Abstract
Context: Inflammation plays a critical role in the development and progression of obstructive sleep apnea (OSA). Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) activation is involved in the pathophysiology of inflammatory process-related disorders. Objective: This study aims to investigate whether serum soluble LOX-1 (sLOX-1) levels are associated with the presence and severity of OSA. Materials and Methods: A total of 137 OSA patients and 78 controls were recruited in this study. Serum sLOX-1 levels were measured by enzyme-linked immunosorbent assay. The severity of OSA was assessed by the apnea–hypopnea index (AHI). Results: OSA patients had significantly higher serum sLOX-1 levels compared with controls. Serum sLOX-1 levels elevated with the increment of OSA severity. sLOX-1 was the independent predictor of OSA. Serum sLOX-1 levels were significantly correlated with AHI and high-sensitivity C-reactive protein levels. Conclusions: Serum sLOX-1 levels were independently correlated with the presence and severity of OSA. These findings revealed that sLOX-1 might function as a potential biomarker for monitoring the development and progression of OSA.
Introduction
Obstructive sleep apnea (OSA) is characterized by recurrent episodes of partial or complete obstruction of the upper airway during sleep associated with transient oxygen desaturation (Punjabi, 2008). OSA is a highly prevalent disorder, affecting about 4–20% of adults, with certain subgroups of the population bearing a higher risk (Murase et al., 2013). Accumulating clinical evidence suggests that OSA is an independent risk factor for cardiovascular diseases and cerebrovascular events. Therefore, it is of great importance to assess the risk of OSA earlier because it could lead to improved patient or physician adherence to risk-reducing behaviors or interventions.
Although the exact mechanism of OSA remains unclear, it is now well appreciated that inflammation plays a crucial role in the development and progression of OSA (Inancli and Enoz, 2010). Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) is a type II glycoprotein acting as a receptor for oxidized low-density lipoprotein (ox-LDL) (Sawamura et al., 1997). LOX-1 was mainly expressed by endothelial cells (ECs) and later found also to be inducibly expressed by vascular smooth muscle cells (VSMCs), macrophages, and platelets (Zhao et al., 2011). Recent studies have verified that LOX-1 activation is involved in the inflammatory process and inflammatory-related disorders (Hayashida et al., 2005; Lubrano et al., 2008; Yan et al., 2011; Takanabe-Mori et al., 2013).
Like many cell surface receptors with a single transmembrane domain, LOX-1 can be secreted in a soluble form (sLOX-1) by proteolytic cleavage at its membrane-proximal extracellular domain (Zhao et al., 2011). Therefore, sLOX-1 holds the potential to be assessed as a blood biomarker. Noninvasive blood biomarkers are represented as important alternatives for diagnosis and risk stratification of disease. Akinnusi et al. (2011) have demonstrated that LOX-1 expression was significantly increased in apoptotic circulating ECs in OSA patients. However, the correlation between sLOX-1 levels and the presence and severity of OSA has not been assessed. Therefore, the present study aimed to measure serum sLOX-1 levels in OSA patients and to investigate their correlation with the presence and severity of OSA.
Materials and Methods
Study subjects
From July 2012 to October 2014, a total of 137 OSA patients during hospitalization were recruited in the present study. All OSA patients were diagnosed by polysomnography (PSG) in our hospital. Seventy-eight age-, sex-, and body–mass index (BMI)-matched volunteers who visited our hospital for routine physical examination during the same period were enrolled as controls. Patients were excluded on the basis of having coronary artery disease, diabetes, chronic obstructive pulmonary disease, asthma, malignant disease, connective tissue diseases, advanced renal disease, or on dialysis. Informed consent was obtained from all participants. This study was approved by the ethics committee of our hospital.
Sleep study
All subjects underwent overnight PSG monitoring using a digital polygraph (Alice 5 polysomnographic Sleep System; Philips Respironics, Andover, MA) in our hospital. Polysomnographic monitoring was done with standard technique. Apnea was defined as complete cessation of airflow ≥10 s. Hypopnea was defined as a reduction in airflow with a 50% from baseline for at least 10 s, a 3% drop in oxygen saturation from the preceding stable saturation, and/or arousal. Apnea–hypopnea index (AHI) was calculated as the number of apneas plus hypopneas per hour of sleep. The diagnosis of OSA was based on AHI>5, which was further subdivided into mild (5≤AHI<15), moderate (15≤AHI<30), and severe (≥30) OSA.
Biochemical investigation
Blood samples were taken from the cubital vein of all participants in the fasting state. Blood samples were centrifuged and stored at −80°C until investigation. Serum fasting blood glucose, total cholesterol, triglyceride, low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol, and high-sensitivity C-reactive protein (hs-CRP) were measured by standard laboratory techniques on a Hitachi 7060 Automatic Biochemical Analyzer (Hitachi Co., Tokyo, Japan). Serum sLOX-1 levels were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit according to the protocol of the manufacturer (USCN Life Science, Wuhan, China). The intra-assay and interassay CV of the ELISA kits were both <5%. All the samples were routinely analyzed in triplicate, and the results were averaged.
Statistical analyses
SPSS software package 15.0 for Windows (SPSS, Inc., Chicago, IL) was used for statistical analyses. The distribution of continuous variables for normality was tested with the Kolmogorove–Smirnov test, and data are presented as mean standard deviation (SD) or median and interquartile ranges, as appropriate. Categorical variables are reported as frequencies and group percentages. Differences between patients with and without OSA were evaluated by the unpaired t-test, the Mann–Whitney U-test, or chi-square test, as indicated. Differences among OSA patients with different degrees of severity were analyzed by one-way analysis of variance, followed by Tukey's post hoc test or the Kruskal–Wallis test when appropriate. Multiple logistic regression analysis was used to determine the independent predictors of OSA. Significant univariate variables with p<0.05 were included in the multiple logistic regression analysis. Spearman rank correlation coefficient was employed to determine the correlation between serum sLOX-1 levels and AHI scores. Multivariate linear analysis was performed to determine the independent predictors of AHI. As the serum sLOX-1 levels and AHI scores were not normally distributed, log transformation values were performed. All p-values are two-sided, and a p-value of <0.05 was considered significant.
Results
Baseline clinical characteristics
The baseline clinical and laboratory characteristics of the subjects are presented in Table 1. OSA patients had significantly higher systolic blood pressure (SBP), diastolic blood pressure (DBP), LDL-c, and hs-CRP levels compared with controls. There were no significant differences in other baseline characteristics between the two groups.
Table 1.
Baseline Clinical Characteristics
| Variables | Control group (n=78) | OSA group (n=137) | p-Value |
|---|---|---|---|
| Age (years) | 57.35±8.08 | 58.33±8.61 | 0.41 |
| Male, n (%) | 54 (69.23%) | 96 (70.07%) | 0.90 |
| Smoking, n (%) | 41 (52.56%) | 81 (59.12%) | 0.35 |
| BMI (kg/mm2) | 25.92 (24.82–26.57) | 26.07 (24.65–27.58) | 0.22 |
| SBP (mmHg) | 127.23±17.36 | 138.31±17.53 | <0.001 |
| DBP (mmHg) | 80.95±10.48 | 89.17±11.24 | <0.001 |
| FBG (mM) | 5.71±0.73 | 5.73±0.88 | 0.86 |
| TC (mM) | 4.45±1.15 | 4.64±1.04 | 0.23 |
| TG (mM) | 1.67 (1.28–2.25) | 1.84 (1.32–2.43) | 0.65 |
| LDL-c (mM) | 2.66±0.95 | 3.00±0.80 | 0.005 |
| HDL-c (mM) | 1.11±0.29 | 1.05±0.22 | 0.10 |
| hs-CRP (mg/L) | 0.56 (0.32–0.78) | 0.89 (0.62–1.71) | <0.001 |
All values are mean±SD, median with interquartile range or n (%).
BMI, body–mass index; DBP, diastolic blood pressure; FBG, fasting blood glucose; HDL-c, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; LDL-c, low-density lipoprotein cholesterol; OSA, obstructive sleep apnea; SBP, systolic blood pressure; SD, standard deviation; TC, total cholesterol; TG, triglycerides.
Serum sLOX-1 levels in subjects
OSA patients had significantly higher serum sLOX-1 levels compared with controls (0.68 [range 0.42–0.99] vs. 0.37 [range 0.18–0.92] ng/mL, p<0.001; Fig. 1A). In OSA patients, the Kruskal–Wallis test showed that there was a significant difference in serum sLOX-1 levels among OSA patients of different severity (sLOX-1 levels of 0.55 [range 0.33–0.87] ng/mL in the mild OSA group, 0.68 [range 0.42–0.99] in the moderate OSA group, and 0.82 [range 0.50–1.20] in the severe OSA group, p=0.015; Fig. 1B).
FIG. 1.
Box-and-whisker plot showing serum soluble LOX-1 (sLOX-1) levels in controls and obstructive sleep apnea (OSA) patients (A) and in mild, moderate, and severe OSA patients (B). LOX-1, lectin-like oxidized low-density lipoprotein receptor-1.
Logistic regression analysis for the presence of OSA
Simple logistic analysis demonstrated that SBP, DBP, LDL-c, and serum sLOX-1 levels were significantly associated with the presence of OSA. Multivariate logistic regression, including these variables, revealed that sLOX-1 was the independent predictor of OSA (odds ratio 3.06, 95% confidence interval 1.55–6.02; p<0.01; Table 2).
Table 2.
Logistic Regression Analysis for the Presence of OSA
| Variables | Simple regression OR (95% CI) | p-Value | Multiple regression OR (95%CI) | p-Value |
|---|---|---|---|---|
| Age (per year) | 1.01 (0.98–1.05) | 0.41 | ||
| Male (no/yes) | 0.96 (0.53–1.76) | 0.90 | ||
| Smoking (no/yes) | 0.77 (0.44–1.34) | 0.35 | ||
| BMI (per kg/mm2) | 1.08 (0.96–1.21) | 0.20 | ||
| SBP (per mmHg) | 1.04 (1.02–1.06) | <0.001 | 1.02 (1.00–1.05) | 0.03 |
| DBP (per mmHg) | 1.07 (1.04–1.10) | <0.001 | 1.05 (1.02–1.09) | 0.005 |
| FBG (per mM) | 1.03 (0.74–1.45) | 0.86 | ||
| TC (per mM) | 1.18 (0.90–1.53) | 0.23 | ||
| TG (per mM) | 0.96 (0.77–1.20) | 0.69 | ||
| LDL-c (per mM) | 1.64 (1.15–2.32) | 0.006 | 1.34 (0.92–1.95) | 0.13 |
| HDL-c (per mM) | 0.39 (0.13–1.21) | 0.10 | ||
| hs-CRP (per mg/L) | 1.06 (0.92–1.21) | 0.41 | ||
| sLOX-1 (per ng/mL) | 2.49 (1.34–4.61) | 0.004 | 3.06 (1.55–6.02) | 0.001 |
OR, odds ratio; CI, confidence interval.
The association of serum sLOX-1 levels with AHI
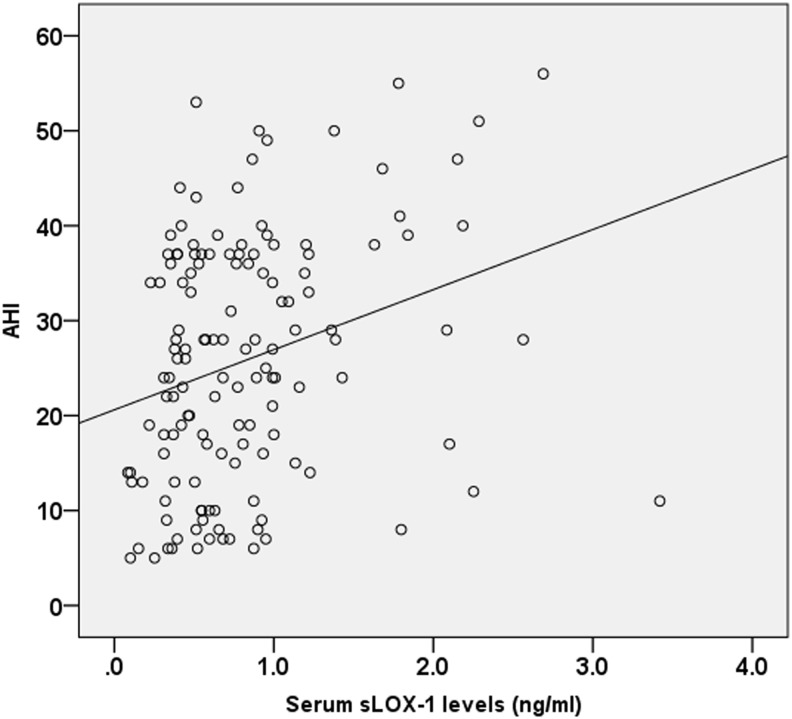
Spearman correlation analysis indicated that serum sLOX-1 levels were significantly correlated with AHI scores (r=0.30, p<0.001; Fig. 2). Multivariate linear regression analysis demonstrated that there was still a positive association between serum sLOX-1 levels and AHI after adjusting for all other variables as potential confounders (β 0.26, t=3.26, p=0.001).
FIG. 2.
The correlation between serum sLOX-1 levels and apnea–hypopnea index (AHI) scores.
The association of serum sLOX-1 levels with other clinical characteristics
The relationship between serum sLOX-1 and other clinical characteristics was also assessed. We demonstrated that serum sLOX-1 levels were weakly correlated with hs-CRP levels (r=0.16, p=0.017).
Discussion
In the present study, we demonstrated that OSA patients had higher serum sLOX-1 levels compared with controls. Multivariate logistic regression demonstrated that the elevated sLOX-1 level was an independent predictor of the presence of OSA. In OSA patients, those with higher AHI scores had higher serum sLOX-1 levels. Serum sLOX-1 levels were independently associated with disease severity. The relationship between sLOX-1 and OSA suggests a crucial role of LOX-1 activation in the pathogenesis of OSA.
We demonstrated, in the present study, that serum sLOX-1 levels elevated in OSA patients, which is in accordance with a previous study (Akinnusi et al., 2011). The elevated levels of sLOX-1 in serum may reflect the increased expression of membrane-bound LOX-1 in OSA patients (Brinkley et al., 2008). The cell surface expression of LOX-1 could be induced by many inflammatory cytokines, oxidative stress, and hemodynamic stimuli (Kume et al., 1998; Li et al., 2004). OSA is almost always accompanied by inflammatory cell infiltration in the upper airway (Shi et al., 2014). Therefore, LOX-1 may be synthesized and released from ECs and differentiated VSMCs in response to the inflammatory process and hypoxia. Whether other mechanisms contribute to the increased LOX-1 expression is yet to be further investigated.
Serum sLOX-1 levels are elevated in many inflammatory diseases (Hayashida et al., 2005; Lubrano et al., 2008; Yan et al., 2011; Takanabe-Mori et al., 2013). Its clinical importance is gradually increasing as a potential biomarker of various inflammatory diseases. In the present study, we also demonstrated that serum sLOX-1 levels were correlated with levels of hs-CPR, a sensitive marker of systemic inflammation. This result concurred with those of earlier studies linking LOX-1 to proinflammatory cytokines.
The major finding of the present study was that the serum sLOX-1 level was an independent predictor for the presence of OSA. This result indicated that LOX-1 activation may be involved in the pathophysiology of OSA, and sLOX-1 appears to be a potential serum biomarker for early diagnosis of OSA. LOX-1 is a multiligand receptor with high specificity for ox-LDL. In spite of this specificity, LOX-1 recognizes multiple ligands, including apoptotic cells, aged red blood cells, leukocytes, inflammatory cytokines, activated platelets, phosphatidylcholine, and advanced glycation end products (AGEs) (Navarra et al., 2010). The binding of ligands to LOX-1 induces the activation of p38 mitogen-activated protein kinase (p38MAPK), phosphatidylinositol-3-kinase, and extracellular signal-regulated kinase (ERK1/2), which leads to activation of transcription factor nuclear factor-κB (Li et al., 2000; Tanigawa et al., 2006). These signal pathways promote the production of proinflammatory cytokines (such as tumor necrosis factor-α, interleukin-6,10) and elicit expression of adhesion molecules such as intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 (Li et al., 2002; Hashizume and Mihara, 2012; Yin et al., 2013). These mediators lead to macrophage/monocyte attachment and activation, further inducing the expression of LOX-1. By this positive feedback, LOX-1 thus contributes to the pathogenesis of many inflammatory diseases, including OSA.
We further investigated the relationship between serum sLOX-1 levels and the severity of OSA. We found that serum sLOX-1 levels elevated with the increment of AHI. Moreover, serum sLOX-1 levels were significantly correlated with AHI. The correlation was still significant after adjusting for potential confounders such as age and BMI in multivariate linear analysis. These results revealed that LOX-1 activation might contribute to the progression of OSA, and sLOX-1 holds the potential to be assessed as a biomarker for risk stratification of OSA. However, the correlation between serum sLOX-1 levels and AHI was weak and its clinical significance should be interpreted cautiously.
Some inherent limitations of this study may have affected the results and may limit generalization of the findings. First, this cross-sectional study was conducted at a single center and had a relatively small sample size. Therefore, it is necessary to validate these results in multicenter, randomized, prospective longitudinal studies with a larger sample size. Second, we did not monitor other proinflammatory cytokines (e.g., tumor necrosis factor-α) and assess the association between those cytokines and sLOX-1 in this study. Such investigations may reveal more valuable information on the possible pathogenic role of LOX-1 in OSA. Third, controls recruited in the present study did not undergo PSG to rule out OSA. This might lead to a potential bias.
In conclusion, we demonstrated that serum sLOX-1 levels were independently correlated with the presence and severity of OSA. These findings revealed that sLOX-1 might function as a potential biomarker for monitoring the development and progression of OSA. Therapeutic interventions by inhibiting LOX-1 signaling pathways to delay the progressive process of OSA warrant further investigations.
Acknowledgment
This study was supported by the Yiwu Municipal Science and Technology Project (Grant 11-3-27).
Author Disclosure Statement
No competing financial interests exist.
References
- Akinnusi ME, Laporta R, El-Solh AA. (2011) Lectin-like oxidized low-density lipoprotein receptor-1 modulates endothelial apoptosis in obstructive sleep apnea. Chest 140:1503–1510 [DOI] [PubMed] [Google Scholar]
- Brinkley TE, Kume N, Mitsuoka H, et al. (2008) Variation in the human lectin-like oxidized low-density lipoprotein receptor 1 (LOX-1) gene is associated with plasma soluble LOX-1 levels. Exp Physiol 93:1085–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume M, Mihara M. (2012) Atherogenic effects of TNF-α and IL-6 via up-regulation of scavenger receptors. Cytokine 58:424–430 [DOI] [PubMed] [Google Scholar]
- Hayashida K, Kume N, Murase T, et al. (2005) Serum soluble lectin-like oxidized low-density lipoprotein receptor-1 levels are elevated in acute coronary syndrome: a novel marker for early diagnosis. Circulation 112:812–818 [DOI] [PubMed] [Google Scholar]
- Inancli HM, Enoz M. (2010) Obstructive sleep apnea syndrome and upper airway inflammation. Recent Pat Inflamm Allergy Drug Discov 4:54–57 [DOI] [PubMed] [Google Scholar]
- Kume N, Murase T, Moriwaki H, et al. (1998) Inducible expression of lectin-like oxidized LDL receptor-1 in vascular endothelial cells. Circ Res 83:322–327 [DOI] [PubMed] [Google Scholar]
- Li D, Chen H, Romeo F, et al. (2002) Statins modulate oxidized low-density lipoprotein-mediated adhesion molecule expression in human coronary artery endothelial cells: role of LOX-1. J Pharmacol Exp Ther 302:601–605 [DOI] [PubMed] [Google Scholar]
- Li D, Saldeen T, Romeo F, et al. (2000) Oxidized LDL upregulates angiotensin II type 1 receptor expression in cultured human coronary artery endothelial cells: the potential role of transcription factor NF-kappaB. Circulation 102:1970–1976 [DOI] [PubMed] [Google Scholar]
- Li L, Roumeliotis N, Sawamura T, et al. (2004) C-reactive protein enhances LOX-1 expression in human aortic endothelial cells: relevance of LOX-1 to C-reactive protein-induced endothelial dysfunction. Circ Res 95:877–883 [DOI] [PubMed] [Google Scholar]
- Lubrano V, Del Turco S, Nicolini G, et al. (2008) Circulating levels of lectin-like oxidized low-density lipoprotein receptor-1 are associated with inflammatory markers. Lipids 43:945–950 [DOI] [PubMed] [Google Scholar]
- Murase K, Mori K, Yoshimura C, et al. (2013) Association between plasma neutrophil gelatinase associated lipocalin level and obstructive sleep apnea or nocturnal intermittent hypoxia. PLoS One 8:e54184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarra T, Del Turco S, Berti S, et al. (2010) The lectin-like oxidized low-density lipoprotein receptor-1 and its soluble form: cardiovascular implications. J Atheroscler Thromb 17:317–331 [DOI] [PubMed] [Google Scholar]
- Punjabi NM. (2008) The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 5:136–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamura T, Kume N, Aoyama T, et al. (1997) An endothelial receptor for oxidized low-density lipoprotein. Nature 386:73–77 [DOI] [PubMed] [Google Scholar]
- Shi YK, Chen JX, Huang Y, et al. (2014) Serum S100A12 levels are associated with the presence and severity of obstructive sleep apnea syndrome in male patients. Sleep Breath 18:269–274 [DOI] [PubMed] [Google Scholar]
- Takanabe-Mori R, Ono K, Wada H, et al. (2013) Lectin-like oxidized low-density lipoprotein receptor-1 plays an important role in vascular inflammation in current smokers. J Atheroscler Thromb 20:585–590 [DOI] [PubMed] [Google Scholar]
- Tanigawa H, Miura S, Zhang B, et al. (2006) Low-density lipoprotein oxidized to various degrees activates ERK1/2 through Lox-1. Atherosclerosis 188:245–250 [DOI] [PubMed] [Google Scholar]
- Yan M, Mehta JL, Zhang W, et al. (2011) LOX-1, oxidative stress and inflammation: a novel mechanism for diabetic cardiovascular complications. Cardiovasc Drugs Ther 25:451–459 [DOI] [PubMed] [Google Scholar]
- Yin Y, Liu W, Ji G, et al. (2013) The essential role of p38 MAPK in mediating the interplay of oxLDL and IL-10 in regulating endothelial cell apoptosis. Eur J Cell Biol 92:150–159 [DOI] [PubMed] [Google Scholar]
- Zhao ZW, Zhu XL, Luo YK, et al. (2011) Circulating soluble lectin-like oxidized low-density lipoprotein receptor-1 levels are associated with angiographic coronary lesion complexity in patients with coronary artery disease. Clin Cardiol 34:172–177 [DOI] [PMC free article] [PubMed] [Google Scholar]