Abstract
Background
Usher syndrome (USH) is a clinically and genetically heterogeneous disease. The three recognised clinical phenotypes (types I, II and III; USH1, USH2 and USH3) are caused by mutations in nine different genes. USH2C is characterised by moderate to severe hearing loss, retinitis pigmentosa and normal vestibular function. One earlier report describes mutations in GPR98 (VLGR1) in four families segregating this phenotype.
Objective
To detect the disease-causing mutation in an Iranian family segregating USH2C. In this family, five members had a phenotype compatible with Usher syndrome, and two others had nonsyndromic hearing loss.
Methods
Mutation analysis of all 90 coding exons of GPR98.
Results
Consistent with these clinical findings, the five subjects with USH carried a haplotype linked to the USH2C locus, whereas the two subjects with nonsyndromic hearing loss did not. We identified a new mutation in GPR98 segregating with USH2C in this family. The mutation is a large deletion g.371657_507673del of exons 84 and 85, presumably leading to a frameshift.
Conclusions
A large GPR98 deletion of 136 017 bp segregates with USH2C in an Iranian family. To our knowledge, this is only the second report of a GPR98 mutation, and the first report on male subjects with USH2C and a GPR98 mutation.
Keywords: Usher syndrome type II, USH2C, VLGR1
INTRODUCTION
Usher syndrome (USH) is a genetically and clinically complex autosomal recessive disorder with an estimated prevalence of 3–6.2 per 100 000. [1] Three distinct types of USH (types I, II and III; USH1, USH2 and USH3) are recognised, all of them characterised by hearing loss and retinitis pigmentosa (RP). These three types can be distinguished based on specific clinical characteristics such as the presence or absence of vestibular symptoms, and the degree and progression of the hearing impairment. Although, this classification is generally adequate, atypical clinical types have been described, which defy easy classification. This limitation has been overcome in part by the identification of genes causing USH. To date, 12 different USH loci have been mapped, for which 9 genes have been identified. At least five of these genes have also been implicated in nonsyndromic hearing loss (MYO7A, USH1C, CDH23, PCDH15 and DFNB31).
USH type II (OMIM 605472) is characterised by congenital, stable hearing loss, which is most pronounced in the mid to high frequencies. The onset of RP generally occurs in the second decade of life or later and no vestibular symptoms are observed. Four USH2 loci have been mapped and three genes have been identified: USH2A (OMIM 276901), GPR98 (OMIM 602851; VLGR1) and DFNB31 (OMIM 607928). [2–5] GPR98 is a large gene encompassing 605 kb. Three human GPR98 mRNA isoforms are known, of which isoform b is the largest and contains all 90 exons. Isoform a starts in intron 64 of isoform b, and contains the last 26 exons. Isoform c has the same start codon as isoform b but only has 31 exons. All three isoforms are expressed during the development of the central nervous system and are also found in the fetal retina.
The translated GPR98 protein is one of the largest proteins found in humans. It belongs to the large N-terminal family B (LNB) of seven-transmembrane segment (7TM) receptors; specifically, to the subfamily with a G-protein-coupled proteolysis site (GPS) for G-protein signalling. The protein consists of several domains, each with a specific function. The N-terminal 29 amino acids of isoform b are hydrophobic and may act as a signal sequence for cleavage in the mature protein. Next, there are 35 Calxβ-repeat domains, which are responsible for protein–protein interactions, possibly Ca2+-mediated. Exons 20 and 21, in between the Calxβ repeats, partly code for a region with similarity to a pentraxin (PTX) domain, although its functional significance is uncertain. In aggregate, these domains form the large ectodomain at the N-terminal side of the protein, after which there is the GPS and the 7TM near the carboxyterminus. Exons 82–88 encode for the GPS and the 7TM and are widely spread over an area about 300 kb in length. [6]
To study the function of GPR98 in the inner ear, Gpr98/del7TM mutant mice were constructed by deleting the transmembrane and cytoplasmic domains of Gpr98.7 The Gpr98/del7TM homozygous mutant mice have a normal vestibular function but with hearing loss, as is also seen in human patients with USH2C. However, the mice become profoundly deaf by postnatal day 20 and they do not have a retinal phenotype. Studies of the mouse mutant have shown that the murine protein is probably critical in maintaining the form of the hair bundle in the later developmental stages. [7] The protein has also been implicated as a possible component of the ankle link complex.
In 2002, a mutation in a gene called MASS1 was reported to cause febrile and afebrile seizures in one family and the gene was afterwards found to be part of GPR98. [8] The spontaneous mouse mutant Frings has a mutation in Mass1, making this mouse prone to audiogenic seizures.[9] Subsequent reports verified that GPR98 was responsible for USH type II and to date, four USH2C mutations have been identified in GPR98.3 Three of these mutations, Q2301X, I2906FS and M2931FS, are located in the Calxβ repeat domains and are only present in isoform b of GPR98. The fourth mutation, T6244X, is located in exon 89 downstream of the 7TM and is present in both isoforms a and b. In this report, we have identified a new mutation in GPR98, causing USH2C in both male and female members of an Iranian family.
MATERIALS AND METHODS
This study was approved by the ethics committee of the University of Antwerp, Belgium and the University of Social Welfare and Rehabilitation Sciences in Tehran, Iran, and written informed consent was obtained from all participants before inclusion into the study.
Clinical data
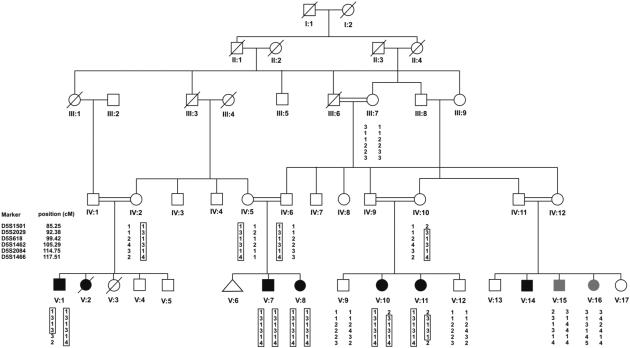
The study was carried out on an Iranian family in which affected members have a phenotype compatible with USH2C (Fig 1). In total, 13 family members participated in the study. The diagnosis of USH was based on a clinical examination of five affected family members (V:1, V:7, V:8, V:10 and V:11). Audiograms were obtained by pure-tone audiometry at frequencies of 0.25, 0.5, 1, 2, 4 and 8 kHz. To investigate the presence of vestibular dysfunction, infrared/video electronystagmography (ENG) (Eye Dynamics Inc., Torrance, CA.) was performed for all members except patient V:1. To evaluate the presence of eye abnormalities, a variety of methods were used including a general ophthalmic examination (slit-lamp biomicroscopy), Goldmann kinetic perimetry, funduscopy to detect pigmentary changes, and full-field electroretinography (ERG) of the cones by measuring both photoptic responses and 30 Hz flicker responses (Metrovision, Pérenchies, France). In one subject, we also obtained cross-sectional images of the central retina using optical coherence tomography (OCT) to study the central retinal microstructure (Topcon, Newbury, UK). Retinal thickness measurements were made at the fovea and at the perifoveal temporal retinal loci. Retinal thickness was quantified at all loci. All subjects were investigated for the presence of febrile or afebrile seizures.
Figure 1.
Pedigree of the Iranian family with Usher syndrome type IIC. Black symbols indicate individuals with USH2C, open symbols represent unaffected individuals. The grey symbols indicate individuals with a nonsyndromic form of hearing loss. On the left, the markers are listed which were analysed to define the candidate region. The haplotypes for all analysed individuals are shown, with the linked haplotype indicated by a box. VLGR1 is located between markers D5S618 and D5S1462. Individual V:2 and V:14 were thought to be affected, based on anamnestical data, but they were not fully examined.
Genetic analysis
Based on an autosomal recessive mode of inheritance and on the clinical diagnosis of all participants, SLINK simulations were performed using the Easylinkage V.4.01 (Easylinkage, Berlin, Germany). [10] SLINK scores were .3.3, indicating that the family was sufficiently informative for linkage analysis. [11] All genotyping was performed by PCR and polyacrylamide gel electrophoresis. A genome-wide search was performed by the company Prevention Genetics (Marshfield, Wisconsin, USA), using a polymorphic set of 400 short tandem repeat (STR) markers spread across the genome. Linkage analysis was performed by calculating two-point and multipoint LOD scores with the Easylinkage software (linkage parameters: disease allele frequency= 0.001, with phenocopy rate=0% and penetrance= 0% for wild-type/wild-type and wild-type/mutant and 100% for mutant/mutant). Two-point LOD scores >3.3 were considered significant for linkage and scores, [22] were considered significant for exclusion of a locus. If the two-point LOD score was between 3.3 and 22, multipoint LOD scores were calculated to confirm or exclude linkage. [11]
Primers were developed for all 90 exons and exon–intron boundaries of GPR98 (Genbank NG_007083). In addition, extra primers were developed in intron 83 and 85 to characterise the deletion breakpoints. The non-coding exon 1 and the coding exon 2 of GJB2 (RefSeq NM_004004.4) were amplified by PCR for V:15 and V:16. DNA sequencing of exon 2 was performed using four additional sequencing primers. PCR products were sequenced using an automated DNA sequencer (ABI 3130; Applied Biosystems, California, USA).
RESULTS
Clinical data
Based on clinical examination, five subjects showed a phenotype compatible with USH (females: V:8, V:10 and V:11; males: V:1 and V:7). Individuals V:2 and V:14 were reported to be affected by family members. One of them was deceased (V:2) and the other chose not to participate in this study (V:14). For individual V:1 (53 years old), an audiogram was obtained but no eye or balance tests could be performed. The patient was almost completely blind. Table 1 summarizes all clinical data of the five investigated members with Usher syndrome and of V:15 and V:16, who had atypical hearing loss.
Table 1.
Summary of all clinical symptoms of five patients with Usher syndrome type IIC and individuals V:15 and V:16 with atypical hearing loss.
| Individual | genotype | hearing loss | fundoscopic examination |
ERG | OCT | ENG |
|---|---|---|---|---|---|---|
| V:1 | del/del | moderate-severe across all frequencies |
loss of vision | / | / | / |
| V:7 | del/del | moderate-severe across all frequencies |
retinitis pigmentosa | cone degeneration |
increased inner retinal thickness |
directional preponderance 27% left, unilateral weakness 36% left |
| V:8 | del/del | moderate-severe across all frequencies |
retinitis pigmentosa | cone degeneration |
/ | directional preponderance 35% right |
| V:10 | del/del | moderate-severe across all frequencies |
retinitis pigmentosa | / | / | directional preponderance 13% right |
| V:11 | del/del | moderate-severe across all frequencies |
retinitis pigmentosa | / | / | normal |
| V:15 | wt/wt | severe-profound in mid to high frequencies |
normal | / | / | / |
| V:16 | wt/wt | severe-profound in mid to high frequencies |
normal | / | / | / |
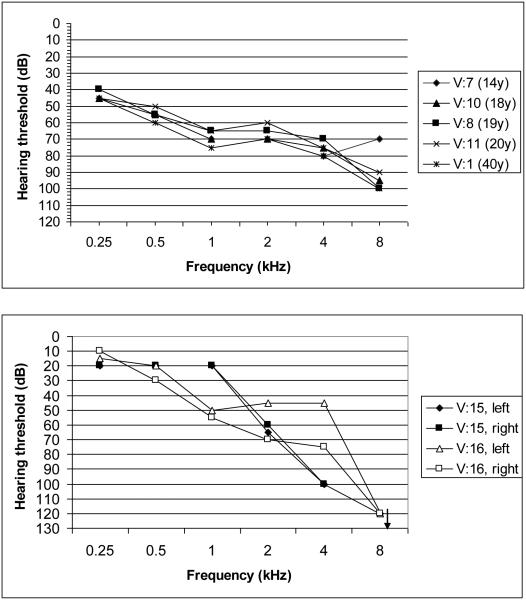
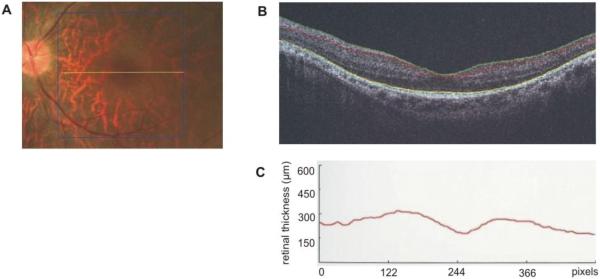
The audiograms of the five members with Usher syndrome and of V:15 and V:16 are shown in Figure 2. Audiograms from all subjects with USH showed moderate to severe sensorineural hearing loss across all frequencies, with an increasing degree of hearing loss toward the higher frequencies. V:15 and V:16 had a much more downward-sloping audiogram, with normal thresholds in the low frequencies, progressing towards profound hearing loss in the high frequencies. Funduscopic examination in these members did not reveal any abnormalities, although V:15 had myopia. As this phenotype was not similar to the clinical picture observed in the other affected family members, we believe that their hearing loss was of a different genetic aetiology. ENGs of four affected members did not show marked abnormalities. Two members had normal test results and two others had mild unilateral weakness. Under fundoscopy, an RP pattern was observed in the retina of all four investigated members with Usher syndrome, with an age of diagnosis in the first to second decade. ERG revealed a cone dystrophy (CD) in V:7 and V:8, which was symmetrical for both eyes. Both of these people also had myopic astigmatism. For both eyes of subject V:7, we performed OCT, which showed increased inner retinal thickness with a maximum of 245 mm for the right eye and 248 mm for the left eye (normal range 150–200 mm) (Fig 3). No cystic changes were seen in the central foveal region. V:7 also had anosmia and patient V:8 had asthma. There was no history of seizures in any subject.
Figure 2.
A: Audiograms of the best ear of 5 Usher patients of the family. The audiograms are all slightly downsloping and show a moderate-to-severe hearing loss across all frequencies. B: Audiograms of both ears of individuals V:15 and V:16 who have another type of hearing loss. The audiogram is clearly downsloping, with normal hearing thresholds in the low frequencies and profound hearing loss in the high frequencies.
Figure 3.
OCT of the fovea and perifoveal area of the left eye of patient V:7. (A) The location were the cross-section was made (B) OCT image, showing increased inner retinal thickness. The pit in the middle of the picture is the fovea. (C) A graph showing the thickness of the inner retinal layer. A maximal thickness of 248 μm was seen, while normal values range between 150 and 200 μm.
Linkage analysis
As individuals V:15 and V:16 showed an atypical phenotype, they were coded as unaffected for linkage analysis. Persons V:2 and V:14 were coded as affected based on anamnestic data. SLINK calculations revealed a maximal LOD score of 6.17, indicating that the family is informative enough for genome-wide linkage analysis. A genome-wide scan was performed and revealed linkage to a region on chromosome 5q14. The analysis of extra microsatellite markers allowed refinement of the linked region to 12.91 cM between markers D5S2029 (centromeric) and D5S1462 (telomeric). This region shows partial overlap with the known USH2C locus and shows complete overlap with nonsyndromic locus DFNB49. [12] The haplotypes for the linked region are shown in Figure 1. The maximum two-point LOD score was 3.76 at O=0 for marker D5S2029 and the maximal multipoint LOD score was 4.6. Individuals V:15 and V:16 did not carry the same genotype as the Usher patients, as was predicted based on the clinical data.
Mutation analysis of VLGR1 and GJB2
GPR98 has been identified as the disease-causing gene for locus USH2C and was located in the linked region of this family. Therefore, DNA sequencing of all 90 exons and intron–exon boundaries of the gene was performed. Three new variants were identified, but none of them was considered pathogenic as they were found in both affected and unaffected family members. However, we did find that exons 84 and 85 could only be amplified by PCR in control samples but not in affected samples. Exons 83 and 86 could be amplified normally, suggesting the presence of a homozygous deletion comprising exons 84 and 85. Additional PCR assays were performed with new primers located in introns 83 and 85 to locate the deletion breakpoints. Using primers 5’-CCAGAGGCATATCATGAGTCC-3’ and 3’-GTTGGGCCAGAATGTTAAGG-5’, the deletion could be spanned in affected samples and resulted in a PCR fragment of approximately 630 bp. The predicted fragment in control samples was 136 653 bp (theoretically) and could not be amplified by PCR. The PCR product obtained from affected samples was purified and sequenced, and revealed the proximal and distal deletion breakpoints at positions 371 657 and 507 673, respectively. The deletion g.371657_507673del is 136 017 bp long and deletes exons 84 and 85 and part of introns 83 and 85. At the protein level, it causes the amino acid change p.Ile5954ValfsX42 and affects both GPR98 isoforms a and b. The deletion segregated with the phenotype and was not present in 100 Iranian control samples. The two subjects, V:15 and V:16, with an atypical clinical picture did not carry the GPR98 mutation. As GJB2 is the most common cause of autosomal recessive hearing loss, DNA sequencing of the non-coding and coding exon of the gene was performed for V:15 and V:16. No mutation could be identified, indicating that GJB2 is probably not responsible for the hearing loss in these subjects.
DISCUSSION
We describe the phenotype and genotype of an Iranian family with type IIC Usher syndrome. The clinical evaluation indicated moderate to severe hearing loss, as has also been observed in other people with USH2C. The hearing loss is remarkably similar in all members, with slow to no progression below the age of 40 years (Fig 2A). Pronounced vestibular dysfunction was not seen in any of the members, which is consistent with the phenotype in other people with USH2C. With respect to the ocular findings, a detailed description of the retinal phenotype of USH2C has been reported by Schwartz et al. [13] The main changes these investigators observed were RP, characterized by rod–cone degeneration or abnormalities of the retinal pigment epithelium. In addition, they found a reduced outer nuclear layer at the perifoveal locus, an increased inner retinal layer, and a central retinal architecture that was normal but could be distorted by macular cystic changes. The ocular phenotype we observed in V:7 was very similar to that described by Schwartz et al, with an increased inner retinal thickness (245–248 mm) and no central retinal changes in the absence of cystic changes. Both V:7 and V:8 also had cone degeneration as shown by ERG. For V:10 and V:11, a funduscopic examination indicated an ocular phenotype compatible with RP. The age of diagnosis of RP was in the first to second decade in all family members, which is earlier than in most other patients with USH2C previously reported. [3]
Deletion g.371657_507673del in GPR98 was found to be the cause of Usher syndrome in this family. This deletion, which results in omission of exons 84 and 85, probably arose due to forward replication slippage caused by the two perfect short direct 7 bp repeats we found at the deletion breakpoints. [14] The slippage will cause the elimination of the intervening sequence between the repeats, and the loss of one repeat. The consequence is a frameshift and a premature termination codon, p.Ile5954ValfsX42. If the abnormal mRNA is not degraded by nonsense-mediated mRNA decay, the protein function is predicted to be grossly abnormal as the deleted exons encode part of the 7TM domain of GPR98. Furthermore, the intracellular region of this domain is thought to be involved in intracellular signal transduction, which may be crucial for proper protein function. [15] V:15 and V:16 were not diagnosed with the same disease as the other affected family members, based on several findings. First, the phenotype of their hearing loss was quite different from their relatives with Usher syndrome, with normal thresholds in the low frequencies and profound hearing loss at the high frequencies. Second, the patients had no other symptoms characteristic of Usher syndrome. This clinical impression was confirmed by the genetic results. Neither V:15 nor V:16 carries the disease-linked haplotype, nor do they have the GPR98 mutation that was identified as disease-causing in other members of the family. Because the hearing loss was nonsyndromic in these members, GJB2 was analysed by DNA sequencing but no mutations were found.
We looked for a possible genotype–phenotype correlation between mutation type and USH2C phenotype. However, the clinical picture appears similar for all USH2C members described, regardless of the mutation. Furthermore, the localization of the mutation in a specific domain of the protein does not seem to influence the phenotype significantly. It is interesting to note that this family is the first in which male subjects have been identified with USH2C and a GPR98 mutation; all 13 people with USH2C previously reported were female. We believe that the lack of male patients is probably due to chance, as we have no reason to assume a female preponderance for USH2C, based on the clinical data available.
Usher syndrome represents an important cause of deaf–blindness, and because clinical data can be ambiguous, comprehensive and robust molecular diagnostics for USH are needed. Although an Usher syndrome genotyping microarray has been developed that fulfils some of these requisites, a great disadvantage of the platform is that it detects only known variants (429 different known variants in 8 Usher syndrome genes). [16] Clearly, new technologies are needed to allow screening of the Usher syndrome genes with detection of known and new variants.
In conclusion, we have identified a large deletion in GPR98 that probably causes a frameshift, resulting in Usher syndrome type IIC in this family. For the first time, male patients have been identified with USH2C and a GPR98 mutation. This report is the first to report on a GPR98 deletion in USH2C and only the second molecular report on GPR98 mutations in USH2C. The lack of additional families with GPR98 mutations could be attributed to the fact that GPR98 mutations are not a frequent cause of USH type II (~15%). Alternatively, USH2C patients may be clinically misclassified and therefore, the need for mutation screening of GPR98 is unrecognized. Certainly, screening this gene is very labor intense. Optimizing the genetic diagnoses of the USH should clearly be a priority in the clinical management of deaf and deaf-blind patients.
Acknowledgments
This study was supported by grants from EUROHEAR (LSHG-CT-20054-512063) by the Iranian National Science Foundation (Grant 85033/10), by the Fund for Scientific Research Flanders (FWO-F, Grant G.0138.07) and by a grant from the National Institutes of Health (DC02842, RJHS). Nele Hilgert is a fellow of the Fund for Scientific Research Flanders (FWO-F).
REFERENCES
- [1].Cohen M, Bitner-Glindzicz M, Luxon L. Int J Audiol. 2007;46:82–93. doi: 10.1080/14992020600975279. [DOI] [PubMed] [Google Scholar]
- [2].Eudy JD, Weston MD, Yao S, Hoover DM, Rehm HL, Ma-Edmonds M, Yan D, Ahmad I, Cheng JJ, Ayuso C, Cremers C, Davenport S, Moller C, Talmadge CB, Beisel KW, Tamayo M, Morton CC, Swaroop A, Kimberling WJ, Sumegi J. Science. 1998;280:1753–7. doi: 10.1126/science.280.5370.1753. [DOI] [PubMed] [Google Scholar]
- [3].Weston MD, Luijendijk MW, Humphrey KD, Moller C, Kimberling WJ. Am J Hum Genet. 2004;74:357–66. doi: 10.1086/381685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pieke-Dahl S, Moller CG, Kelley PM, Astuto LM, Cremers CW, Gorin MB, Kimberling WJ. J Med Genet. 2000;37:256–62. doi: 10.1136/jmg.37.4.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ebermann I, Scholl HP, Charbel Issa P, Becirovic E, Lamprecht J, Jurklies B, Millan JM, Aller E, Mitter D, Bolz H. A novel gene for Usher syndrome type 2: mutations in the long isoform of whirlin are associated with retinitis pigmentosa and sensorineural hearing loss. Hum Genet. 2007;121:203–11. doi: 10.1007/s00439-006-0304-0. [DOI] [PubMed] [Google Scholar]
- [6].McMillan DR, Kayes-Wandover KM, Richardson JA, White PC. Very large G protein-coupled receptor-1, the largest known cell surface protein, is highly expressed in the developing central nervous system. J Biol Chem. 2002:785–92. doi: 10.1074/jbc.M108929200. [DOI] [PubMed] [Google Scholar]
- [7].McGee J, Goodyear RJ, McMillan DR, Stauffer EA, Holt JR, Locke KG, Birch DG, Legan PK, White PC, Walsh EJ, Richardson GP. J Neurosci. 2006;26:6543–53. doi: 10.1523/JNEUROSCI.0693-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nakayama J, Fu YH, Clark AM, Nakahara S, Hamano K, Iwasaki N, Matsui A, Arinami T, Ptacek LJ. Ann Neurol. 2002;52:654–7. doi: 10.1002/ana.10347. [DOI] [PubMed] [Google Scholar]
- [9].Skradski SL, Clark AM, Jiang H, White HS, Fu YH, Ptacek LJ. A novel gene causing a mendelian audiogenic mouse epilepsy. Neuron. 2001;31:537–44. doi: 10.1016/s0896-6273(01)00397-x. [DOI] [PubMed] [Google Scholar]
- [10].Lindner TH, Hoffmann K. Bioinformatics. 2005;21:405–7. doi: 10.1093/bioinformatics/bti009. [DOI] [PubMed] [Google Scholar]
- [11].Lander E, Kruglyak L. Nat Genet. 1995;11:241–7. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- [12].Ramzan K, Shaikh RS, Ahmad J, Khan SN, Riazuddin S, Ahmed ZM, Friedman TB, Wilcox ER, Riazuddin S. A new locus for nonsyndromic deafness DFNB49 maps to chromosome 5q12.3–q14.1. Hum Genet. 2005;116:17–22. doi: 10.1007/s00439-004-1205-8. [DOI] [PubMed] [Google Scholar]
- [13].Schwartz SB, Aleman TS, Cideciyan AV, Windsor EA, Sumaroka A, Roman AJ, Rane T, Smilko EE, Bennett J, Stone EM, Kimberling WJ, Liu XZ, Jacobson SG. Invest Ophthalmol Vis Sci. 2005;46:734–43. doi: 10.1167/iovs.04-1136. [DOI] [PubMed] [Google Scholar]
- [14].Chen JM, Chuzhanova N, Stenson PD, Ferec C, Cooper DN. Hum Mutat. 2005;25:207–21. doi: 10.1002/humu.20133. [DOI] [PubMed] [Google Scholar]
- [15].Stacey M, Lin HH, Gordon S, McKnight AJ. Trends Biochem Sci. 2000;25:284–9. doi: 10.1016/s0968-0004(00)01583-8. [DOI] [PubMed] [Google Scholar]
- [16].Cremers FP, Kimberling WJ, Kulm M, de Brouwer AP, van Wijk E, te Brinke H, Cremers CW, Hoefsloot LH, Banfi S, Simonelli F, Fleischhauer JC, Berger W, Kelley PM, Haralambous E, Bitner-Glindzicz M, Webster AR, Saihan Z, De Baere E, Leroy BP, Silvestri G, McKay GJ, Koenekoop RK, Millan JM, Rosenberg T, Joensuu T, Sankila EM, Weil D, Weston MD, Wissinger B, Kremer H. J Med Genet. 2007;44:153–60. doi: 10.1136/jmg.2006.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]