Abstract
This study examined the association between prenatal tobacco exposure (PTE) and infant cortisol reactivity at 9 months of infant age. Child sex and maternal parenting behavior were hypothesized moderators. The sample included 217 (148 tobacco-exposed, 69 non-exposed) mother-child dyads. Data used were obtained from pregnancy assessments, mother-infant feeding interactions at 2 months, and salivary cortisol at 4 time points in response to frustration at 9 months. Results indicated a significant association between PTE and infant cortisol that was moderated by infant sex and maternal intrusiveness. That is, PTE boys had lower cortisol than control boys, but there was no association between PTE and cortisol among girls. There was a significant association between PTE and cortisol among infants of intrusive mothers, but not among infants with non-intrusive mothers. Thus, PTE was associated with cortisol hypo-reactivity such that boys and non-exposed infants experiencing high maternal intrusiveness were at greater risk.
Keywords: Infant cortisol, Prenatal tobacco exposure, Sex differences, Mother-infant interactions
Introduction
Prenatal tobacco exposure (PTE) may represent a condition of toxic stress (Shonkoff et al., 2012) due to the combination of high prenatal stress from fetal hypoxia and ischemia, and continued postnatal stress from cumulative environmental and maternal risks. Toxic stress for children has been defined as strong or chronic activation of the stress response system in the absence of a buffer posed by a supportive relationship with a caring adult. Recent theoretical frameworks such as the adaptive calibration model of the stress response system (Giudice, Ellis, & Shirtcliff, 2011) have included the possibility of both over and under activation of the hypothalamic-pituitary-adrenocortical (HPA) system (Badanes, Watamura, & Hankin, 2011; McEwen, 1998). Cortisol is the primary glucocorticoid hormone produced by the HPA system in humans, and is considered a major indicator of physiological states in response to negative affect (Kirschbaum & Hellhammer, 1989). Therefore, cortisol plays an essential role in the individual’s ability to cope with stressors of daily life. Under non-stressful conditions, there is a diurnal pattern of activity in the HPA axis, with greater concentrations of cortisol in the morning hours, a steep decrease in concentration toward midday, and slowly decreasing concentrations throughout late afternoon into the evening hours. Under stressful conditions, there is an increase in cortisol production approximately 15–20 minutes after the onset of a stressor, followed by return to pre-stressor levels approximately 40 minutes after stress exposure.
To date, only a handful of studies have examined the association of PTE and HPA functioning. One study conducted in the fetal period (Divers, Wilkes, Babaknia, & Yen, 1981) reported significantly higher concentrations of epinephrine and norepinephrine in the amniotic fluid of pregnant smokers compared to non-smokers. Three studies of PTE and stress hormones were conducted in the neonatal period (McDonald et al., 2006; Varvarigou, Petsali, Vassilakos, & Beratis, 2006; Varvarigou, Liatsis, Vassilakos, Decavalas, & Beratis, 2009), with one indicating higher levels of ACTH, but not cortisol among PTE infants compared to controls (McDonald et al., 2006), and the other two reporting higher cortisol in cord blood for PTE compared to controls (Varvarigou et al., 2006, 2009). However, results have been mixed in later childhood, with one report of lower stress reactivity due to higher pre-stressor cortisol among PTE infants compared to control at 2 months (Ramsay, Bendersky, & Lewis, 1996) but not at 6 months of infant age, one report of higher cortisol reactivity at 7 months (Schuetze, Lopez, Granger, & Eiden, 2008) of infant age, and two studies indicating no associations, one at 4–6 months of infant age (Granger et al., 2007) and one at 10.6 years of child age (Huijbregts, van Berkel, Swaab-Barneveld, & van Goozen, 2011). There are several issues of note here. First, the literature is quite small, with only a handful of studies on this topic. Second, there is variability not only in age of measurement and sample size, but also in how PTE was measured. Third, studies vary in cortisol measurement, from measures taken from amniotic fluid and cord blood, to reactivity in response to frustration or pain (from inoculations). Fourth, there is only one study of older children, although it should be noted that the number of smokers in this study was quite small (n = 14; Huijbregts et al., 2011), and the remaining studies reflect a pattern of findings with consistent over-activity of stress hormones in the fetal/neonatal period, and inconsistent results in infancy. This changing pattern from neonatal to later childhood may reflect a changing stress regulation system as a function of accumulating fetal, neonatal, and later postnatal risk from hyper to hypo reactive patterns (Badanes et al., 2011; Giudice et al., 2011). Indeed, theoretical frameworks such as the Adaptive Calibration Model indicate that a period of chronic elevation of the HPA axis is often followed by a period of hypocortisolism that may last months (Giudice et al., 2011).
These conflicting findings may also be due to environmental moderators that may exacerbate or ameliorate the effects of prenatal stress. One of the most significant influences on managing stress and/or arousal in infancy is the quality of mother-infant interactions (Blair, Granger, Willoughby, & Kivlighan, 2006). The stress buffering hypothesis suggests that caregiving experiences characterized by high levels of nurturance may buffer the effects of stress exposure. Research with non-human primates indicates that this is most apparent among those who are genetically predisposed to high stress reactivity (Suomi, 2011). Indeed, the Adaptive Calibration Model (Giudice et al., 2011) and theories such as differential susceptibility (Belsky & Pluess, 2009) suggest that children with highly reactive stress response systems posed by tobacco exposure may also benefit the most from the buffering effects of responsive parenting. Human studies indicate that by the latter part of the first year, sensitive, responsive parenting may buffer the HPA system from responding negatively, even under conditions that elicit marked distress and fearful behavior (Spangler & Schieche, 1998). This is supported in tobacco exposed samples with outcomes other than stress reactivity. For instance, among boys experiencing high maternal responsiveness in infancy, there was no association between PTE and conduct disorder at age 10, while this association was strong and significant among boys who experienced maternal unresponsiveness during infancy (Wakschlag & Hans, 2002).
In addition to maternal parenting behavior, child sex may also moderate the association between tobacco exposure and stress reactivity. The Adaptive Calibration Model (Giudice et al., 2011) emphasizes sex differences in the stress response system that are evolutionarily adaptive and that vary as a function of the level of stress. However, the empirical literature on sex-related differences in tobacco exposed infants is relatively sparse. Indeed, most studies of tobacco exposure have used sex as a covariate, or have reported aggregated results without considering the potential for sex to moderate the effects of PTE on child outcomes (Coles, Kable, & Lynch, 2012). Since interventions are most beneficial if they are targeted at sex/gender specific risks and biological mechanisms that explain those risks, this is a critical gap in our knowledge. Evidence from animal research indicates that males are more biologically vulnerable to prenatal substance exposure effects on the CNS, particularly with respect to areas responsible for regulation (Lewis & Kestler, 2012). Some human studies also indicate greater biological vulnerability for boys exposed to tobacco for outcomes such as stress reactivity and disruptive behavior problems. For instance, Schuetze et al. (2008) reported significantly higher peak cortisol reactivity for tobacco exposed boys compared to non-exposed infants or exposed girls at 7 months of infant age. Two studies indicated that tobacco exposed boys, but not girls, displayed low sociability, increased negative emotionality, and higher reactivity in infancy (Wakschlag & Hans, 2002; Willoughby, Greenberg, Blair, Stifter, & Group, 2007). Others have found that PTE boys, but not girls, were more likely to develop conduct problems and to display antisocial behaviors later in childhood (Fergusson, Woodward, & Horwood, 1998; Weissman, Warner, Wickramaratne, & Kandel, 1999). In addition, PTE has been associated with higher behavioral stress reactivity in response to life events from ages 2 to 6 years, and higher stress reactivity was a significant mediator of the association between PTE and poor mental health outcomes between ages 7–11 years (Park, O’Malley, King, & Picciotto, 2014). However, little is known about the role of child sex as a moderator of PTE effects on stress reactivity.
In addition to these gaps in the literature, few studies have examined the role of postnatal tobacco exposure on infant stress reactivity. Postnatal exposure is consistently associated with poor respiratory health and sudden infant death syndrome (Surgeon General Report, 2006). However, associations with other developmental outcomes associated with reactivity and regulation, such as behavior problems and ADHD have been mixed and fraught with methodological problems such as retrospective design and poor assessment of tobacco exposure. Consequently, there is a need for prospective studies with careful, multi-method assessments of prenatal and postnatal exposure to understand whether continued postnatal exposure increases risk for outcomes, such as infant stress reactivity.
Based on this literature and theoretical frameworks, we hypothesized that tobacco exposed infants would have higher concentrations of cortisol compared to control group infants and that this association would be stronger for tobacco exposed boys. We also hypothesized that maternal parenting behavior would moderate the association between PTE and stress reactivity, such that tobacco exposed infants experiencing low maternal sensitivity and/or high maternal intrusiveness during mother-infant interactions would have higher cortisol reactivity compared to non-exposed infants or those with lower levels of these risk variables. We examined the role of postnatal tobacco exposure as a significant additional predictor of stress reactivity, tentatively hypothesizing that there would be a significant interaction of prenatal and postnatal exposure indicating compounding effects on cortisol reactivity.
Method
Sample Selection
Women who presented for care at a large urban hospital’s prenatal clinic were asked to complete a screening form during their first prenatal appointment. Eligible women were invited to participate in an ongoing longitudinal study of maternal health and child development. Initial exclusionary criteria included: less than 20 weeks gestation, maternal age of less than 18 years, and multiple fetuses. Additional eligibility criteria were: no illicit drug use (other than cannabis based on maternal self-reports, salivary assays in each trimester, and infant meconium assays), no heavy alcohol use (more than 1 drink/day on average or 4 drinks on one occasion based on maternal self-reports on a calendar based interview) after pregnancy recognition, and no heavy marijuana use (more than 1 marijuana joint/day on average based on maternal self-reports) after pregnancy recognition (see below for measurement details). Women who agreed to participate were scheduled for four appointments: one at the end of each trimester of pregnancy and one at 2 months postpartum (at age corrected for prematurity). A second postpartum assessment was scheduled for 9 months postpartum (at age corrected for prematurity). At the end of each month of recruitment, the closest matching non-smoker (based upon age and education) was invited to participate. Smokers were over-sampled so that one non-smoker was recruited for every two smokers (taking the average of age and education of both). Participants included a total of 258 mothers and their infants. One mother-infant dyad was dropped from analyses because infant meconium was positive for methamphetamine, resulting in a sample size of 257 infants. Of these dyads, 181 were infants prenatally exposed to tobacco through maternal smoking, and 76 were infants not exposed to tobacco. Only dyads with cortisol assessments at the 9-month visit were included in these analyses. Of the 257 infants recruited into the study, 22 did not attend the 9-month assessment after repeated reschedules (8 tobacco-exposed), 6 were unable to be located (2 tobacco-exposed), and 6 (4 tobacco-exposed) were dropped (severe medical problems) or withdrew from the study. An additional 7 infants did not have saliva samples that could be analyzed due to infant irritability (5 tobacco-exposed). Consequently, the final sample for cortisol analysis was 217 (148 tobacco-exposed, 69 non-exposed) dyads. There were no significant differences between families with complete versus missing data at 9 months on demographic or substance use variables.
Mothers ranged in age from 19 to 40 (M = 25.39, SD = 4.99). Maternal race was 52% African-American, 30% Caucasian, 18% Hispanic, and 8% other or mixed race with several mothers reporting more than one race. Approximately 44% percent of women were married or living with their partner at the first prenatal appointment, 35% were in a relationship but not living with their partner, and 21% were single. By 9 months of infant age, about 26% of women had less than a high-school education, 60% completed high-school, 10% completed some college courses, and 4% had a vocational degree or technical training degree. Thus, the sample consisted of primarily young, unmarried, minority women with low education.
Procedure
Informed written consent was obtained from interested, eligible women at their first laboratory visit in the first trimester of pregnancy. Prenatal assessments were conducted once in each trimester of pregnancy, and at 2 and 9 months of infant ages at age corrected for prematurity. Data from the prenatal interviews, from mother-infant feeding interactions at 2 months, and from the maternal interview and cortisol assessments conducted at the 9-month visit were included in these analyses.
The study protocol was approved by the Children and Youth Institutional Review Board at the State University of New York. Participants were informed that data confidentiality was protected by a Federal Certificate of Confidentiality issued by the National Institute on Drug Abuse. Participants received payments for completed assessments at all visits.
Study Measures
Maternal prenatal substance use
Maternal pregnancy smoking status was determined through a combination of self-report, meconium, and maternal saliva samples taken once in each trimester of pregnancy. At each prenatal interview and at the postnatal interviews, the Timeline Follow-Back Interview (TLFB; Sobell & Sobell, 1992) was used to gather daily tobacco, alcohol, and cannabis use for the previous three months. Participants were provided a calendar and asked to identify events of personal interest (e.g., holidays, birthdays, vacations, etc.) as anchor points to aid recall. The method has been established as a reliable and valid method of obtaining data on substance use patterns including cigarettes, has good test-retest reliability, and is highly correlated with other intensive self-report measures (Brown et al., 1998). The TLFB was also used at each postpartum visit (2 and 9 months of infant age) to assess postnatal substance use. The TLFB yielded data on average number of cigarettes smoked per day, average number of drinks per day, and average number of joints per day during each trimester and overall pregnancy.
Maternal saliva was collected at each prenatal interview to provide objective evidence of recent exposure. The saliva specimens were analyzed by a commercial laboratory for cotinine, a metabolite of nicotine that indicates exposure to nicotine (Jarvis et al., 2003). Maternal salivary cotinine ranged from 0 to 569ng/ml of saliva. Following previous recommendations for cotinine cut points (Jarvis et al., 2008), mothers were assigned to the PTE group if they had salivary cotinine levels greater than 12ng/ml.
After birth, meconium specimens were collected from soiled diapers twice daily until the appearance of milk stool, transferred to storage containers, and frozen until transport to the National Institute on Drug Abuse for analysis. Meconium specimens were assayed with a validated LC-MS/MS method (Gray et al., 2010) for the presence of nicotine, cotinine, or trans-3-hydroxycotinine (OHCOT) as evidence of prenatal nicotine exposure (see Gray et al., 2010 for assay details). Infants were assigned to the PTE group if their meconium was positive for nicotine, cotinine, or OHCOT. Mothers were assigned to the PTE group if they reported smoking cigarettes after conception on the TLFB, if they had salivary cotinine levels greater than 12ng/ml in any trimester of pregnancy, and/or if the infants had any tobacco metabolites in their meconium.
Postnatal tobacco exposure
Postnatal tobacco exposure was assessed for infants during their 2 and 9-month visits using infant saliva samples. Infant saliva samples were assayed for cotinine levels. Salivary cotinine concentrations are highly correlated to those in the blood (Jarvis, Primatesta, Erens, Feyerabend, & Bryant, 2003) and, thus, are an accurate, yet noninvasive, way of measuring passive exposure. Saliva samples were collected by placing eye spears (BD Opthalmology “Visispears” (product #581089), marketed by Salimetrics as “Sorbettes” (product #5029)) in the mouth of infants. These samples were placed in a storage vial and immediately placed in −80°C freezer and sent to the Center for Interdisciplinary Salivary Bioscience at Johns Hopkins University for assay. The advantage of saliva testing is that it quantifies exposure to cigarette smoke from all possible sources including other household smokers.
Infant cortisol
At 9 months, infant reactivity and regulation were assessed during a positive and negative affect paradigm taken from the Laboratory Temperament Assessment Battery (LabTAB; Goldsmith & Rothbart, 1996). These included the puppet show designed to elicit positive affect and an arm restraint paradigm to elicit anger/frustration. The order of procedures was as follows: The Time 1 or pretask saliva sample (T1) was collected after the infant arrived at the laboratory; the infant was then seated in a high chair, hooked up to electrodes for measurement of heart rate, and watched a Baby Einstein video for 3 minutes for a baseline assessment of physiology (see Calkins, 1997) followed by a 6 minute focused attention paradigm (presentation of 4 novel toys for 90 seconds each), a 2-minute puppet show, and a second 3-minute baseline interval (watching a video). This was followed by the arm restraint paradigm designed to elicit anger/frustration. The Time 2 (T2) saliva sample was collected at the end of the arm restraint paradigm. This was followed by another 3 minutes of video. The infant was then unhooked from the heart rate monitor and placed on a play mat on the floor with a variety of toys for measurement of infant activity level. Mothers were asked to respond to their infants as they normally would but not to initiate interaction. This was followed by an 8-minute free play procedure and another 3 minutes of the infant interacting with toys in a basket. The third saliva sample was collected 20 minutes after the end of arm restraint (T3) and the fourth sample was collected 40 minutes after the end of arm restraint (T4).
As with measurement of cotinine, saliva samples were collected by placing eye spears (BD Opthalmology “Visispears” (product #581089), marketed by Salimetrics as “Sorbettes” (product #5029)) in the mouth of infants. These samples were placed in a storage vial and immediately placed in −80°C freezer and sent to the Center for Interdisciplinary Salivary Bioscience at Johns Hopkins University for assay. Samples were assayed for cortisol using a highly sensitive enzyme immunoassay the U.S. Food and Drug Administration (510k) cleared for use as an in vitro diagnostic measure of adrenal function. The test uses 25µL of saliva and has a lower limit of sensitivity from .007 to 3.0 µg/dl. All samples were assayed in duplicate and averaged duplicate scores were used in all statistical analyses. Cortisol values at all 4 time points were available for 212 infants. There were no differences in demographic or substance use variables between infants with missing vs. complete cortisol data.
Maternal parenting behavior
Parenting was assessed using behavioral observations during feeding interaction at 2 months of infant age. Mothers were asked to feed their infants as they normally would at home. The first 10 minutes of these interactions were coded using a collection of global 5-point rating scales developed by Clark et al. (Clark, 1999; Clark, Musick, Scott, & Klehr, 1980). These scales have been found to be applicable for coding mother-child interactions for children ranging in age from 2 months to 5 years (Clark, 1999). Three composite scales for maternal behavior were derived from these items: warmth/sensitivity (e.g., warm, kind tone of voice, expressed positive affect, contingent responsiveness to infant behavior, connectedness), negative affect (e.g., angry, hostile tone of voice, displeasure, disapproval, criticism), and intrusiveness (e.g., flexibility/rigidity, intrusiveness). These scales had high internal consistencies with Cronbach’s alpha of .88 for warmth/sensitivity, .84 for negative affect, and .80 for intrusiveness.
Two coders blind to group status rated maternal behavior. Both coders were trained on the Clark scales by the first author. Inter-rater reliability was conducted on a random selection of 15% (n = 33) of the tapes and were .88 for warmth/sensitivity, .72 for negative affect, and .84 for intrusiveness.
Data Transformations
We first examined cortisol data for outliers, (defined as +3SD from the mean; Gunnar, Broderson, Krueger, & Rigatuso, 1996). There were 7 infants with Time 1 values, 11 infants with Time 2 values, 10 infants with Time 3 values, and 10 infants with Time 4 values that were 3 SD above the mean, and none that were 3 SD below the mean. Following recommendations by Tukey (1977), these values were winsorized by replacing values that were 3 SD above the mean with the value of 3 SD above the mean, as in previous studies (Haley, Handmaker, & Lowe, 2006).
Analytic Strategy
We first examined associations between cortisol values at different time points using Pearson correlations. Analyses of potential confounds were conducted next using correlations or analysis of variance (ANOVAs) as appropriate. Confounds with significant bivariate associations with cortisol values or those of theoretical interest (e.g., other substance use) were included as covariates in all remaining analyses (if they were associated at p < .10). Sex-related differences in cortisol were examined using ANCOVA. Repeated measures ANCOVA with significant theoretical or empirical covariates were conducted to examine group differences on cortisol values. Correlations and repeated measures ANCOVA were also used to examine the associations between postnatal exposure and cortisol values and to examine whether the combination of prenatal and postnatal exposure was associated with the most significant effects on cortisol values. Hierarchical multiple regressions were used to examine whether maternal parenting behavior moderated the association between PTE and infant cortisol in order to not artificially dichotomize the continuous measures of maternal behavior using median splits.
Results
Pearson correlations were computed to examine the associations among the cortisol values. Cortisol values at each of the time points were positively correlated with each other. The correlations among cortisol samples at the four time points were high, ranging from r = .74, p < .001 to r = .85, p < .001. To examine whether the arm restraint paradigm was effective in increasing stress/arousal in infants, we conducted repeated measures ANOVA with heart rate measures (3-minute baseline, average HR during trial 1 of arm restraint, average HR during trial 2 of arm restraint, and 3-minute recovery), with PTE group status and child sex as the between subjects factors. Results indicated a significant effect of time, F (3, 183) = 52.81, p = .00, with a significant quadratic trend, but no associations with PTE, child sex, or interaction effects. There was a significant linear increase in heart rate from baseline to trial 1 and trial 2 of arm restraint, and a sharp decrease from trial 2 to recovery. Thus, the arm restraint paradigm was effective in increasing sympathetic arousal for infants in general, but there were no differences as a function of PTE or child sex.
Substance Use, Demographics, and Infant Growth Outcomes
Results from MANOVA with demographic variables (maternal age, education, parity) as the dependent measures and PTE group status as the independent variable yielded no significant group difference on these demographic variables (see Table 1). Chi-square analyses indicated no differences on percentage of families in Temporary Assistance for Needy Families, Medicaid, food stamps, or marital status. However, smokers were more likely to be White and to have partners who smoked. Table 1 also includes group differences in prenatal and postnatal maternal substance use variables. Results from MANOVA yielded significant group differences in prenatal substance use variables. As would be expected, univariate analyses indicated that mothers who smoked during pregnancy were heavier users of cigarettes, alcohol, and marijuana during pregnancy than mothers who did not smoke during pregnancy (see Table 1). Similarly, results from MANOVA with postnatal substance use variables as the dependent measures yielded a significant group difference. Univariate analyses indicated that mothers in the tobacco group continued to be heavier users of cigarettes, alcohol, and marijuana in the postnatal period compared to pregnancy non-smokers (see Table 1). Infants in the prenatal tobacco group also had significantly higher levels of cotinine at 9 months compared to infants in the control group (see Table 1).
Table 1.
Group Differences in Demographic Variables and Substance Use.
| Exposure Group: | Non-Smokers | Smokers | F value | Partial η2 |
||
|---|---|---|---|---|---|---|
| M | SD | M | SD | |||
| Pregnancy (MANCOVA) |
25.25** | .27 | ||||
| Cigarettes/Day | 0 | 0 | 5.08 | 5.03 | 69.18** | .25 |
| Drinks/Day | .01 | 0 | .08 | .20 | 6.85** | .03 |
| Joints/Day | .07 | .31 | .32 | .73 | 7.40** | .03 |
| Postnatal (MANCOVA) | 32.08** | .29 | ||||
| Cigarettes/Day | .14 | .71 | 6.08 | 5.28 | 85.26** | .29 |
| Drinks/Day | 1.08 | 1.48 | 2.83 | 2.84 | 22.88** | .10 |
| Joints/Day | .13 | .52 | .82 | 1.75 | 10.21** | .05 |
| Infant Cotinine | 2.82 | 3.23 | 7.06 | 8.00 | 17.50** | .08 |
| Parenting (MANCOVA) | .16 | .02 | ||||
| Warmth/Sensitivity | 4.11 | .61 | 3.96 | .66 | 2.26 | .01 |
| Negative Affect | 1.13 | .26 | 1.27 | .48 | 5.16* | .02 |
| Intrusiveness | 1.50 | .74 | 1.62 | .76 | 1.39 | .01 |
| Demographics: | ||||||
| Maternal age | 23.30 | 4.90 | 24.28 | 5.27 | 1.91 | .00 |
| Parity | 2.04 | 1.47 | 2.18 | 1.56 | .99 | .00 |
| Years education | 12.22 | 1.65 | 11.99 | 1.69 | .44 | .00 |
| Race (% White) | 35% | 17% | χ2=7.13** | |||
| TANF | 18% | 12% | χ2=.47 | |||
| Medicaid | 65% | 66% | χ2=.00 | |||
| Food Stamps | 51% | 55% | χ2=.16 | |||
| Married/Cohabiting | 52% | 54% | χ2=1.24 | |||
| Partner Smoking | 22% | 60% | χ2=13.53** | |||
| Medication Use | 29% | 18% | χ2=3.80* | |||
Note. TANF: Temporary Assistance to Needy Families.
p < .05,
p < .01
Potential Confounds and Sex Differences
There were no significant associations between infant cortisol values (values at each time point at alpha conservatively set at p < .10) and maternal education, parity, and age; whether the child woke during the night, time of day, race, birth weight, gestational age, head circumference, small for gestational age status, or preterm birth status. There were also no significant associations between infant cortisol values and average number of drinks per day or average number of joints per day during each trimester of pregnancy, and no associations between infant cortisol values and the overall measure of pregnancy alcohol and marijuana use.
Given recent studies on the importance of considering medication use in cortisol studies (Hibel, Granger, Cicchetti, & Rogosch, 2007), we examined medication status as a potential confound (current medications vs. none). About 22% of infants in the study had been administered medication within 24 hours of the laboratory visit. There was a significant association between PTE group status and medication use (see Table 1). Repeated measures ANOVA with the four cortisol values as the dependent measures indicated a significant main effect of medication status (use vs. no use), F (1,207) = 9.67, p = .002. Infants with current medications had higher cortisol values overall. Means (with standard errors in parentheses for medicated and unmedicated infants) were 0.33 (.05) and 0.62 (.09). Medications ranged from over the counter cold products to asthma medication, acetaminophen, anti-inflammatories, antibiotics, and teething gels. Consequently, infant medication status was used as a covariate in the main analyses.
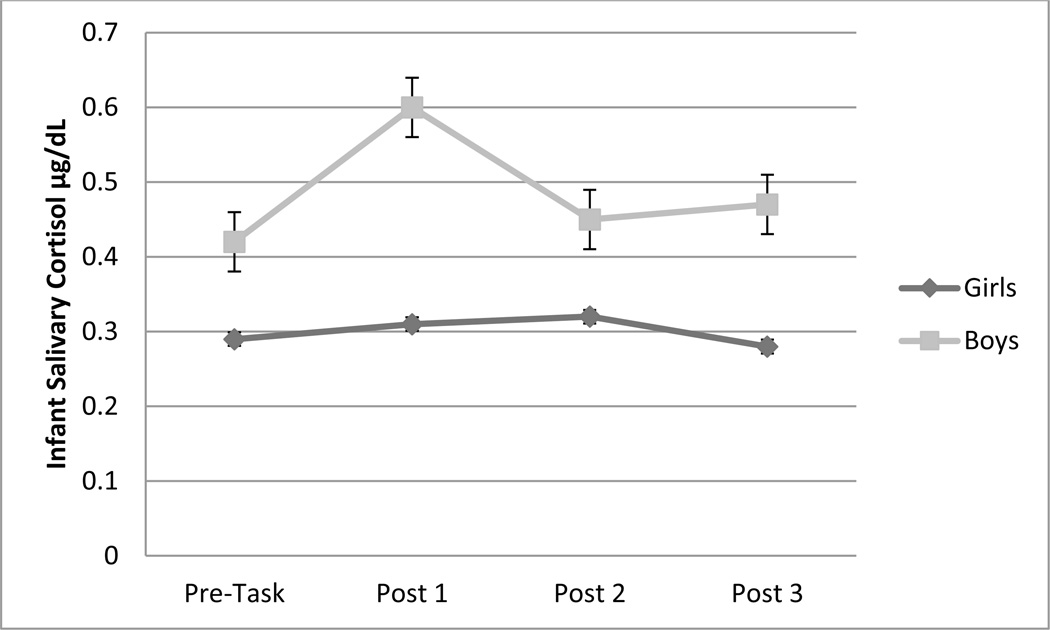
Repeated measures ANOVA was used to examine sex-related differences in cortisol over time. Results indicated a significant sex by time interaction, F(3, 512.6) = 3.11, p = .035, with a significant cubic trend (see Figure 1). Simple effects analyses indicated that boys had significantly higher cortisol values compared to girls at Times 2 and 4, but not at Times 1 and 3. There was no significant effect of time on cortisol for girls, but a significant increase in cortisol from Time 1 to 2 and a significant decline between Time 2 and 3 for boys.
Figure 1.
Child sex by time interaction effect on infant cortisol at 9 months of infant age.
Main Analyses
Change over Time
In order to examine if there was a cortisol response to the arm restraint paradigm for the sample as a whole, we conducted repeated measures ANOVA with time as the within subjects factor. Results indicated a significant effect of time, F (3, 341.7) = 3.10, p = .02, ηp2 = .02. There was a significant increase in cortisol from Pre-Task to Post-Task 1 (Ms = .25 and .35, SD = .37 and .60 respectively), but no significant change from Post Task 1 to 2 (M for Post-Task 2 = .31, SD = .53), or Post-Task 2 to 3 (M for Post-Task 3 = .30, SD = .42).
Association between PTE and infant cortisol
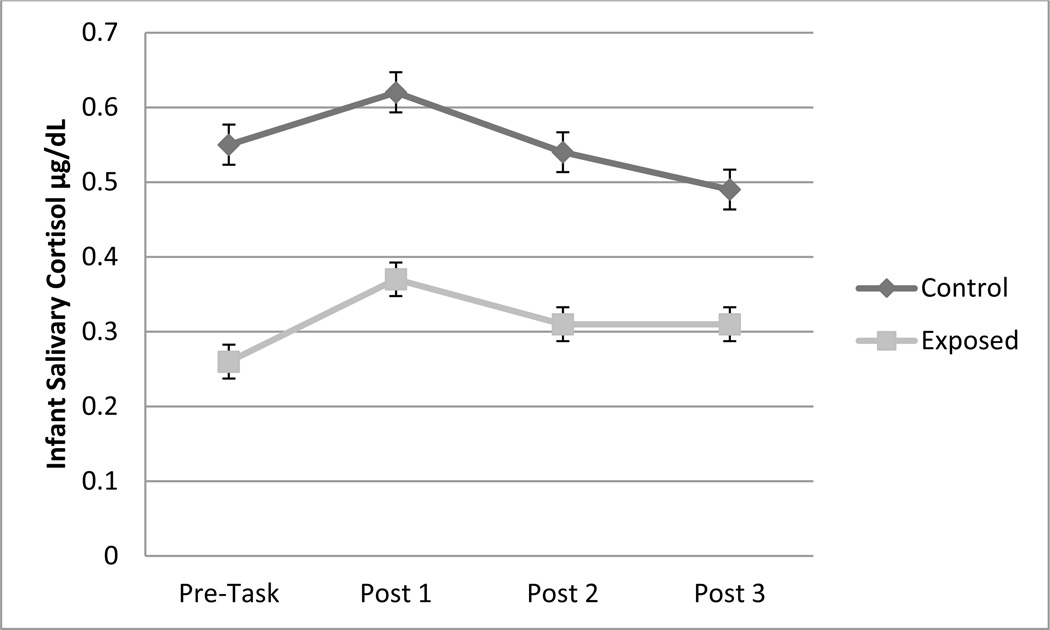
A repeated measures ANCOVA was conducted to examine the association between PTE group status and change in cortisol over time with infant medication status included as a covariate. Results indicated a significant main effect of PTE group status, F (1, 206) = 7.26, p = .008, ηp2 = .04 (see Figure 2). Exposed infants (M = .31, SE = .05) had significantly lower cortisol compared to non-exposed infants (M = .55, SE = .07). Potential dose-response associations were examined next by examining the correlations between cortisol values and the average number of cigarettes per day in each trimester of pregnancy. As noted in Table 2, in general, there were small but significant associations between average number of cigarettes in Trimesters 1 and 2 and cortisol values across time, with higher number of cigarettes per day associated with lower cortisol across time. Thus, there was some indication of dose response associations, but the magnitude of these associations did not differ by timing of exposure and were generally small. In addition to maternal self-reports, infant meconium is a reliable indicator of fetal third trimester exposure (see Gray et al., 2010). When the dichotomous (positive vs. negative) measure of meconium was used, only Pre-Task cortisol was significantly associated with infant meconium positive for nicotine metabolites (r = −.15, p = .05). Infants with meconium positive for nicotine and metabolites had lower Pre-Task cortisol values. The magnitude of this association was similar to those obtained using maternal self-reports in the first two trimesters. Finally, when the nanomolar equivalent nicotine per gram of meconium was used as a continuous measure of total biomarker concentration, correlational analyses indicated that infants with higher amounts of nicotine in meconium had lower Pre-Task cortisol and marginally lower Post-Task 2 cortisol (see Table 2).
Figure 2.
Main effect of smoking group status on infant cortisol at 9 months of infant age for the sample as a whole, but no interactions involving time.
Table 2.
Pearson correlation coefficients between number of cigarettes per day in each trimester of pregnancy, continuous measure of total biomarker in meconium, and cortisol values.
| Pre-Task | Post 1 | Post 2 | Post 3 | |
|---|---|---|---|---|
| Trimester 1 cigarettes per day | −.15* | −.12 | −.14* | −.15* |
| Trimester 2 cigarettes per day | −.16* | −.15* | −.13* | −.13* |
| Trimester 3 cigarettes per day | −.12 | −.13* | −.12 | −.12 |
| Nicotine molar equivalent/g Meconium | −.17* | −.12 | −.14+ | −.10 |
p < .05
p < .10
Moderation by infant sex
Infant sex was included as a between subjects factor in repeated measures ANCOVA to examine whether the association between PTE and cortisol differed for boys and girls. Medication status was included in the analyses as a covariate. Results indicated a significant interaction of PTE group status and infant sex, F(1, 203) = 8.97, p = .003, ηp2 = .04. Simple effects analyses indicated a significant effect of PTE for boys, F(1,100) = 11.90, p = .001, ηp2 = .11 (Ms = .83 and .33, SEs = .12 and .08 for control and PTE groups respectively), but not girls, F(1, 102) = .18, p = .60 (Ms = .33 and .29, SEs = .07 and .06 for control and PTE groups respectively).
Associations between PTE, postnatal tobacco exposure, and infant cortisol
Repeated measures ANCOVA was used to examine whether the association between PTE status and cortisol reactivity was moderated by postnatal tobacco exposure. Infant sex and medication status were used as covariates and PTE group status and 9 month infant salivary cotinine results (positive vs. negative) were used as the between subjects factors. Results yielded no significant interaction or main effect of postnatal tobacco exposure at 9 months.
Associations between PTE, parenting, and infant cortisol
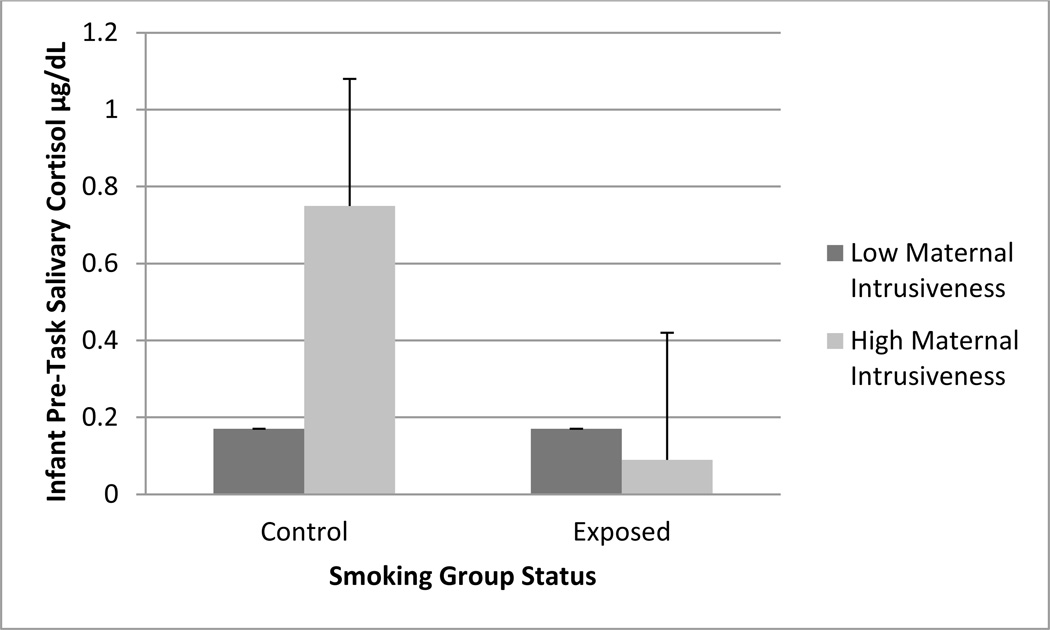
The correlations among the three maternal behavior measures were significant, but in the small to moderate range (Pearson r = .30 to .34, p = .00). Thus, they were related but different aspects of maternal behavior and the magnitude of the association was not supportive of combining the three measures. In order to not artificially dichotomize the continuous measures of maternal behavior, hierarchical regression analyses were used to examine whether maternal warmth, negative affect, and intrusiveness during feeding interactions at 2 months of infant age moderated the association between PTE and infant cortisol at each time point. Infant sex and medication status were entered on the first step of regression equation, followed by the main effects of PTE and each of the three maternal behavior variables on the second step of the regression equation, and the interaction terms of PTE by maternal behavior on the final step of the regression equation. Results yielded a significant interaction of PTE and maternal intrusiveness for cortisol at each time point (βs ranged from −.33 for Time 1 to −.64 for Time 2 cortisol, p < .01 for all time points). Given the similarity of interaction effects for each time point, we have depicted Time 1 cortisol in Figure 3. As indicated in Figure 3, there was no association between PTE and infant cortisol at Time 1 when maternal intrusiveness was low. However, among mothers with high levels of intrusiveness, PTE infants had significantly lower cortisol compared to those in the control group (see Figure 3). Maternal negative affect and sensitivity did not moderate the association between PTE and cortisol at any time point, although the overall pattern of associations were similar.
Figure 3.
Interaction of PTE and maternal intrusiveness on Time 1 cortisol.
Discussion
PTE was associated with lower concentrations of cortisol in this sample of high-risk 9 month old boys, but not girls. There was some evidence of dose response associations, with higher amounts of cigarettes associated with lower cortisol, although associations with maternal report variables were strongest for the first two trimesters and the association with infant meconium reflecting third trimester exposure significant for the pre-task cortisol. This may partly be a reflection of the skewed nature of the variables in the last trimester. Results overall did not provide strong evidence that specific timing of exposure was associated with differential pattern of cortisol responses to laboratory stressors.
According to the Adaptive Calibration Model (Giudice et al., 2011), hypo-activation of the HPA system would be the expected pattern in response to severe or traumatic stress. In PTE samples, this may be a reflection of chronic prenatal stress due to fetal ischemia and hypoxia and continued postnatal stress due to non-optimal caregiving environments. While studies have consistently found significantly higher concentrations of stress hormones in PTE infants during the fetal and neonatal period (McDonald et al., 2006; Varvarigou et al., 2006, 2009), results have been quite variable in the postnatal period with some studies demonstrating higher stress reactivity at 2 (Ramsay et al., 1996) and 7 months (Schuetze et al., 2008) of infant ages, and other studies finding no associations at 6 months (Ramsay et al., 1996), 4–6 months of infant ages (Granger et al., 2007), and 10.6 years of child ages (Huijbregts et al., 2011). One explanation for these changes may be differences in how PTE was measured and relative sample sizes. For instance, in the study by Ramsay and colleagues (1996), women who used any cigarettes or alcohol during pregnancy were grouped into the exposed group. In the study by Schuetze and colleagues (2008), PTE was measured by maternal self-report alone, with salivary cotinine measures taken postnatally, and thus reflecting postnatal exposure. In contrast, the study by Granger et al (2007) grouped mothers into the smoking group if they smoked at least 1 cigarette in the past 48 hours or had salivary cotinine levels above 10ng/mL when their infants were 6 months of age, and into the control group if they did not smoke at least 1 cigarette in the past 48 hours and did not have salivary cotinine levels above 10ng/mL. Thus, women in either group may or may not have smoked cigarettes prenatally. Finally, the study by Huijbregts et al. (2011), consisted of a small sample of smokers (n = 14) and PTE was based on retrospective reports of maternal smoking 8–13 years after delivery. Thus, discrepancies in results beyond the fetal/neonatal period may be due to these differences in measurement, sample size, and the nature of the independent variable.
The mixed findings may also be due to potential changes in the stress response system from chronic hyper-activation to hypo-activation. There is suggestion the literature that there may be periods of rebound effects from chronic hyper-activation (Giudice et al., 2011) that have long term implications for health and disease (McEwen, 1998). Future studies with prospective assessments over time may be better able to address this issue. When and under what circumstances does potential hyper-activation of the stress response system change to hypo-activation. Our findings also suggest that the lack of cohesion of these findings may be due to the presence of significant moderators, such as infant sex and maternal parenting.
Indeed, results from the present study indicated that the direct association between PTE and infant cortisol was qualified by two significant interactions, revealing that all of the infants did not experience equal risk for the consequences of PTE in regard to HPA reactivity. First, the association between PTE and infant cortisol was moderated by infant sex, such that only boys demonstrated hypoactivation of the HPA system. This pattern of sex-related differences is supported by theoretical frameworks suggesting that the shift toward hypocortisolism would be more prevalent among males due to the evolutionary benefits posed by this stress response pattern under conditions of high environmental risk (Giudice et al., 2011). These findings are also supportive of both the animal and human literature and provide additional evidence for the notion that boys may be particularly vulnerable to prenatal stresses associated with PTE, such as fetal hypoxia. Indeed, studies that have examined prenatal exposure to nicotine in rodents have found that male rodents are more prone to demonstrate poorer behavioral functioning as a result of hypoxic insult (Tashima, Nakata, Anno, Sugino, & Kato; Tizabi et al., 1997). This male biased pattern of hyporesponsivity under conditions of high environmental stress is hypothesized to be associated with high levels of risk taking (Guidice et al., 2011). Indeed, this may be one mechanism explaining the stronger association between PTE and behavioral disorders for boys, but not girls (Coles et al., 2012). Thus, the present findings add to this growing body of literature by providing preliminary evidence that sex-related differences in the association between PTE and HPA reactivity may exist as early as the first year of life.
Findings from the present work also provided evidence to support the notion that the association between PTE and HPA reactivity varies as a function of maternal intrusiveness. Results indicated that PTE infants had stress response systems that were not as responsive to the added environmental adversity posed by high maternal intrusiveness. These finding are particularly noteworthy, as there is a relative paucity of studies that have examined outcomes of PTE in the context of parenting, despite theories highlighting the importance of the caregiving environment for shaping consequential outcomes in children who have experienced prenatal substance exposure (Mayes & Bornstein, 1997). More specifically, to our knowledge no studies to date have examined whether parenting influences the association between PTE and HPA functioning. This is surprising given that pregnancy smoking has been associated with greater risk of poorer parenting. Studies, for instance, have demonstrated that pregnancy smoking is associated with higher rates of parent-child conflict (Weissman et al., 1999), less maternal warmth, and parenting behavior characterized by poor communication, ineffective discipline, and poor supervision (Wakschlag et al., 1997). Moreover, the quality of the mother-infant relationship has been shown to be a pivotal influence on managing stress and/or arousal in infancy, with theories, such as the stress buffering hypothesis and the stress inoculation hypothesis highlighting the important role of the caregiving environment for stress reactivity in infancy (Blair et al., 2006). While we are not aware of previous studies that have directly examined whether parenting confers greater vulnerability for infant stress reactivity among PTE infants, our findings are consistent with those of Wakschlag and Hans (2002) who found that there was no association between PTE and conduct disorder at age 10 among boys experiencing high maternal responsiveness in infancy, but that there was a strong positive association between PTE and conduct disorder among boys who experienced maternal unresponsiveness during infancy. These results may help to clarify, in part, why cigarette-exposed boys who experience unresponsive parenting are at a higher risk for developing conduct disorder as children (Wakschlag & Hans, 2002).
Several studies have noted that early maternal intrusiveness is an important aspect of maternal behavior that is predictive of infant disorganized behaviors in the Strange Situation paradigm (Lyons-Ruth et al., 1999), greater child negativity in the toddler period (Ispa et al., 2004), and greater peer directed hostility in kindergarten (Fearon, Bakermans-Kranenburg, van IJzendoorn, Lapsley, & Roisman, 2010; Lyons-Ruth, Alpern, & Repacholi, 1993), and in middle childhood (Lyons-Ruth, Easterbrooks, & Cibelli, 1997). In addition to its predictive role for infant behaviors and mother-infant relationship, maternal intrusiveness during mother-infant interaction seems to be reflective of greater maternal stress and anxiety. For instance, Atzil and colleagues (2011) reported that intrusive mothers had greater activation in brain areas associated with stress-related mechanisms and greater neural disorganization compared with sensitive attuned mothers. Authors posited that maternal intrusiveness may be an indicator of stress or anxiety. Similarly, Kiel and Buss (2013) reported that maternal intrusiveness was associated with higher maternal stress response among inhibited infants and reflected greater maternal anxiety and embarrassment about infant inhibition. Thus, the results from these studies suggest that unlike maternal sensitivity, maternal intrusiveness may be a reflection of greater maternal stress, anxiety, and hostility with corresponding increases in infant stress. This may be associated with greater cortisol reactivity among infants with normally reactive HPA systems. However, this conclusion remains speculative, and hence should be interpreted with caution. Firmer conclusions await replication of our work and the accumulation of converging evidence.
Contrary to expectations, postnatal tobacco exposure did not exacerbate the association between PTE and infant cortisol at 9 months. One explanation may be that given the narrow range of cortisol and the hypoactive HPA system associated with PTE, there may be floor effects. All infants with postnatal exposure had prenatal exposure as well and if the effects of PTE are to reduce HPA activation, the levels may already be too low for additional effects of postnatal exposure to be evident. Few studies have examined the role of postnatal exposure as a moderator of prenatal effects. However, Granger et al (2007) reported no associations between postnatal exposure and infant cortisol reactivity. Thus, another possibility is that postnatal exposure was not severe enough for an impact on cortisol activity at 9 months of age.
Theoretical frameworks regarding developmental changes from hyper- to hypo-activation of the stress response system (Giudice et al., 2011) can only be examined with prospective data from samples characterized by chronic and/or severe stress. Given lack of data, it is unclear whether the associations between PTE and lower cortisol concentrations persist throughout infancy and into the childhood years. Prospective designs would also be better able to address when and under what conditions do initially high levels observed in a few studies change to low cortisol reactivity. Future studies with developmental and health outcomes beyond infancy are also needed to understand at what level and under which contexts is low reactivity associated with harmful consequences. Studies have noted that hypoactivity of the HPA system is associated with adult psychopathology (O’Leary, Loney, & Eckel, 2007) and disease {McEwen, 1998 #4608}, Fries, Hesse, & Hellhammer, 2005). Prospective studies following these children over time may address the issue of the HPA system being one mechanism linking prenatal exposure to adult health and disease in cigarette exposed samples. Finally, future studies of fetal origins of disease may be better focused on diurnal rhythms as opposed to laboratory based measures. Loss of diurnal rhythm may be a better index of a distressed HPA system than laboratory measures and may be more predictive of developmental and health outcomes, or lack of resilience in the context of high stress (Gunnar & Vasquez, 2001).
Other limitations of the current study must be acknowledged. First, Time 1 cortisol taken after arrival at the lab reflects some level of cortisol reactivity to the environmental stressors associated with travel to the laboratory setting. Thus, it does not reflect baseline conditions. Nonetheless, our results suggest that our lab-based procedures assess meaningful individual differences. A second limitation is that accurate assessment of substance use both prenatally and postnatally is complicated. Pregnant and postpartum women are often hesitant to divulge substance use information. However, an important strength of this study was the use of multiple methods to ascertain prenatal and postnatal substance use, which partially mitigated this limitation. A third limitation is that the measure of maternal parenting behavior was limited to a single feeding session and was relatively brief. However, a strength of this assessment approach is that it provides an objective measure of parenting, as opposed to parent reports of their own parenting behavior.
Notwithstanding these limitations, the present work is important because it offers further empirical support to a growing body of literature documenting significant consequences of PTE on the HPA axis. This study also contributes to the field because of its examination of moderating factors, such as infant sex and maternal parenting behavior following recent theoretical frameworks such as the Adaptive Calibration Model of stress response. The present findings with regard to HPA reactivity indicate that PTE infants have stress response systems that are not responsive to negative environmental adversity posed by high maternal intrusiveness, and that male PTE infants are at higher risk for hyporesponsivity. These findings indicate that the link between PTE and HPA reactivity is rather complex, suggesting that we need to move away from linear models examining the association between PTE and HPA functioning to models that include relevant mediators and moderators. Current findings also lend support to fetal programming of stress response mechanisms that may lead to increased adverse outcomes as development progresses and underscore the importance of considering fetal origins in theoretical frameworks of stress and illness.
Clinical Implications
The results from this study suggest that cigarette exposed boys may be particularly vulnerable to hypoactivation of the HPA system and exposed children may have blunted HPA systems that are not responsive to additional environmental stressors. Apart from the importance of reducing maternal smoking during pregnancy, these findings underscore the importance of interventions that may help exposed children, especially boys, regulate responses to environmental stressors appropriately.
Acknowledgements
The authors are grateful to the families who participated in the study and to Research Technicians for data collection and coding. Special thanks goes to Dr. Amol Lele at Women and Children’s Hospital of Buffalo for her collaboration on data collection. The study was supported by the National Institute on Drug Abuse at the National Institutes of Health under award number R01DA019632 and the Intramural Research Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: With the exception of Dr. Granger, none of the other authors have any conflicts of interest to declare.
Conflict of Interest
In the interest of full disclosure, Dr. Granger is founder and Chief Scientific and Strategy Advisor at Salimetrics LLC. Dr. Granger’s relationships with these entities are managed by the policies of the Conflict of Interest Committee at the Johns Hopkins University and the Office of Research Integrity and Assurance at Arizona State University. None of the other authors have any conflict of interest to declare.
Contributor Information
Rina D. Eiden, Research Institute on Addictions, State University of New York at Buffalo, Buffalo, NY
Danielle S. Molnar, Research Institute on Addictions, State University of New York at Buffalo, Buffalo, NY
Douglas A. Granger, Institute for Interdisciplinary Salivary Bioscience Research, Arizona State University, Tempe, AZ and School of Nursing, Johns Hopkins University, and Bloomberg School of Public Health
Craig R. Colder, Department of Psychology, State University of New York at Buffalo, Buffalo, NY
Pamela Schuetze, Department of Psychology, Buffalo State College, Buffalo NY.
Marilyn A. Huestis, Chemistry and Drug Metabolism, National Institute on Drug Abuse National Institutes of Health, Baltimore, MD
References
- Atzil S, Hendler T, Feldman R. Specifying the neurobiological basis of human attachment: brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology. 2011;36(13):2603–2615. doi: 10.1038/npp.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanes LS, Watamura SE, Hankin BL. Hypocortisolism as a potential marker of allostatic load in children: associations with family risk and internalizing disorders. Dev Psychopathol. 2011;23(3):881–896. doi: 10.1017/S095457941100037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009;135(6):885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Blair C, Granger D, Willoughby M, Kivlighan K. Maternal sensitivity is related to hypothalamic-pituitary-adrenal axis stress reactivity and regulation in response to emotion challenge in 6-month-old infants. Ann N Y Acad Sci. 2006;1094:263–267. doi: 10.1196/annals.1376.031. [DOI] [PubMed] [Google Scholar]
- Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors. 1998;12(2):101–112. [Google Scholar]
- Calkins SD. Cardiac vagal tone indices of temperamental reactivity and behavioral regulation in young children. Dev Psychobiol. 1997;31(2):125–135. doi: 10.1002/(sici)1098-2302(199709)31:2<125::aid-dev5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Clark R. The Parent-Child Early Relational Assessment: A factorial validity study. Educational and Psychological Measurement. 1999;59(5):821–846. [Google Scholar]
- Clark R, Musick J, Scott F, Klehr K. The mothers' project rating scale of mother-child interaction. 1980 [Google Scholar]
- Coles CD, Kable JA, Lynch ME. Examination of gender differences in effects of tobacco exposure. In: Lewis M, Kestler L, editors. Gender differences in prenatal substance exposure. Washington, DC US: American Psychological Association; 2012. pp. 99–120. [Google Scholar]
- Divers WA, Wilkes MM, Babaknia A, Yen SSC. Maternal smoking and elevation of catecholamines and metabolites in the amniotic fluid. Am. J. Obstet Gynecol. 1981;141:625–628. doi: 10.1016/s0002-9378(15)33301-9. [DOI] [PubMed] [Google Scholar]
- Fearon RP, Bakermans-Kranenburg MJ, van Ijzendoorn MH, Lapsley A-M, Roisman GI. The significance of insecure attachment and disorganization in the development of children’s externalizing behavior: A meta-analytic study. Child Development. 2010;81:435–456. doi: 10.1111/j.1467-8624.2009.01405.x. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Woodward LJ, Horwood LJ. Maternal smoking during pregnancy and psychiatric adjustment in late adolescence. Archives of General Psychiatry. 1998;55(8):721–727. doi: 10.1001/archpsyc.55.8.721. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calibration Model of stress responsivity. Neurosci Biobehav Rev. 2011;35(7):1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith HH, Rothbart MK. Laboratory assessment of infant temperament. 1996 [Google Scholar]
- Granger DA, Blair C, Willoughby M, Kivlighan KT, Hibel LC, Fortunato CK Family Life Project, Investigators. Individual differences in salivary cortisol and alpha-amylase in mothers and their infants: relation to tobacco smoke exposure. Dev Psychobiol. 2007;49(7):692–701. doi: 10.1002/dev.20247. [DOI] [PubMed] [Google Scholar]
- Gray TR, Eiden RD, Leonard KE, Connors G, Shisler S, Huestis MA. Nicotine and metabolites in meconium as evidence of maternal cigarette smoking during pregnancy and predictors of neonatal growth deficits. Nicotine Tob Res. 2010;12(6):658–664. doi: 10.1093/ntr/ntq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M, Broderson L, Krueger K, Rigatuso J. Dampening of adrenocortical reactivity during earliy infancy: Normative changes and individual differences. Child Development. 1996;67:877–889. [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Developmental Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Haley DW, Handmaker NS, Lowe J. Infant stress reactivity and prenatal alcohol exposure. Alcoholism: Clinical & Experimental Research. 2006;30:2055–2064. doi: 10.1111/j.1530-0277.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- Hibel LC, Granger DA, Cicchetti D, Rogosch F. Salivary biomarker levels and diurnal variation: Associations with medications prescribed to control children's problem behavior. Child Development. 2007;78:927–937. doi: 10.1111/j.1467-8624.2007.01041.x. [DOI] [PubMed] [Google Scholar]
- Huijbregts SC, van Berkel SR, Swaab-Barneveld H, van Goozen SH. Neurobiological and behavioral stress reactivity in children prenatally exposed to tobacco. Psychoneuroendocrinology. 2011;36(6):913–918. doi: 10.1016/j.psyneuen.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Ispa JM, Fine MA, Halgunseth LC, Harper S, Robinson J, Boyce L, Brooks-Gunn J, Brady-Smith C. Maternal Intrusiveness, Maternal Warmth, and Mother-Toddler Relationship Outcomes: Variations Across Low-Income Ethnic and Acculturation Groups. Child Development. 2004;75:1613–1631. doi: 10.1111/j.1467-8624.2004.00806.x. [DOI] [PubMed] [Google Scholar]
- Jarvis Martin J, Primatesta Paola, Erens Bob, Feyerabend Colin, Bryant Andrew. Measuring nicotine intake in population surveys: comparability of saliva cotinine and plasma cotinine estimates. Nicotine & Tobacco Research: Official Journal Of The Society For Research On Nicotine And Tobacco. 2003;5(3):349–355. doi: 10.1080/1462220031000094213. [DOI] [PubMed] [Google Scholar]
- Kiel EJ, Buss KA. Toddler inhibited temperament, maternal cortisol reactivity and embarrassment, and intrusive parenting. Journal of Family Psychology. 2013;27:512–517. doi: 10.1037/a0032892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: an overview. Neuropsychobiology. 1989;22:150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- Lewis Michael, Kestler Lisa. Gender differences in prenatal substance exposure. Washington, DC US: American Psychological Association; 2012. [Google Scholar]
- Lyons-Ruth K, Alpern L, Repacholi B. Disorganized infant attachment classification and maternal psychosocial problems as predictors of hostile-aggressive behavior in the preschool classroom. Child Development. 1993;64:572–585. doi: 10.1111/j.1467-8624.1993.tb02929.x. [DOI] [PubMed] [Google Scholar]
- Lyons-Ruth K, Bronfman E, Parsons E. Maternal frightened, frightening, or atypical behavior and disorganized infant attachment patterns. Monographs of the Society for Research in Child Development. 1999;64:67–96. doi: 10.1111/1540-5834.00034. [DOI] [PubMed] [Google Scholar]
- Lyons-Ruth K, Easterbrooks MA, Cibelli CD. Infant attachment strategies, infant mental lag, and maternal depressive symptoms: Predictors of internalizing and externalizing problems at age 7. Developmental Psychology. 1997;33:681–692. doi: 10.1037//0012-1649.33.4.681. [DOI] [PubMed] [Google Scholar]
- Mayes LC, Bornstein MH. Attention regulation in infants born at risk: Prematurity and prenatal cocaine exposure. In: Burack JA, Enns JT, editors. Attention, development, and psychopathology. New York: Guilford Press; 1997. pp. 97–122. [Google Scholar]
- McDonald SD, Walker M, Perkins SL, Beyene J, Murphy K, Gibb W, Ohlsson A. The effect of tobacco exposure on the fetal hypothalamic-pituitary-adrenal axis. BJOG. 2006;113(11):1289–1295. doi: 10.1111/j.1471-0528.2006.01089.x. [DOI] [PubMed] [Google Scholar]
- McEwen Bruce S. Stress, adaptation, and disease: Allostasis and allostatic load. In: McCann Samuel M, Lipton James M, Sternberg Esther M, Chrousos George P, Gold Philip W, Smith Craig C., editors. Annals of the New York Academy of Sciences, Vol. 840: Neuroimmunomodulation: Molecular aspects, integrative systems, and clinical advances. New York, NY US: New York Academy of Sciences; 1998. pp. 33–44. [DOI] [PubMed] [Google Scholar]
- O'Leary MM, Loney BR, Eckel LA. Gender differences in the association between psychopathic personality traits and cortisol response to induced stress. Psychoneuroendocrinology. 2007;32:183–191. doi: 10.1016/j.psyneuen.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Park A, O’Malley SS, King SL, Picciotto MR. Mediating role of stress reactivity in the effects of prenatal tobacco exposure on childhood mental health outcomes. Nicotine & Tobacco Research. 2014;16:174–185. doi: 10.1093/ntr/ntt131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay DS, Bendersky MI, Lewis M. Effect of prenatal alcohol and cigarette exposure on two- and six-month-old infants' adrenocortical reactivity to stress. Journal of Pediatric Psychology. 1996;21(6):833–840. doi: 10.1093/jpepsy/21.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze P, Lopez FA, Granger DA, Eiden RD. The association between prenatal exposure to cigarettes and cortisol reactivity and regulation in 7-month-old infants. Dev Psychobiol. 2008;50(8):819–834. doi: 10.1002/dev.20334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS Committee on Psychosocial Aspects of, Child, Family, Health, Committee on Early Childhood, Adoption, Dependent, Care, … Behavioral, Pediatrics. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, et al., editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ: Humana Press, Inc; 1992. pp. 41–72. [Google Scholar]
- Spangler G, Schieche M. Emotional and adrenocortical responses of infants to the strange situation: The differential function of emotional expression. International Journal of Behavioral Development. 1998;22(4):681–706. [Google Scholar]
- Suomi SJ. Risk, resilience, and gene-environment interplay in primates. Journal of the Canadian Academy of Child and Adolescent Psychiatry / Journal de l'Académie canadienne de psychiatrie de l'enfant et de l'adolescent. 2011;20(4):289–298. [PMC free article] [PubMed] [Google Scholar]
- Tashima L, Nakata M, Anno K, Sugino N, Kato H. Prenatal influence of ischemia-hypoxia-induced intrauterine growth retardation on brain development and behavioral activity in rats. Biology of the Neonate. 2001;80:81–87. doi: 10.1159/000047125. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Popke EJ, Rahman MA, Nespor SM, Grunberg NE. Hyperacticity induced by prenatal nicotine exposure is associated with an increase in cortical nicotinic receptors. Phamacol Biochem Behav. 1997;58:141–146. doi: 10.1016/s0091-3057(96)00461-3. [DOI] [PubMed] [Google Scholar]
- Tukey JW. Exploratory data analysis. Reading, MA: Addison-Wesley; 1977. [Google Scholar]
- U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office of Smoking and Health. The health consequences of involuntary exposure to tobacco smoke: A report of the surgeon general. Secondhand smoke what it means to you. 2006 Retrieved from www.surgeongeneral.gov/library/reports/secondhand-smoke-consumer.pdf. [PubMed]
- Varvarigou AA, Petsali M, Vassilakos P, Beratis NG. Increased cortisol concentrations in the cord blood of newborns whose mothers smoked during pregnancy. J. Perinat. Med. 2006;34:466–470. doi: 10.1515/JPM.2006.091. [DOI] [PubMed] [Google Scholar]
- Varvarigou AA, Liatsis SG, Vassilakos P, Decavalas G, Beratis NG. Effect of maternal smoking on cord blood estriol, placental lactogen, chorionic gonadotropin, FSH, LH, and cortisol. J Perinat Med. 2009;37(4):364–369. doi: 10.1515/JPM.2009.028. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Hans SL. Maternal smoking during pregnancy and conduct problems in high-risk youth: A developmental framework. Development & Psychopathology. 2002;14:351–369. doi: 10.1017/s0954579402002092. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Lahey BB, Loeber R, Green SM, Gordon RA, Leventhal BL. Maternal smoking during pregnancy and the risk of conduct disorder in boys. Archives of General Psychiatry. 1997;54(7):670–676. doi: 10.1001/archpsyc.1997.01830190098010. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Warner V, Wickramaratne PJ, Kandel DB. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38(7):892–899. doi: 10.1097/00004583-199907000-00020. [DOI] [PubMed] [Google Scholar]
- Willoughby M, Greenberg M, Blair C, Stifter C Group, The Family Life Investigative. Neurobehavioral consequences of prenatal exposure to smoking at 6 to 8 months of age. Infancy. 2007;12(3):273–301. [Google Scholar]