi: 10.1037/0033-2909.127.5.571. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Silk KR, Stavro G, et al. Disinhibition and borderline personality disorder. Dev Psychopathol. 2005;17:1129–1149. doi: 10.1017/s0954579405050534. [DOI] [PubMed] [Google Scholar]
- O’Brien MS, Anthony JC. Risk of becoming cocaine dependent: Epidemiological estimates for the United States, 2000–2001. Neuropsychopharmacology. 2005;30:1006–1018. doi: 10.1038/sj.npp.1300681. [DOI] [PubMed] [Google Scholar]
- O’Leary TA, Rohsenow DJ, Martin R, et al. The relationship between anxiety levels and outcome of cocaine abuse treatment. Am J Drug Alcohol Abuse. 2000;26:179–194. doi: 10.1081/ada-100100599. [DOI] [PubMed] [Google Scholar]
- Rama P, Linnankoski I, Tanila H, et al. Medetomidine, atipamezole, and guanfacine in delayed response performance of aged monkeys. Pharmacol Biochem Behav. 1996;55:415–422. doi: 10.1016/s0091-3057(96)00111-6. [DOI] [PubMed] [Google Scholar]
- Ramanujam VM, Anderson KE, Grady JJ, et al. Riboflavin as an oral tracer for monitoring compliance in clinical research. Open Biomark J. 2011;2011:1–7. doi: 10.2174/1875318301104010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. L`examinen clinique en psychologie. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- Roberts DC. Preclinical evidence for GABAB agonists as a pharmacotherapy for cocaine addiction. Physiol Behav. 2005;86:18–20. doi: 10.1016/j.physbeh.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Brebner K. GABA modulation of cocaine self-administration. Ann N Y Acad Sci. 2000;909:145–158. doi: 10.1111/j.1749-6632.2000.tb06680.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Jimenez R, Avila C, Jimenez-Arriero MA, et al. Impulsivity and sustained attention in pathological gamblers: Influence of childhood ADHD history. J Gambl Stud. 2006;22:451–461. doi: 10.1007/s10899-006-9028-2. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Andrews TC, Grasby PM, et al. Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. J Cogn Neurosci. 2000;12:142–162. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Morris RG, Evenden JL, et al. A comparative study of visuospatial memory and learning in Alzheimer-type dementia and Parkinson’s disease. Brain. 1988;111 ( Pt 3):695–718. doi: 10.1093/brain/111.3.695. [DOI] [PubMed] [Google Scholar]
- Sallee FR, Kollins SH, Wigal TL. Efficacy of guanfacine extended release in the treatment of combined and inattentive only subtypes of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2012;22:206–214. doi: 10.1089/cap.2010.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Hervas E, Llorente del Pozo JM. Relapse in cocaine addiction: a review. Adicciones. 2012;24:269–279. [PubMed] [Google Scholar]
- Scahill L, Chappell PB, Kim YS, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067–1074. doi: 10.1176/appi.ajp.158.7.1067. [DOI] [PubMed] [Google Scholar]
- Seedat YK. Clonidine and guanfacine – comparison of their effects on haemodynamics in hypertension. S Afr Med J. 1985;67:557–559. [PubMed] [Google Scholar]
- Senderecka M, Grabowska A, Szewczyk J, et al. Response inhibition of children with ADHD in the stop-signal task: An event-related potential study. Int J Psychophysiol. 2012;85:93–105. doi: 10.1016/j.ijpsycho.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, et al. The reinstatement model of drug relapse: History, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shang CY, Gau SS. Improving visual memory, attention, and school function with atomoxetine in boys with attention-deficit/hyper-activity disorder. J Child Adolesc Psychopharmacol. 2012;22:353–363. doi: 10.1089/cap.2011.0149. [DOI] [PubMed] [Google Scholar]
- Shields AD, Wang Q, Winder DG. Alpha2A-adrenergic receptors heterosynaptically regulate glutamatergic transmission in the bed nucleus of the stria terminalis. Neuroscience. 2009;163:339–351. doi: 10.1016/j.neuroscience.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, et al. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KI, et al. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry. 2011;68:942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soar K, Mason C, Potton A, et al. Neuropsychological effects associated with recreational cocaine use. Psychopharmacology (Berl) 2012;222:633–643. doi: 10.1007/s00213-012-2666-4. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, et al. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2013;64:452–463. doi: 10.1016/j.neuropharm.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer BR, Mitchell EL, Wroolie TE. Topiramate: Effects on cognition in patients with epilepsy, migraine headache and obesity. Ther Adv Neurol Disord. 2013;6:211–227. doi: 10.1177/1756285613481257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin EM, Heel RC. Guanfacine. A review of its pharmaco-dynamic and pharmacokinetic properties, and therapeutic efficacy in the treatment of hypertension. Drugs. 1986;31:301–336. doi: 10.2165/00003495-198631040-00003. [DOI] [PubMed] [Google Scholar]
- Streeter CC, Terhune DB, Whitfield TH, et al. Performance on the Stroop predicts treatment compliance in cocaine-dependent individuals. Neuropsychopharmacology. 2008;33:827–836. doi: 10.1038/sj.npp.1301465. [DOI] [PubMed] [Google Scholar]
- Summers RJ, Jarrott B, Louis WJ. Comparison of [3H]clonidine and [3H]guanfacine binding to alpha 2 adrenoceptors in membranes from rat cerebral cortex. Neurosci Lett. 1981;25:31–36. doi: 10.1016/0304-3940(81)90096-3. [DOI] [PubMed] [Google Scholar]
- Swann NC, Tandon N, Pieters TA, et al. Intracranial electroencephalography reveals different temporal profiles for dorsal- and ventro-lateral prefrontal cortex in preparing to stop action. Cereb Cortex. 2013;23:2479–2488. doi: 10.1093/cercor/bhs245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz BE, McDonald CR, Patel A, et al. The effects of guanfacine on working memory performance in patients with localization-related epilepsy and healthy controls. Clin Neuropharmacol. 2008;31:251–260. doi: 10.1097/WNF.0b013e3181633461. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Taintor NB, Rivera SN, et al. DRD2 gene transfer into the nucleus accumbens core of the alcohol preferring and non-preferring rats attenuates alcohol drinking. Alcohol Clin Exp Res. 2004;28:720–728. doi: 10.1097/01.alc.0000125270.30501.08. [DOI] [PubMed] [Google Scholar]
- Tice DM, Bratslavsky E, Baumeister RF. Emotional distress regulation takes precedence over impulse control: If you feel bad, do it! J Pers Soc Psychol. 2001;80:53–67. [PubMed] [Google Scholar]
- Troyer AK, Moscovitch M, Winocur G. Clustering and switching as two components of verbal fluency: Evidence from younger and older healthy adults. Neuropsychology. 1997;11:138–146. doi: 10.1037//0894-4105.11.1.138. [DOI] [PubMed] [Google Scholar]
- Troyer AK, Moscovitch M, Winocur G, et al. Clustering and switching on verbal fluency tests in Alzheimer’s and Parkinson’s disease. J Int Neuropsychol Soc. 1998;4:137–143. doi: 10.1017/s1355617798001374. [DOI] [PubMed] [Google Scholar]
- Turner MA. Generating novel ideas: Fluency performance in high-functioning and learning disabled individuals with autism. J Child Psychol Psychiatry. 1999;40:189–201. [PubMed] [Google Scholar]
- Turner TH, LaRowe S, Horner MD, et al. Measures of cognitive functioning as predictors of treatment outcome for cocaine dependence. J Subst Abuse Treat. 2009;37:328–334. doi: 10.1016/j.jsat.2009.03.009. [DOI] [PubMed] [Google Scholar]
- van Holst RJ, Schilt T. Drug-related decrease in neuropsychological functions of abstinent drug users. Curr Drug Abuse Rev. 2011;4:42–56. doi: 10.2174/1874473711104010042. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Perez-Garcia M. Profile of executive deficits in cocaine and heroin polysubstance users: Common and differential effects on separate executive components. Psychopharmacology (Berl) 2007;190:517–530. doi: 10.1007/s00213-006-0632-8. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Betanzos-Espinosa P, Lozano OM, et al. Self-regulation and treatment retention in cocaine dependent individuals: A longitudinal study. Drug Alcohol Depend. 2012;122:142–148. doi: 10.1016/j.drugalcdep.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Verrico CD, Haile CN, Newton TF, et al. Pharmacotherapeutics for substance-use disorders: A focus on dopaminergic medications. Expert Opin Investig Drugs. 2013 doi: 10.1517/13543784.2013.836488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitkovitch M, Bishop S, Dancey C, et al. Stroop interference and negative priming in patients with multiple sclerosis. Neuropsychologia. 2002;40:1570–1576. doi: 10.1016/s0028-3932(02)00022-2. [DOI] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Ye Z, Altena E, Nombela C, et al. Improving response inhibition in Parkinson’s disease with atomoxetine. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.01.024. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]