Abstract
Objective: The research presented here assesses the scientific evidence for the telemedicine intervention in the management of diabetes (telediabetes), gestational diabetes, and diabetic retinopathy. The impetus derives from the confluence of high prevalence of these diseases, increasing incidence, and rising costs, while telemedicine promises to ameliorate, if not prevent, type 2 diabetes and its complications. Materials and Methods: A purposeful review of the literature identified relevant publications from January 2005 to December 2013. These were culled to retain only credible research articles for detailed review and analysis. The search yielded approximately 17,000 articles with no date constraints. Of these, 770 appeared to be research articles within our time frame. A review of the abstracts yielded 73 articles that met the criteria for inclusion in the final analysis. Evidence is organized by research findings regarding feasibility/acceptance, intermediate outcomes (e.g., use of service, and screening compliance), and health outcomes (control of glycemic level, lipids, body weight, and physical activity.) Results: Definitions of telediabetes varied from study to study vis-à-vis diabetes subtype, setting, technology, staffing, duration, frequency, and target population. Outcome measures also varied. Despite these vagaries, sufficient evidence was obtained from a wide variety of research studies, consistently pointing to positive effects of telemonitoring and telescreening in terms of glycemic control, reduced body weight, and increased physical exercise. The major contributions point to telemedicine's potential for changing behaviors important to diabetes control and prevention, especially type 2 and gestational diabetes. Similarly, screening and monitoring for retinopathy can detect symptoms early that may be controlled or treated. Conclusions: Overall, there is strong and consistent evidence of improved glycemic control among persons with type 2 and gestational diabetes as well as effective screening and monitoring of diabetic retinopathy.
Key words: : diabetes, gestational diabetes, diabetic retinopathy, telediabetes telemedicine, telemonitoring, telescreening
Introduction
In this article, we examine the empirical evidence pertaining to the effects of the telemedicine intervention on the management of diabetes (telediabetes), gestational diabetes mellitus (GDM), and diabetic retinopathy (DR). In an earlier article,1 we focused on the evidence for the management of three other chronic diseases: congestive heart failure, stroke, and chronic obstructive pulmonary disease. Our approach here is similar to the one used in the earlier article.1 We begin by providing basic information on the nature of diabetes, including its etiology, epidemiology, and cost. As with other chronic diseases, diabetes has different manifestations in terms of etiology, severity, age of onset, sequelae, comorbidities, and, most importantly to our purpose, its amenability to control, amelioration, and/or prevention through telemedicine-supported interventions. This information establishes a foundation for the inquiry into the nature and relevance of the telemedicine intervention in this domain. This section is followed by a description of the literature search and review process, including the inclusion criteria for the selection of research studies and the manner in which the resulting empirical evidence is organized. In the latter instance, analysis of the evidence in telediabetes research is organized according to diabetes type (type 1 and/or type 2, GDM, and a common diabetes-related complication, DR). For each of these, the evidence is organized by research findings regarding (a) feasibility/acceptance and effectiveness in controlling or ameliorating the course of diabetes, (b) intermediate outcomes (e.g., use of service, compliance with screening guidelines), and (c) health outcomes (control of glycemic level, lipids, body weight, and physical activity).
Diabetes
The term diabetes derives from the Greek diabainein,2 meaning “siphon,” referring to excessive passing of water (urine), and later from the English adoption of the medieval Latin, “diabetes.” The disease is also referred to as “diabetes mellitus”; the latter term was added in 1675 by Thomas Willis.3 “Mellitus” derives from the Latin mel for honey or sweetness (due to the high glucose content of the urine). Diabetes is a complex group of metabolic diseases—referring to disorders in the complex set of chemical reactions the body uses to maintain life, including energy production. As such, it is a clinically heterogeneous group of disorders that have hyperglycemia (or high blood sugar) as a common attribute. Hyperglycemia results from insufficient insulin hormone secretion by the pancreas, the body's inability to respond properly to insulin (insulin resistance), or both. Insulin is a hormone produced by the pancreas that regulates blood glucose and allows the body to convert glucose from carbohydrates to energy. Insulin controls blood sugar levels and keeps them within a normal range. Uncontrolled hyperglycemia results in the body's cells being starved for energy because of a lack of insulin. Early complications of diabetes include damage to the small blood vessels in the eyes, the kidneys, and the nervous system, which can lead to vision problems, possibly blindness, kidney disease, neuropathy, and risk of amputation. A prolonged high blood glucose level damages medium-size and larger blood vessels that supply the heart and the brain, often leading to heart disease and stroke. Other long-term complications include a higher risk for cancer, physical disabilities, depression, and complications in pregnancy.4
Diabetes has been classified into three types:
• Type 1 (also referred to as insulin-dependent diabetes, juvenile diabetes, or early-onset diabetes) occurs when the pancreas cannot make insulin because of damaged or destroyed β cells, thereby leading to absolute insulin deficiency. Although often identified in children and adolescents, type 1 diabetes can occur at any age, accounting for about 5–10% of those with diabetes. Some forms of type 1 diabetes have no known etiology and therefore can be referred to as idiopathic diabetes. Insulin therapy is required for individuals with type 1 diabetes in combination with diet and exercise.
• Type 2 (also referred to as adult-onset diabetes or non–insulin-dependent diabetes) occurs when the pancreas does not produce a sufficient amount of insulin or the cells in the pancreas do not process insulin properly. It is the most common type of diabetes (about 90% of cases), and it afflicts people of all ages. Its incidence is associated with age, obesity, and lack of physical activity. Often, individuals with type 2 diabetes do not need insulin treatment to survive. In some instances, it may be difficult to classify patients as having either type 1 or type 2 diabetes. However, the forms of diabetes that have their onset in young age are different from those that start in adulthood.
• The third type is GDM, which occurs during pregnancy in women without a history of diabetes. GDM is characterized by high blood glucose levels, particularly in the third trimester, due to increased levels of human placental lactogen leading to insulin resistance, which in turn results in high blood glucose levels. Some women may have had diabetes (type 1 or type 2) prior to becoming pregnant. The majority of pregnant women with gestational diabetes can control their glucose with diet and exercise. If glucose is not controlled, oral medication or insulin is used. Additional information regarding GDM is included later in the section on telemonitoring and GDM.
Prediabetes is a condition of high blood glucose levels in which the β cells are becoming resistant to insulin, but not sufficiently commensurate to classify the condition as diabetes. This means that the blood sugar level is higher than normal but not high enough to be classified as type 2 diabetes. It is characterized by a degree of hyperglycemia that can result in pathological and functional changes before the detection of diabetes. The term prediabetes was introduced in 1960s, dismissed in 1980 by the World Health Organization,5 and reactivated in 2003 by the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus6 to draw attention to this condition, which is often a precursor to type 2 diabetes. It is estimated that the vast majority of people with prediabetes (about 90%) do not know they have it. Without changes in life style, 15–30% of them will develop type 2 diabetes within 5 years.7
Diabetes Testing
There are three tests for diagnosing diabetes type 1 or type 2: (1) The most common test is the glycated hemoglobin HbA1c (a subclass of hemoglobin A) or simply A1c, which measures average blood glucose level over a period of 2–3 months. The test became available in 1978, gained popularity in the 1980s, and was formally adopted by the World Health Organization in 2011.8 A normal level (no diabetes) is less than 5.7%. Prediabetes is diagnosed at values between 5.7% and 6.4%. Diabetes is diagnosed at an A1c level of 6.5% or more. (2) Fasting plasma glucose (FPG) measures glucose levels after fasting, thereby improving the reliability of the test. Diabetes is diagnosed at values of 126 mg/dL or higher, and prediabetes is diagnosed between 100 and 125 mg/dL. (3) The oral glucose tolerance test (OGTT) is a 2-h test that measures blood glucose before and after a sweet drink. Values of 200 mg/dL or higher are indicative of diabetes, and 144–199 mg/dL as prediabetes. Blood glucose can also be tested at random or when there are severe symptoms, and is interpreted the same way as the OGTT.
Diabetes Epidemiology
In 2014, the overall prevalence rate of diabetes in the U.S. population was estimated at 9.3%, or about 29.1 million individuals. Of these, 21.0 million have been diagnosed, and the remaining 8.1 million were not. That is, about 27.8% of the population who have diabetes do not know they have the condition.9 In 2012, it was estimated that about 86 million people had prediabetes, but only 10% of them were aware of it. Hence, in the aggregate, nearly one-third of the U.S. population may have either diabetes or prediabetes but do not know it. This is important because many of these individuals would be able to control, if not reverse, the ravages of diabetes through rigorous monitoring of their glucose levels, focusing on medication management, as well as weight loss and moderate physical exercise.
The incidence of diabetes increases with age. For instance, the estimated rate is 12.3% among people 20 years of age or older and 25.9% of those who are 65 years of age or older. The percentage difference in the prevalence of diabetes between adult men and women is rather small (13.6% and 11.2%, respectively).9
The incidence of type 2 diabetes has been increasing during the last few decades, especially among the young. Historically, type 2 diabetes among persons younger than 20 years of age was extremely rare, but it is now on the rise, particularly among Native American and African American populations. This is attributed primarily to increased numbers of people who are overweight/obese and an increasingly common sedentary life style. During the last decade, among young people between the ages of 10 and 20 years, the incidence of new cases of type 1 diabetes was 18.6 per 100,000, and that of type 2 diabetes was 8.5 per 100,000. On the other hand, about 25% of those over the age of 65 years had type 2 diabetes. On average, African Americans tend to have a higher level of A1c compared with whites, suggesting that their glycemic burden may be higher.9
On a global scale, it is estimated that 387 million individuals have diabetes, representing 8.3% of the world population. This is projected to increase to 600 million by 2035.10 Whereas diabetes used to be considered a problem for developed countries, “77% of the world's diabetes population will be from low-income and middle-income countries, with over half from Southeast Asia and the Western Pacific regions.”10
Diabetes is the seventh leading cause of death and a major cause of disability in the United States. However, this ranking may be underestimated because diabetes is a major cause of heart disease and stroke. It is also the leading cause of kidney failure, lower limb amputations, and new cases of blindness among adults.11 According to the Centers for Disease Control and Prevention, “the risk for death among people with diabetes is about twice that of people of similar age but without diabetes.”4
As mentioned above, the major risk factors in type 2 diabetes are overweight/obesity and sedentary life style. It is important that significant weight loss and a healthy life style can reduce the risk of type 2 diabetes by nearly 50%.4 Indeed, there is a potential for “reversal” of the disease in some cases. Also, the risk of diabetes among persons with prediabetes can be decreased by nearly 60% if they lose a significant amount of body weight (about 7%) and increase moderate physical activity to minimum of 150 min/week.4,12 Among pregnant women with GDM, the risk for developing diabetes after delivery is 35–60% higher compared with their counterparts.4
Diabetes Cost
A study commissioned by the American Diabetes Association, entitled ”Economic Costs of Diabetes in the U.S. in 2012,”13 estimated the total cost of diagnosed diabetes at $245 billion. This was up from $174 billion in 2007, representing a 41% increase in 5 years, at an annual rate of increase of 8.2% or $14 billion. Of this amount, $176 billion were spent on direct medical care, and $69 billion were attributed to productivity loss. The largest components of direct medical expenditures were hospitalization (43%), medications (18%), agents and supplies (12%), physician office visits (9%), and nursing/residential facility stays (8%). During the same 5-year period (2007–2012), the average annual medical expenditures for a person with diabetes were estimated at about $13,700. Nearly two-thirds, or 62.4%, of the direct medical cost of diabetes is borne by government programs (including Medicare, Medicaid, and the military). However, these estimates do not include the cost of undiagnosed diabetes, the burden of pain and suffering, or the care provided by unpaid caregivers. For example, it is estimated that families having a child with diabetes devote about 10% of their family income for diabetic care.14 Overall, medical expenses for people with diabetes are more than twice (2.3 times) that for people without diabetes.4
Telediabetes
The lives of the vast majority of people with diabetes—including those diagnosed with the disease, those at the threshold of developing diabetes, and those who have diabetes and do not know it—are adversely affected by this pernicious disease. Yet, this is one chronic disease (especially type 2 diabetes) that can be controlled, if not prevented, by appropriate behavioral changes on the part of those who are affected or at risk. Type 2 diabetes is progressive, often requiring treatment with one or more non-insulin therapies. Insulin therapy may be needed in order to achieve optimal glycemic control. Type 1 diabetes requires frequent monitoring and medication titration. Regardless of type, in order for patients with diabetes to take appropriate measures for managing their condition, they need early diagnosis, regular glucose monitoring, and individualized treatment plans that address their emerging needs, as well as ongoing clinical support and guidance that prompts and assists them to take appropriate preventive/maintenance measures to optimize their health and well-being.
In general terms, telediabetes serves two related goals: (1) control of blood glucose through a healthy life style, including proper diet and weight reduction among overweight or obese individuals, as well as increased regular physical exercise, and (2) medication management, including insulin titration. Telediabetes provides the tools for routine and ongoing monitoring of blood glucose levels, patient-specific management plans, and educational materials, instruction, and reinforcement/support strategies for the adoption of a healthy life, as indicated by an individual's relative risk. Once the system is in place, its components serve as an effective communication link between patients and a professional support team that responds promptly to patients' needs and inquiries.
Telediabetes shares some of the same attributes of telemonitoring for other chronic conditions, such as congestive heart failure, stroke, and chronic obstructive pulmonary disease. In fact, all these interventions constitute essential elements of comprehensive chronic disease management wherein patients are expected to assume substantial responsibility for managing their own health and healthcare while being monitored and guided actively by clinical providers through telephony, videoconferencing, or other electronic devices on a routine basis. The typical telediabetes system provides patients with electronic tools to measure blood glucose levels, to report this information to a care coordination clinic, staffed by nurses and/or dietitians, and to receive guidance on proper steps to control their glucose levels, thus enhancing their health and well-being. The electronic system collects, stores, and processes the data provided by patients, establishes trends in the progression of the disease for each patient, and gathers other case-specific information over time. It has built-in and protocol-driven trigger alerts for response or action when certain clinical markers are exceeded. In brief, the ultimate aim of chronic disease management systems is to assure that patients receive the “appropriate care at the appropriate time and place in the most appropriate manner.”15 Telediabetes brings this goal closer to fruition.
In general, telediabetes incorporates essential elements of patient-centered care, as it focuses on the patients' specific health needs and engages them in their own care. The corollary concept of the medical home is intended to coordinate the services and referrals across a continuum of care for the patient.16,17 These two concepts are often combined in the patient-centered medical home to describe an innovative model18 of care coordination and delivery that serves the full spectrum of healthcare needs of a patient. Telemonitoring also incorporates the concept of shared decision-making, which has been advocated since the 1970s as the ideal model for decision-making on the part of patients when confronted with a consequential range of choices in their care.19 Shared decision-making embodies the principle of informed consent, which stipulates that the patient has to agree to any proposed medical treatment prior to its initiation. It reinforces the patient's right to informed choice and the role of the provider as an agent of the patient. Accordingly, the patient would be given explicit information regarding treatment options and their potential consequences in terms of benefits and risks. The same principle is extended to other critical decisions confronting patients such as going to the emergency department or other options. Indeed, the informed consent requirement is now commonplace in healthcare delivery.
Telemonitoring systems facilitate the patient-centered medical home by linking patients and providers through a variety of telecommunications options and systems. These systems may be automated or not, fixed or mobile, possibly wearable or implantable. Some require the patient to collect and transmit the data to a designated clinical center, where they are stored, processed, and subsequently used to help the patient. In addition, some systems contain educational materials that are tailored to the individual patient needs, typically focusing on glycemic control, weight control, physical activity, and smoking cessation. The providers have ready access not only to the monitored information on glucose levels, lipid levels, and other clinically relevant parameters, but also to the patients' medical histories where electronic personal health records are available. The system contains interactive tools to help patients with critical decisions, such as the interpretation of symptoms and conditions as well as the options regarding treatment modalities.
There is near consensus in the field that type 2 diabetes can be controlled (if not prevented), that treatment and guidance protocols can and should be customized to fit the needs of individual patients, and that patients with diabetes should be monitored on a regular basis.20
The Review Process
The analysis of the empirical evidence in telediabetes research entailed four steps: (1) an initial literature search for all publications using the key terms “telemedicine,” “telehealth,” “telemonitoring,” and “diabetes” to identify all relevant publications from January 2005 to December 2013; (2) a culling of references to select research articles only, eliminating editorials, case reports, and project descriptions; (3) a review of abstracts of research articles to determine eligibility for inclusion in the final list, using the two inclusion criteria, namely (a) a randomized clinical trial (RCT) or another robust research design and (b) a sample size of 150 or more cases to assure statistical power; and (4) a detailed review of complete manuscripts of publications in the final list of eligible articles. A few exceptions were made in the selection of the final list for analysis where sample size was smaller than 150, the RCT design was not used, or both. The decision to include these articles was based on their contribution to our understanding of innovative methods in diabetes monitoring such as the use of an interactive diary, or when an RCT design was not the method of choice for the particular research objectives under investigation, such as the estimation of incidence or prevalence rates, the measurement of other population-based parameters, or feasibility assessment of certain monitoring modalities or interventions. However, studies that did not investigate the effects of telediabetes or produced empirical evidence of the merit of telediabetes were not included in the discussion and tabular presentation of the empirical evidence. The rationale for the time limit to one preceding decade has to do with the rapid pace of technological development, whereby older technologies have become obsolete in terms of functionality, ease of use, cost, and ubiquity.
Step 1 in the review of the past 10 years yielded approximately 17,000 articles. Of these, 770 appeared to be research articles. A review of the abstracts of these 770 yielded 73 articles that met the inclusion criteria for this analysis. Of these, 21 were focused on telediabetes, plus 9 studies conducted at the Veteran Health Administration (VHA) and 16 reports from one project, the Informatics for Diabetes Education and Telemedicine (IDEATel). An additional set of four studies was focused on GDM, and 23 focused on DR.
One final note regarding the complexity of the evidence in telediabetes research is in order. The empirical findings reported here derive from a rich mixture of research studies that were conducted in different countries and settings, with different patient populations, using different configurations of technologies and human resources, and also using different research protocols. More specifically, the studies differed in terms of (a) the selection of patient populations by diabetes type (type 1 diabetes, type 2 diabetes, or both; GDM; and patients with DR), (b) the technological configuration of the intervention (telephone or video, automated or manual, machine captured or patient reported, with or without trend displays, provider-only accessed or shared with the patient), (c) provider mix and healthcare setting, and (d) the duration and intensity of the intervention. Hence, it is important to interpret the findings from this research in relation to the context, the particular research configuration, and the specific input variables that were used. Moreover, there were variations in the specific effects measured, including intermediate outcomes, such as use of service and compliance with screening, as well as health outcomes, including glycemic control, desirable lipid levels, weight and/or body mass control, and health knowledge pertaining to diabetes and its management.
In view of the variety of diabetes types, methodological designs, and outcome measures, we decided to organize the evidence of telediabetes initially by categories of the disease: (1) diabetes type 1 and/or type 2, (2) GDM, and (3) DR. Subsequently, we report on the empirical evidence of telediabetes for each of these disease categories in terms of three sets of findings: (a) feasibility and/or acceptance, (b) intermediate outcomes (e.g., adherence to screening or other prescribed regimen, use of service), and (c) health outcomes. We limited the analysis of the empirical evidence to categories (b) and (c). In addition, where available, we included studies of cost-effectiveness.
We encountered one prolific project, called IDEATel, that produced 18 publications. We decided to review all the articles published from this project as a separate set, as will be seen later in this review. The VHA presented another context where multiple research articles were published using a similar context. Most of these are combined as a set in the discussion.
Telediabetes for Type 1 and/or Type 2 Diabetes
A recent “review of reviews” of the evidence from studies in telediabetes was published in 2013.21 It was designed to assess the relative effectiveness of four quality improvement strategies—namely, patient education and support, telemedicine, provider role changes, and organizational changes. The selection of only “high-quality systematic reviews” resulted in 21 studies on patient education and support, 10 on telemedicine, 7 on provider role changes, and 4 on organizational changes. However, the authors noted that several studies investigated combinations of strategies, and they classified eight as such. In any case, the authors used a “voting method” to determine the effectiveness of each of these strategies on glycemic control, retinopathy, and vascular risk factors such as blood pressure, low-density lipoprotein (LDL) cholesterol, and/or diabetic foot outcomes. Among the 10 reviews that focused on telemedicine in this review, the number of studies included in each individual review varied from five to eight, and all were conducted between 1976 and 2011. However, based on the evidence from “credible reviews” dealing with three of the four strategies, the authors concluded “that patient education and support, provider role changes, and telemedicine are associated with improvements in glycemic control and vascular risk factor control in patients.”21 Apparently, there was no such evidence for organizational changes.
The authors noted considerable overlap among these three strategies, as many of the interventions/strategies did not fit into a single category. This raises questions regarding the validity of their classification scheme because the input variables did not fit into mutually exclusive categories. Hence, there is no way to ascertain the relative effectiveness of a specific intervention or strategy or, more critically for our purposes, how to interpret the telemedicine effect when it is stripped of some of its essential components, such as patient education, provider participation, and organizational realignment.
Feasibility and Acceptance of Telediabetes
As mentioned earlier, this initial section of the analysis focuses on studies that investigated the feasibility and/or acceptance of the telemedicine intervention in type 1 and/or type 2 diabetes (telediabetes). The findings are presented in historical order, covering those published from 2005 to 2013. As mentioned earlier, many of these of these studies did not meet the requisite sample size for inclusion in the analysis of empirical findings. Hence, the studies that were solely concerned with feasibility and/or acceptance of telediabetes are discussed here, but they are not included in the analysis of the empirical evidence of the effects of telediabetes.
We identified 11 feasibility-related studies that met the selection criteria for this set. These were conducted in six countries: (United States [n=4], Australia [n=2], Germany [n=2], Italy [n=1], Canada [n=1], and the United Kingdom [n=1). Sixty percent of these studies were based on an RCT design. Sample size varied from 120 to 538.
From Australia (2006), an RCT (n=139 pediatric patients with type 1 diabetes) investigated the effectiveness/feasibility of making scheduled telephone calls from a “pediatric diabetes educator” on a bimonthly basis.22 The mean age of the patients was about 12 years. Hence, it can be assumed that parents/guardians provided much of this information. The educator inquired about glucose level, hospital admissions, diabetes knowledge, compliance, and psychological well-being. After 7 months of observation, there were no significant differences between the experimental and control groups on the measured variables, but the patients or their caregivers reported that the telephone calls were helpful.
A Canadian study (RCT, n=193) investigated the feasibility of an automated telephone reminder system, using interactive voice recognition, and its effects on medication and appointment adherence among adult patients (18 years of age or older) who had access to a telephone.23 In total, 253 patients were enrolled from 47 physician practices, and they were randomly assigned to the intervention or usual care group. The enrollment process was facilitated by the use of electronic medical records. In total, 193 patients were successfully registered in the system, and they were subsequently observed over a period of 7.5 months. “Success” was defined as the actual receipt/completion of automated telephone reminders without the intervention of a human operator. The study revealed that the average percentage of successful reminders declined as the number of messages increased. Overall, 81% of patients in the intervention group received/completed at least three successful reminders, and 50% received/completed at least five reminders. Overall, the main finding from this study documented the feasibility of the automated telephone system as a substitute for a human operator.
Another telephone-based self-management telediabetes program evaluated the incidence of adverse events among ambulatory diabetes patients in 2008 (n=111).24 This U.S. study was unique in terms of focusing on safety issues and adverse event characteristics, including detection triggers, preventability, potential for amelioration, and primary care provider awareness of such events. The findings suggest that telephone surveillance “facilitated self-management support program…to detect adverse events and potential adverse events.”24 Often, without this system, providers were not aware of such events. Thus, the authors concluded that the system “can improve patient safety for chronic disease patients.”24
In 2009, a British RCT (n=137) evaluated the effects of a mobile phone telediabetes system on glycemic control among patients with complicated diabetes.25 Patients were asked to measure their blood glucose level with a sensor on a regular basis. The values were subsequently transmitted to a mobile phone via a wireless Web-based application. The usual providers received the readings on mobile phones and responded with appropriate guidance via a mobile phone. After a follow-up of 9 months, patients in the intervention group had a lower A1c level compared with the control group: 7.7% versus 8.4%, respectively. However, this study is not included in the tabular material because of the small sample size (n=137). The findings supported the utility of mobile devices in the management of diabetes.
Three relevant studies were conducted in 2011. The first was a composite, or multipart, study in Germany. The first component of this study was a survey of physicians who provided care for 538 patients with type 1 or type 2 diabetes (only 4.1% of the sample had type 1) for more than 1 year in the Karlsburg Diabetes Management System. The survey focused on the acceptance of the system by the physicians who provided care for these patients.26 The second component was a retrospective analysis of data for a subset of 289 patients who completed two glucose monitoring sessions during 1 year. The program was open for patients with diabetes who were 18 years of age or older and also diagnosed with cardiovascular disease (including angina, history of myocardial infarct, or heart failure [New York Heart Association class 3 or 4]). Nearly three-quarters (74%) of the participating physicians (n=276) accepted (or were satisfied with) the system. Among the 289 patients who completed two sets of routine glucose measurements, 214 used the telemedicine system, and 75 did not. The authors concluded that the monitoring system “in combination with telemedicine has high potential to improve the outcome of routine outpatient diabetes care.”26
From the United States (2011), an observational study of 117 patients with diabetes type 1 or type 2 reported on the feasibility of incorporating a telediabetes system into an existing telephonic diabetes management program.27 The primary outcome measure was change in glucose level, and the secondary outcomes were patient knowledge and engagement. The unique feature of this study was the use of clinical pharmacists as providers. A survey of patient satisfaction with the program reported high levels of satisfaction but no improvement in knowledge about diabetes management. The clinical findings confirmed the feasibility of this intervention as indicated by a decrease in A1c of 1.3% during a period of 4 months. Moreover, the users were satisfied with the service.
Another feasibility study, also from the United States, was conducted over a 3-year period (n=206 in year 1, but the number declined to 135 in year 2). The findings were reported in 2011.28 This was a nonrandomized, pre–post study of patients with type 2 diabetes who also had at least one uncontrolled vascular risk factor. It compared participants from two rural clinics in Montana who were enrolled in a telediabetes program versus those receiving usual care. The project was aimed at determining (a) the feasibility of a team approach in telediabetes and (b) its effects on clinical outcomes. Feasibility was assessed in terms of patient acceptance, patient self-management behaviors, and diabetes knowledge. Clinical outcomes were measured by control of diabetes risk factors. The authors concluded that telediabetes “proved to be an effective mode for the provision of diabetes care to rural patients,” by achieving results comparable to in-person care while “addressing the unique challenges faced by patients living in rural communities.”28
An Australian RCT (2012) (n=120 patients with type 2 diabetes) investigated the feasibility and accessibility of an interactive voice recognition telephone system among children and adolescents.29 After 6 months of observation, patients in the telediabetes group had a significantly greater decline in A1c, from 8.7% to 7.9%, compared with 8.9% to 8.7% in the control group.
In the same year, a German observational study was conducted among 124 children and adolescents who were overweight or obese.30 The purpose of that study was to determine the acceptance and effectiveness of a sophisticated mobile motion sensor device and a digital camera integrated into a mobile phone. Outcome measures included physical activity and eating habits. The subjects readily accepted the movement detection system. More important is the finding that the use of the system resulted in significant weight reduction in this young population.
Two feasibility studies were published in 2013. The first was an RCT (n=100) conducted in the United States. It used telephone and Internet links to connect providers with diabetes patients, and it was aimed at determining the congruence between routine clinical telediabetes assessments and in-person care assessments.31 Patients were randomly assigned to experimental or control groups in equal proportions. After 12 months of observation, no differences were observed between the two groups in terms of glycemic control, lipids, or body mass index. In terms of feasibility, the authors concluded that the use of “telemedicine-based treatment protocols in diabetes patients is feasible and efficient.…”31
The second study published in 2013 was also an RCT (n=127, all with type 1 diabetes), conducted in Italy.32 In this study, patients used an automated carbohydrate/bolus calculator (referred to as Diabetes Interactive Diary [DID]) to calibrate the individual's appropriate insulin dose for each meal. The patients were instructed by physicians and/or dietitians on how to record the results of glucose profiles, daily insulin doses, and hypoglycemic episodes. Additionally, participants were required to complete a quality of life questionnaire at the start of the project and 6 months after randomization. The results demonstrated the feasibility and comparability of using the DID in terms of the outcome equivalence to in-person traditional carbohydrate counting insofar as A1c levels were concerned. The risk of moderate/severe hypoglycemia was reduced while quality of life improved. Three years earlier (2010), the same senior author and a different set of co-authors published results on the effectiveness of an Interactive Diary for Diet Management (DAI [Un Diario Alimentare Interattivo]).33 That was a prospective observational study of 140 participants who participated in the DAI and were trained and monitored by dietitians to determine its impact on body weight management and nutritional status. The DAI software was designed to support the patients in their daily management of food intake, and it was installed on the patients' mobile phones. The study documented the feasibility of this technology in supporting people who need to lose body weight and also to promote the healthy properties of the Mediterranean diet and consumption of local produce.
Still earlier, in 2010, a different kind of study was published in Italy, using the same DID.34 This was an RCT with a sample of only 130 patients with type 1 diabetes. The sample was somewhat smaller than the required size, but we include this study here because it used a novel approach for metabolic and weight control—namely, the DID. The purpose of the study was to determine whether the use of this tool would improve glycemic control in a shorter time and with greater ease compared with the standard educational approach. The DID had several functions, including a carbohydrate/insulin bolus calculator, an information technology device, and a text messaging system between provider and patient. It contained a complex educational tool to help patients with diabetes follow a flexible insulin therapy and dietary carbohydrate intake: “Bolus insulin is adjusted to match the dietary carbohydrate at each meal.”34 The software was installed on patients' mobile phones to record blood glucose levels and insulin injection in real time and to receive advice on daily carbohydrate and calorie intake (facilitated by visual images). The results of the study demonstrated the safety and effectiveness of the DID, as well as its time efficiency and ease of use.
Telediabetes and Intermediate Outcomes: Adherence and Use of Service
Data on intermediate outcomes of telediabetes are shown in Table 1.
Table 1.
Telediabetes Intermediate Outcomes
| INTERMEDIATE OUTCOMES | ||||||
|---|---|---|---|---|---|---|
| REFERENCE (YEAR) | COUNTRY | RESEARCH DESIGN | SAMPLE SIZE | ADHERENCE | USE OF SERVICE | COMMENTS |
| Orr et al.35 (2006) | United States | Observational | 36,327 | ↑ | NM | 29% increase in A1c testing |
| Jia et al.36 (2011) | United States | Case-control | 387 | NM | ↓ | Inpatient and outpatient use of service ↓ |
| Graham et al.37 (2012) | United States | Case-control | 875 | NM | ↓ | 44% reduction in rehospitalization |
| Fischer et al.38 (2012) | United States | RCT | 762 | NM | ↓ | Average per patient cost↓$2,816; LDL↓6.5%, hospital admission rate↓5.55%; A1c and BP no difference |
A downward arrow indicates decreased.
A1c, glycated hemoglobin A1c; BP, blood pressure; LDL, low-density lipoprotein; NM, not measured; RCT, randomized clinical trial.
To start, a large-scale observational study, conducted in Tennessee (n=36,327), was published in 2006; it investigated the effectiveness of a nurse-led telephonic management program on adherence to prescribed glycemic testing.35 Frequency of calls was determined on the basis of disease severity. In addition to telephone calls, the program provided quarterly newsletters, reminder mailings, and disease-specific educational materials. Participants in the program had moderate to severe risks for diabetes complications. Data on adherence to testing were gathered at 6 months before and 6 months after the start of the program. The findings revealed a significant increase of 29% in A1c testing among the participants, potentially reducing the risk of exacerbation.
Three studies meeting the inclusion criteria investigated the effects of telediabetes on use of service. All three were conducted in the United States and published in 2011 and 2012.
A 4-year case-control study assessed the effects of the VHA Care Coordination Home-Telehealth Program on use of service among veterans diagnosed with diabetes (type unspecified).36 An intervention sample of 387 patients was selected from one of four clinics in a single VHA region. This group was matched by age and gender to create a control group of 387 at the date of enrollment. A “propensity score” was used in matching the two groups in order to reduce selection bias. However, this process did not result in full equivalence between the intervention and control groups. At baseline, the former group had a significantly higher comorbidity score, signifying a sicker population. Also at baseline, the number of inpatient days was similar in the two groups, but the number of outpatient visits was greater in the intervention group. At the conclusion of 48 months, “there was a general pattern of reduced inpatient and outpatient use by the intervention group compared to the control group.…”36
Another case-control study investigated the effects of postdischarge monitoring through the use of interactive voice response on 30-day hospital re-admission rates in 2012.37 A sample of 875 patients enrolled in a case management Medicare Advantage program using the sophisticated telephone system was compared with a matched group of 2,420 controls who had case management only. Matching was at the rate of 1 (case):3 (controls). The major result of this study indicated a 44% reduction in rehospitalization among patients who received a combination of telemonitoring and case management compared with patients receiving case management only.
An RCT (n=762), also U.S.-based, investigated the effects of a nurse-monitored algorithm-driven telephone care management program on several dependent variables, including lipid management, blood pressure, glycemic control, primary and subspecialty care follow-up, cost to the system, and hospital admissions.38 The subjects were adults with diabetes (type unspecified) receiving care at a Federally Qualified Community Health Center that served a primarily indigent Latino population. Patients in the intervention group received calls from nurses to help them initiate and titrate lipid-lowering medications using standard guidelines and to encourage them to adopt healthy lifestyle behaviors. The usual care group received standard diabetes management by primary care providers, which consisted of regular clinic visits. After 20 months, the intervention group had substantially lower hospital admission rates relative to the control group (19.6% and 25.2%, respectively). Furthermore, the average cost per patient for the health system was $6,217 for the intervention group versus $9,033 for the control group. As well, the intervention resulted in improved lipid control (from 52.0% to 58.5%) in the intervention group versus from 55.6% to 46.7% in the control group, but it had no effect on glycemic level or blood pressure.
Telediabetes and Health Outcomes
The vast majority of studies that investigated the effects of telediabetes on health outcomes focused on glycemic control as the primary metric, often in combination with other diabetes risk factors, such as body weight, diet, lipids, blood pressure, and insulin titration. These data are summarized in Table 2. Few studies measured intermediate health outcomes, such as frequency of reporting blood glucose levels, emotional distress, and knowledge of diabetes risk factors. One study48 used the term “informed meals” as a measure of appropriate diet for persons with diabetes.
Table 2.
Telediabetes Outcomes
| OUTCOMES | ||||||||
|---|---|---|---|---|---|---|---|---|
| REFERENCE (YEAR) | COUNTRY | RESEARCH DESIGN | SAMPLE SIZE | A1C | BP | LDL | WEIGHT/BMI | COMMENTS |
| Harno et al.39 (2006) | Finland | RCT | 175 | ↓ | ↓ | ↓ | NM | Fewer physician visits |
| Rodríguez-Idígoras et al.40 (2009) | Spain | RCT (parallel groups) | 328 | ↓ | ↓ | ↓ | ↓ | Control group showed improvement but less |
| Berg et al.41 (2009) | United States | Case-control | 980 | ∼↓ | NM | O | NM | ROI 3.8:1; inpatient bed days↓48%; cost ↓ |
| Anderson et al.42 (2010) | United States | RCT | 295 | ↓ | O | O | O | No difference in A1c at 6 and 12 months; lifestyle changes need longer duration; A1c↓for those with depression |
| Davis et al.43 (2010) | United States | RCT | 165 | ↓ | O | ↓ | O | Self-reported data |
| Jordan et al.44 (2011) | United Kingdom | Case-control | 473 | ↓ | ↓ | NM | ↓ | BMI ↓ 0.7 units; BP ↓; A1c ↓ 0.3%; small ↓ in LDL |
| Musacchio et al.45 (2011) | Italy | Observational | 1,004 | ↓ | ∼ ↓ | ↓ | O | Metabolic and cardiovascular risk↑(face-to-face visits ↓) |
| van Bastelaar et al.46 (2011) | The Netherlands | RCT | 255 | O | NM | NM | NM | Duration=1 month; depression↓; Web-based cognitive therapy helpful |
| Charpentier et al.47 (2011) | France | RCT | 180 | ↓ | NM | NM | NM | Less patient travel |
| Franc et al.48 (2014) | France | RCT | 180 | ↓ | NM | NM | NM | Portion and carbohydrate counting ↑ |
| Stamp et al.49 (2012) | France | Descriptive observational | 330 | ↓ | O | NM | NM | A1c ↓ 1.8% |
| Tang et al.50 (2013) | United States | RCT | 415 | ↓ at 6 months | O | ↓ at 12 months | O | Intervention group better at 6 months but even at 12 months; medication management ↑ |
| Ellis et al.51 (2012) | United States | RCT | 146 | ↓ | NM | NM | NM | Adolescent adherence ↑ |
| Kesavadev et al.52 (2012) | India | Retrospective record review | 1,000 | ↓ | ↓ | ↓ | ↓ | Used no controls |
| Chen et al.53 (2013) | Taiwan | Longitudinal case-control | 162 | ↓ | NM | NM | NM | Invervention group ↑blood glucose monitoring;↑physical activity, diet, medication compliance, coping, problem solving; A1c stabilized at 18 months |
| Brown-Guion et al.54 (2013) | United States | Survey | 1,797 | NM | NM | NM | NM | Urban/rural ethnicity differences in diabetes education |
| Lee et al.55 (2013) | Korea | Tech assessment | 1,568 | NM | NM | NM | NM | Voice diagnostic tools for predicting BMI |
A downward arrow indicates down or decreased; an upward arrow indicates up or increased.
A1c, glycated hemoglobin A1c; BMI, body mass index; CR, cluster randomization; LDL, low-density lipoprotein; NM, not measured; O, no difference; QE, quasi-experimental; ROI, return on investment.
Published in 2006, an RCT (n=175) conducted in Finland involved patients with type 1 or type 2 diabetes who were served by primary care clinics and university hospital outpatient departments.39 The patients were randomly assigned to the intervention (n=101) or control (n=74) group. The intervention group was supplied with an electronic disease management system and a home care link. This allowed both patients and providers to send and receive short message service (text messages) on either mobile phones or the Internet. Patients in the intervention group downloaded their measurements directly, and the data were available to the care team as well as the patients themselves. In addition, providers had access to the patients' diaries, which contained daily entries. Those in the control group made regular visits to their usual providers about once every 3 months. After 12 months in the program, both groups experienced a decline in their A1c lelvel, but the decline in the intervention group was greater (and statistically significant) than that in the control group (from 7.8% to 7.3% versus from 8.2% to 7.8%, respectively). In addition, only patients in the intervention group experienced significant declines in cardiovascular risk factors such as blood pressure and lipids. The other benefit was a reduction in demand for care, that is, “fewer visits [were made] by study patients to doctors and nurses.”39
Four research articles that met the inclusion criteria were published in 2009.
A study in Spain used “a controlled randomized two-parallel group trial” to ascertain the effects of a telediabetes program on glycemic control, lipids, body weight, and blood pressure.40 A two-step selection method was used. The first step identified a sampling frame consisting of 35 physicians and 24 nurses from the Province of Malaga who agreed to participate in the study. The second step consisted of selecting 8–10 subjects from each medical practice. Subsequently, these patients were randomly assigned to the intervention (n=161) or control group (n=167). All patients were 30 years of age or older and had type 2 diabetes. At the beginning of the study, all study patients received a glucose meter. Patients in the intervention group and their physicians also received mobile phones, which were linked to a call center. The patients used the mobile phones to report their glucose measurements. An alarm was triggered when prespecified values were exceeded, and these alarms were addressed promptly by a physician or a nurse providing appropriate instructions. Physicians had access to all information submitted by patients. At 6 months, both intervention and control groups experienced significant reductions in A1c. However, at 12 months, the decrease in A1c was statistically significant in the intervention group but not in the control group (from 7.62% to 7.40% versus from 7.44% to 7.36%, respectively). Similar trends were observed in lipids and blood pressure, but these improvements were sustained in the intervention group only.
A case-control study (n=980) in Puerto Rico investigated the effects of a telediabetes program on use of service, selected clinical outcomes, and financial impact.41 A sample of 490 (age range, 18–64 years) Medicaid patients with diabetes was matched with 490 controls. Composite “propensity scores” were used for matching the two groups, which included demographic characteristics, comorbidities, use of service, medications, diagnostic tests, immunization history, and medical and pharmacy costs. The use of these scores was intended to maximize the similarity between the intervention and control groups. The intervention group received a customized self-management disease program that contained risk stratification (i.e., levels of risk), organized nurse-led educational sessions, 24-h access to nurse counseling, and organized information sources for advice on symptoms, as needed. In addition, patients in this group received individualized assessment letters and reminders for medication compliance and vaccinations. The control group received usual care without the disease management program. Contacts were made primarily by telephone. At 12 months, the intervention group experienced significant improvements, including “48% reduction in inpatient bed days, and a 23% increase in ACE [angiotensin-converting enzyme] inhibitor use, resulting in a return on investment of 3.8:1.”41 Overall, “the intervention group had costs of $122,306, and generated gross savings of $463,814”41 or net savings of $341,508. The intervention resulted “in a 24.2% reduction in the cost of care.”41
Two more studies were published in 2009 by authors at the VHA. These will be discussed later under a separate section dealing with VHA studies.
The first of four RCTs published in 2010 (n=295, all type 2 diabetes) focused on a medically underserved population (80% were below the poverty level). Most were Hispanic or African American, nearly half spoke a language other than English at home, and all were served by a Federally Qualified Community Health Center.42 In total, 1,754 patients were eligible for inclusion in the study. Of these, 1,704 were contacted, 333 were interviewed, and 295 consented to participate and met the criteria for inclusion in the study. This final group was randomly assigned to the intervention (n=146) or control (n=149) group. Only 94 of the intervention group completed the study, whereas 117 did so in the control group. The intervention group received telephone calls in addition to usual care, which were intended to promote a healthy life style. The control group received usual care only. Assessment of A1c at 6 and 12 months showed no difference between the intervention and control groups. The only exception was an improvement of A1c value among patients with an established diagnosis of depression, but the difference was not statistically significant. However, the control group had a significantly higher A1c than the intervention group at baseline, thereby suggesting selectivity bias in the choice of the two groups. The authors explained that 1-year duration “was insufficient to realize benefits from an intervention geared at promoting life style changes. Such changes may take time to enact and show benefits.”42
A somewhat similar study of telediabetes (RCT; n=165) was conducted in a medically underserved community in South Carolina.43 Here again, the patients had diabetes (predominantly type 2) and were using a Federally Qualified Health Center. Most were African American (75%), female (73%), and obese (average of 101 and 96 kg, respectively, for the two groups). Initially, telephone contacts were attempted with a large pool of 1,984 patients who were identified from billing records as tentatively eligible for the study. Of those meeting eligibility criteria and also consenting to participate in the study, 165 completed two in-person screening visits and consented to participate. These were randomly assigned to the telemedicine intervention or in-person group. The intervention group received structured and comprehensive educational materials delivered by videoconferencing in group sessions. The materials were based on a theoretical health belief and health behavior model. Each patient in the usual care group received one 20-min diabetes education session from a licensed practical nurse during the randomization process. High retention rates of 90.9% and 82.4% were observed among both groups at 6 and 12 months, respectively. Results showed significant reductions in A1c and LDL in the intervention group only, but no statistically significant differences were observed in body weight between the two groups.
In 2011, four studies from four countries (United Kingdom, Italy, The Netherlands, and France) met the inclusion criteria for analysis and focused on health outcomes.
The first British case-control study examined the effects of a telephone-based, nurse-delivered motivational coaching and support system for self-management and life style changes among patients with poorly controlled diabetes. The study used a unique case-control methodology.44 A sample of 473 patients 18 years of age or older with poorly controlled diabetes was enrolled in a telephone care management service. The patients were matched with a comparable disease status group pool of 21,052 (selected at the rate of almost 1:50) as a control cohort. The choice of such a large control group would ensure the control sample's representativeness of the population from which it was drawn. However, the true test of an effective case-control design is the comparability of the two groups with each other. A very large sample of controls means that this group will have a negligible sampling error in relation to its population. Eligibility for inclusion in this study was based on age (18 years or more) and one or more of the following conditions: noncompliance with medication, hypertension, diabetic complications, and an assessment by a clinician of the likelihood to benefit from the service. This latter criterion was not explicitly defined.
Each consenting adult was assigned a nurse care manager who had completed a 6-week training program in all aspects of diabetes care management and education. Once enrollment was implemented, the nurses initiated individualized advice on the telephone in a supportive fashion and encouraged the patients to set their own goals. The program was ongoing as of the date of the publication of the article, but the data for the publication were based on 90 days or more of observation. After adjusting for confounding factors, “the intervention showed significant reductions in A1c, average of 0.3% (0.1 versus 0.4); 3.5 mmHg in systolic blood pressure and 1.6 mmHg in diastolic blood pressure; and 0.7 unit reductions in BMI [body mass index], over a follow-up period averaging around 10 months.”44 It is important that a stronger effect was observed among the subset of patients in the intervention group who had poorer baseline levels and among the most disadvantaged populations compared with their counterparts.
An Italian evaluation of the clinical effectiveness of a program, SINERGIA, was designed to help stable patients manage their type 2 diabetes, thereby decreasing unnecessary demand on limited specialist resources and contributing to a sustainable health system.45 The rationale for this study is interesting in terms of aiming at reducing unnecessary demand for physicians. It posits that if patients with stable diabetes can be empowered to self-manage their disease through this patient-focused intervention, then expert clinical personnel can be freed to serve sicker patients. The intervention consisted of nurse- and dietitian-led education, remote monitoring, and electronic medical records. Patients were provided expert guidance to develop their own therapeutic goals, recognize the significance of their symptoms, monitor their glucose level, manage emergencies that may arise, improve their life style, and deal with diabetes-related problems. In total, 1,004 patients with type 2 diabetes were recruited into the SINERGIA program. The impact of the program on patient self-management was followed up for a median of 12 months (range, 6–24 months). The most significant findings were a very substantial decrease in A1c level, from 10.5% to 4.3%, as well as a decrease in personal visits, from 2.8 to 2.3 per year. Thus, the authors concluded, “The SINERGIA model is effective in improving metabolic control and major cardiovascular risk factors, while allowing “diabetologists to dedicate more time for patients with more acute disease.”45
A third study was conducted in The Netherlands with a unique focus on the effects of cognitive behavioral therapy delivered via the Internet on depression and glycemic control.46 This was an RCT with a sample of 255 adult diabetic (type 1 or 2) patients with elevated depression symptoms. After a period of only 1 month, the intervention proved to be effective in reducing these symptoms, but it had no effect on A1c. However, the findings cannot be considered reliable because of the very short duration of the study.
The fourth study was conducted in France in 2011 with a follow-up in 2014. The initial study47 was a 6-month RCT conducted in 17 sites throughout France with the aim of evaluating the efficacy of a smartphone application coupled with an Internet system (Diabeo) for metabolic control among adult patients with type 1 diabetes (n=180).
The Diabeo system consists of a personalized bolus calculator that takes into account the patient's glucose targets, amount of carbohydrate consumed, premeal glucose value, and anticipated physical activity. It has an automated algorithm that recommends adjustment in insulin-to-carbohydrate ratios and basal insulin, data transmission capability, and a secure Web site for telemonitoring and teleconsultation. Patients were randomly assigned to three equal groups: (1) usual care consisting of quarterly follow-up clinic visits, (2) smartphone with Diabeo and quarterly visits, or (3) smartphone with Diabeo and access to teleconsultations every 2 weeks but no medical office visits. The primary end point was the difference in A1c level at 6 months among groups. Use of Diabeo with teleconsultation (Group 3) resulted in a 0.9% reduction in A1c level compared with controls (Group 1) and a 0.7% reduction in Group 2. There was no difference in the rate of nonsevere hypoglycemia among groups at end point. Additionally, there were no differences among the three groups in terms of the time spent in face-to-face visits or in telephone consultations. Patients in Groups 1 and 2 spent more time traveling to and from hospital visits compared with those in Group 3, thereby demonstrating an added benefit for patients using Diabeo with teleconsultation. The authors concluded that the Diabeo system significantly improved glycemic control in patients with type 1 diabetes with less medical time and at less cost to the patient compared with controls.
A post hoc subanalysis of the data from this study48 was published in 2014. Its purpose was to ascertain the role of the Insulin Dose Advisor (IDA) in Diabeo and telemedicine support in reducing A1c levels. High users (n=56) and low users (n=57) in Group 2 and Group 3 were identified using median “informed meals” consumed (defined as when the system proposed an appropriate insulin dose). For “high users,” informed meals remained stable throughout the 6-month study period, whereas they declined among “low users.” A1c levels declined among “high users” from 8.7% to 8.2%. “Low users” also experienced A1c reduction, from 9.0% to 8.5%. Patients receiving teleconsultation support tended to show greater improvement, but the difference was not statistically significant. The subgroup analysis suggested that frequent communication with healthcare professionals among “low users” may have contributed to the A1c improvement. The authors concluded that “the Diabeo system improved glycemic control in both high and low users who avidly used the IDA function, while the greatest improvement was seen in the low users who had the motivational support of teleconsultations.”48
Four studies meeting the inclusion criteria for analysis were published in 2012. With one exception, all were conducted in the United States.
Beginning in 2006, the New York City Health and Hospitals Corporation initiated HouseCalls, a diabetes-telehealth program in conjunction with its Medicaid Health Plan. Patients with poorly controlled type 2 diabetes (A1c >7%) were referred to the program. This descriptive observational study (single group/no controls) was based on the experience of 330 patients enrolled in HouseCalls for a 2-year period (2008–2009) and published in 2012.49 These patients were given in-home monitoring devices including glucose meters and blood pressure monitors (for patients with hypertension), each connected to a telemonitoring modem. Patients were trained to take readings, upload the information, and transfer the data to a secure Web site. Readings were monitored by a telehealth nursing team. Automated “high alerts”—when certain values were exceeded—resulted in calls by nurses within a 2-h period on weekdays. Nurses tracked and managed patients' status, documented care plans, and recorded communications with patients' primary care physicians. Average change in A1c for the entire population over a 12-month assessment was 1.8%. This was both statistically and clinically significant. Patients who completed the program had a larger reduction (−2.2% versus −1.4%) in A1c compared with those who dropped out of the program.
A U.S. RCT (n=415) was designed to determine whether a multifaceted intervention would help patients with uncontrolled type 2 diabetes (initial online publication was in 2012).50 The intervention consisted of seven components: (1) an automated wireless upload of glucose readings with graphical feedback, (2) a status summary report, (3) nutrition and exercise logs, (4) insulin record, (5) team messaging, (6) advice by care manager and dietitian, including medication management, and (7) personalized education by video. Glycemic control was the primary outcome. After 6 months, patients in the intervention group “achieved greater decreases in A1c as compared to those in the usual care group, but the differences were not sustained at 12 months.”50 The authors attributed this finding to “significant improvements in the usual care group in [this] setting. More patients in the intervention group achieved clinically meaningful improvement in A1c than the usual care group.”50 In other words, both groups experienced improvements in their blood glucose levels, but the intervention group experienced greater improvement. Other significant differences were observed between the two groups favoring the intervention, including medication management (number of medication orders and number of insulin orders). There were no differences in the number of physician visits made by the two groups during the 12-month study period.
A study of adolescents with poor metabolic control (diabetes type 1 or 2) conducted an RCT (n=146) to ascertain whether a tailored, intensive, home-based, multipart treatment program was superior to weekly telephone support in terms of adherence to prescribed regimen.51 The intervention group received a complex multipart regimen that promoted self-management across family, school, and community settings through intensive patient, caregiver, and peer education and training. The control group received telephone support only. It should be noted that sample size was slightly below 150. Nonetheless, we include this study in this analysis because it focused on a population of interest—namely, adolescents with type 1 or type 2 diabetes and poor metabolic control. Most of the patients were African American and living in single-parent families. The mean A1c value at baseline was 11.7%. However, results showed substantial improvement in glycemic control (a decrease of 1.01% at 7 months and 0.74% at 12 months compared with those receiving telephone support only). Moreover, when asked, the parents of these adolescents reported significant improvements in their children's adherence to diabetes management such as insulin and dietary management, blood glucose monitoring, and symptom response.
A prospective record analysis of 1,000 patients in India with type 2 diabetes was published in 2012.52 The intervention consisted of a Diabetes Telemanagement System in which a team of physicians, educators, dietitians, nurses, pharmacists, and psychologists provided monitoring functions as well as educational programming and customized guidance for patients in the program. The team maintained electronic records for the patients that included detailed medical history, target glucose values, and life style factors. Each patient was given three options for follow-up care: telephone, e-mail, or secure Web site. Advice was provided on a patient-specific, “as needed” basis. After 6 months, patients in this cohort experienced improvement in A1c, blood pressure, LDL cholesterol, and total cholesterol. However, this study did not use any controls to ascertain whether the changes could be fully attributable to the intervention.
In 2013, three published studies met the inclusion criteria for this analysis, one each from Taiwan, the United States, and Korea. They are reported here in that order.
A longitudinal case-control study of the effects of online self-management education and telemedicine on the adoption of seven self-care behaviors was conducted in Taiwan (n=162).53 The patients had either type 1 or type 2 diabetes without severe complications. The intervention consisted of a third-generation mobile telecommunications glucose meter, an online diabetes self-management system, and a teleconsultation service. It enabled the patients to see online their glucose test results, blood pressure values, body weight, insulin injection record, and daily diet and physical activity. Outcomes were measured in terms of physical activity level, diet, medication compliance, coping skills, problem-solving abilities, risk reduction, and glucose monitoring compliance. Fifty-nine patients participated in the study, and they were matched by demographic characteristics with 103 who did not. After 18 months, patients in the intervention group were more likely than those in the matched control group to adopt healthy behaviors in five areas: physical activity, diet, medication compliance, coping, and problem solving. In addition, they were more likely to monitor their blood glucose compared with their counterparts.
A somewhat related study on the differential receipt (i.e., who does and who does not receive) of diabetes education was conducted in the United States.54 The analysis was based on data from the National Medical Expenditure Survey (n=1,797). All subjects had type 2 diabetes. Results from a logistic regression revealed that being African American increased the likelihood of receiving diabetes education. However, the opposite trends were observed among residents in rural areas and those without health insurance, as well as those living in the South. Perhaps more important is that the majority of the sample (63.7%) reported not receiving any diabetic education. Thus, on a national scale, only 6.3% of persons with diagnosed type 2 diabetes reported having received diabetes education on the telephone.
A novel approach for predicting BMI from voice signals was investigated in Korea in 2013.55 The investigation was based on a sample of 1,568 subjects divided into four groups by age (20–40 and 40–60 years) and gender. Logistical regression analysis of the data revealed significant statistical differences among the four groups in terms of diagnosing BMI from voice signals. The authors concluded that their “results could support the development of BMI diagnosis tools for real-time monitoring.”55
VHA Studies
Several studies were conducted at the VHA on this topic.56–65 Of these, five were reported by the same senior author with different co-authors. These VHA studies are presented here in historical order as a set, shown in Table 3.
Table 3.
Telediabetes and Outcomes in Veterans Health Administration Studies
| OUTCOMES AND EFFECTS | |||||||
|---|---|---|---|---|---|---|---|
| REFERENCE (YEAR) | RESEARCH DESIGN | SAMPLE SIZE | A1C | BP | LIPIDS | WEIGHT | COMMENTS |
| Chumbler et al.56 (2005) | Observational | 445 | NM | NM | NM | NM | Hosp. ↓(50%); ER ↓(11%); bed-days ↓ 3 days; QoL ↑; physical and social functioning ↑; bodily pain ↓; used “Intent to treat” |
| Chumbler et al.57 (2005) | Retrospective case-control | 800 | ↓ A1c=↓ Hosp. | NM | NM | NM | Visits ↑, Hosp. ↓(correlates with A1c) |
| Barnett et al.58 (2006) | Retrospective case-control | 800 | ↓ | NM | NM | NM | Avoidable healthcare services ↓. If A1c ↓, then hospitalization and primary care visits ↓. |
| Chumbler et al.59 (2005) | Record review | 297 | O | O | O | O | After 12 months Hosp. rate ↓33% with daily monitoring. But, BP and BMI baseline significantly worse for Tx group |
| Stone et al.61 (2010) | RCT | 150 | ↓ | O | ↓ | O | Both groups improved but intervention group greater |
| Wakefield et al.62 (2011) | RCT | 302 | ↓ at 6 months | ↓ | NM | NM | A1c difference at 6 months but disappeared at 12 months. Ongoing education, advice, and surveillance improved clinical outcomes. Control A1c significantly improved at 12 months. |
| Wakefield et al.63 (2012) | Survey | 302 | ↓ at 6 months | ↓ | NM | NM | Knowledge, self-efficacy, adherence, and patient perceptions, no difference among Low Tx, High Tx, and Control (except knowledge at 6 months only) |
| Chumbler et al.64 (2009) | QE | 774 | NM | NM | NM | NM | Mortality ↓ 7% |
| Powers et al.65 (2009) | CR | 528 | ↓ | O | ↓ | O | |
A downward arrow indicates down or decreased; an upward arrow indicates up or increased.
A1c, glycated hemoglobin A1c; BMI, body mass index; BP, blood pressure; ER, emergency room; Hosp., hospitalization; NM, not measured; O, no difference; QoL, quality of life; RCT, randomized clinical or controlled trial; Tx, treatment; VHA, Veterans Health Administration.
An observational study (n=445) of the effects of the Care Coordination Home Telehealth program on use of service and quality of life was conducted among patients in the South Florida–Puerto Rico and Georgia VHA region (published in 2005).56 The authors pointed out that diabetes was the second most prevalent condition among veterans in this region and that “4% of the population (more than 1 million veterans, nationwide) were consuming 40% of the healthcare resources.”56 The analysis of utilization data was based on administrative records, starting at baseline and after 12 months of participation in the program. There was no comparison group. In total, 537 patients were recruited to participate in the study. Of these, 92 were lost to follow-up for various reasons, thereby leaving a sample of 445 for analysis. Those who dropped out had poorer health and diminished social functioning compared with those who remained. An “intent to treat” analysis was conducted with the assumption that those who dropped out would have no improvement in any of the outcome measures. A logistic analysis for binary outcomes (used or not used service) and a Poisson regression were used. The results indicated “a statistically significant reduction in the proportion of patients who were hospitalized (50% reduction), emergency room use (11% reduction), reduction in the average number of bed days of care (an average of 3 days, as well as improvement in Health Related Quality of Life, physical functioning, bodily pain, and social functioning.”56 However, the authors cautioned that “the results need to be interpreted with caution because we used a single-group study design that may be influenced by regression to the mean.…”56
Chumbler and co-workers published three articles in 2005,56,57,59 one in 2006,58 and one in 2004,60 each with different objectives but all using a case-control study design. A retrospective case-control study design compared 400 diabetes patients with complex medication regimen and at high risk for expensive, multiple inpatient and outpatient services (including emergency department visits) with a matched group of 400 who did not receive the intervention.57 Eligibility for inclusion in the study included two or more hospitalizations or emergency visits during the preceding 12 months. Patients in the intervention group were enrolled in the patient-centered Care Coordination Home Telehealth program. “Twelve months after enrollment there was a significant difference between the treatment and comparison groups.”57 Need-based and “just in time” visits increased in the treatment group whereby their glycemic level was controlled, but such visits and their attendant benefits decreased in the comparison group. On the other hand, the treatment group “had a lower likelihood of having 1 or more hospitalizations than patients in the comparison group.”57 The treatment effect was measured on the basis of difference-in-differences, which constitutes the net difference between the differences between the two groups, before and after treatment.
In a 2-year follow-up of the same groups,58 the treatment group “exhibited a statistically significant reduction in the likelihood of all-case and [diabetes-related] hospitalization.” The same results were observed after the authors controlled for selection bias and intervening time factors. The intervention resulted in reducing “avoidable healthcare services.” This study was published in 2006.
Also in 2005, Chumbler et al.59 evaluated the effects of two modalities of diabetes management for patients who required close monitoring. Group 1 was monitored weekly with intensive evaluations (n=197), whereas Group 2 was monitored daily but less intensively (n=100). The first modality consisted of patients with active diabetic wounds who were monitored by a care coordinator on a weekly basis in an intensive program. The second group consisted of elderly patients with diabetes, many of whom had wounds that also required close monitoring. These were monitored daily in a less intensive program, while they received general instructions on diabetes care and metabolic control through a home messaging system. A nurse coordinator monitored their symptoms and needs. Telemonitoring in both groups consisted of (a) a hand-held in-home messaging device with disease management dialogues, (b) a telemonitor with two-way audio–video connectivity, and (c) a videophone. Patients in the intervention group were required to answer few questions on a daily basis using the hand-held device. The care coordinator reviewed the data daily. The catchment area was South Georgia and North Central Florida. Patients who had two or more hospitalizations in the preceding year were eligible for participation. However, they were not randomly assigned to the two groups.
At baseline, Group 1 was younger and more likely married than those in Group 2. They also had a substantially lower hospitalization history than their counterparts (30% versus 63%). After 12 months, “the proportion of one or more hospital admissions and number of bed days of care decreased in the daily monitoring group, and increased in the weekly monitoring group, more or less doubling in the former and being halved in the latter.”59 No differences in clinical outcomes were observed between the two groups. The results from this study are not conclusive mostly because of selection bias, but the findings suggest that daily monitoring was more effective in reducing hospitalization compared with weekly monitoring.
In 2004, an earlier study was conducted in the same VHA service area used a case-control design to assess the effects of telemonitoring for patients with hypertension, diabetes, respiratory disease, and/or heart disease.60 A sample of 111 frail, elderly patients was matched with 115 such patients. The results of the study suggest that care coordination can improve functional independence among noninstitutionalized patients with chronic disease. However, because of the performance date of this study it is not included in the analysis of empirical findings.
Another RCT in 2010 (n=150 type 2 diabetes patients) was conducted at the VHA in Pennsylvania. It investigated the effects of an active nurse-led medication management with home telemonitoring (via monthly telephone calls) on glycemic control, blood pressure, and body weight.61 All patients in the study were already on a medication regimen. Those in the intervention group were asked to monitor their blood glucose, blood pressure, and weight on a daily basis and to transmit these data to the clinic via a secure network. In addition, they received monthly calls by the nurse for diabetes education and self-management review. Blood glucose, blood pressure, and weight measures were taken at baseline, 3 months, and 6 months for both groups. Although both groups had improved glycemic control, the intervention group had “significantly larger decreases in A1c at 3 and 6 months with most improvements by 3 months”61 compared with the control group.
Two separate articles were published in 201162 and 201263 stemming from a study that was conducted at the VHA Medical Center in Iowa. This was an RCT (n=302), nurse-managed telemedicine program. Patients were provided with a home device for manually entering blood pressure and blood glucose measurements. Patients with type 2 diabetes and hypertension were assigned randomly into three groups: (1) usual care, (2) low-intensity program, and (3) high-intensity program. The high-intensity group received health information tips on diet, exercise, smoking cessation, foot care, medications, weight measurement, and lifestyle adjustments. The low-intensity group did not receive this information, but both groups were required to send blood glucose and blood pressure measurements and answer two questions daily.
The first report (published in 2011)62 focused on glycemic level (A1c) and systolic blood pressure, whereas the second (published in 2012)63 focused on diabetes knowledge and medication adherence. Overall, patients in both intervention groups had improved A1c during the first 6 months of the study compared with the control group, but this difference disappeared after 12 months. However, patients in Group 2—those who received health information tips—maintained the difference after 12 months, thereby suggesting the importance of education in glycemic control. Moreover, patients receiving the high-intensity intervention had a significant decrease in blood pressure compared with those in the low-intensity and usual care groups. This difference occurred at 6 months and was maintained after 12 months. In terms of secondary outcomes, no significant differences were observed “across the groups in self-efficacy, adherence or patient perceptions of the intervention modes.”63 The authors concluded that “home telehealth can enhance detection of key clinical symptoms that occur between regular physician visits.”63
A case-control study was conducted to assess mortality risk among patients with diabetes (type unspecified) who were enrolled in the VHA Home-based Care Coordination Telehealth program (from June 2004 to December 2005 and published in 2009).64 The intervention consisted of an automated electronic messaging device that queried patients about their diabetes symptoms and general health on a daily basis. The device was connected to a regular telephone line monitored by nurse coordinators. When conditions warranted, nurses called patients for follow-up, to make referrals, and to help with medication management. They consulted with physicians about adjusting medications and scheduling appointments. A sample of 387 patients was enrolled in the program and observed for 4 years. The cases were matched with 387 controls using a propensity score, a summary measure of the patients' background that represents the probability of a patient belonging to the intervention group. Analysis of the 4-year all-cause hazard mortality ratio showed lower mortality in the intervention group compared with the control group (19% versus 26%, respectively).
A related VHA study (RCT; n=528) investigated the effects of “a tailored hypertension self-management intervention” on A1c and LDL cholesterol.65 The study was aimed at evaluating a hypertension self-management program. This subanalysis investigated unintended consequences in relation to A1c and LDL. It compared laboratory values of a subsample of 216 patients with diabetes over a 2-year period. The hypertension self-management had a spillover effect in terms of controlling A1c by a margin of 0.46%, as well as a reduction of 0.9 mg/dL in LDL, but the latter difference was not statistically significant.
IDEATel Reports
IDEATel was a large RCT (n=1,655), of long duration (up to 5 years), and its investigators produced a prolific record of 18 publications,66–83 starting in 2002, all from the project (shown in Table 4). It seemed appropriate to discuss all reports of findings pertaining to this project as a single set. These are presented here in historical order.
Table 4.
Informatics for Diabetes Education and Telemedicine Findings (IDEATel)
| METHODOLOGY | ||||
|---|---|---|---|---|
| REFERENCE (YEAR) | SAMPLE SIZE | RESEARCH DESIGN | FINDINGS | COMMENTS |
| Shea et al.68 (2006) | 1,665 (C=821; I=844) | RCT | After 1 year, A1c ↓, BP ↓, LDL ↓ | A1c improved in both groups, more in intervention group |
| Trief et al.69 (2006) | 1,578 | RCT prospective analysis | Weak relationship between depression and A1c but did not predict change in glycemic control | |
| Palmas et al.70 (2006) | 1,040 | Clustered randomization | Ambulatory pulse pressure may help predict albuminuria progression. | |
| Trief et al.71 (2007) | 1,665 | RCT | Psychosocial outcomes ↑ | Possible spillover effect from education and consultation; not sure about generalizability of findings |
| Izquierdo et al.72 (2007) | 338 (IV group) | Observational | Identification of (1) inappropriate medication, (2) inappropriate timing, (3) contraindication to current medication, and (4) adverse events | |
| Tudiver et al.73 (2007) | 116 | Provider survey | Acceptance ↑, perceived patient knowledge ↑ | Patient activation ↑, excessive paperwork; conflicting advice within team |
| Shea et al.74 (2009) | 1,665a | RCT | A1c ↓, LDL ↓, BP ↓, mortality O | 5-year follow-up, high dropout numbers; used intention-to-treat |
| Lai et al.75 (2009) | Similar to Robinson et al.78 (2010) | |||
| Izquierdo et al.76 (2010) | 890 | Within cluster randomization | Knowledge ↑, exercise ↑, WC ↓, BMI ↓ | 2 years in their analysis; women reduced WC |
| Palmas et al.77 (2010) | 1,665 | RCT | No effect on cost | Cost of implementing program high, need to lower cost of equipment by $622/month/case |
| Robinson et al.78 (2010) | 109 | Survey | In-home training preferred | Preferences for training |
| Weinstock et al.79 (2011) | 1,650 | RCT | Physical decline ↓, more PA (i.e., reduced rate of decline in impairment); improved task performance | Learning curve; remote training is effective; PA associated with↓ comorbidity,↓ depression, ↑ social networking, ↓ BMI,↓ A1c (pedometers) |
| Weinstock et al.80 (2011) | 1,665 | RCT | A1c ↓ | Telediabetes can reduce disparities; BMI not associated with A1c. Hispanics had highest A1c at baseline and greatest improvement. |
| Luchsinger et al.81 (2011) | 2,169 (type 2) | RCT | Slower cognitive decline | Mediated by decline in A1c, not A1c or LDL; post hoc analysis |
| Shea et al.82 (2013) | 1,665 | RCT | Comorbidity ↓, adherence ↑ | Low SES, also worst A1c; lowest-income=more benefits in A1c and BP; Lowest education=more benefits in A1c and BP |
| Trief et al.83 (2013) | 1,665 | RCT | Self-reported adherence improved | Whites more adherent than Hispanics or African Americans |
A downward arrow indicates down or decreased; an upward arrow indicates up or increased.
See comments.
A1c, glycated hemoglobin A1c; BMI, body mass index; BP, blood pressure; C, control group; I, intervention group; LDL, low-density lipoprotein; O, no difference; PA, physical activity; RCT, randomized controlled trial; SES, socioeconomic status; WC, waist circumference.
The initial two articles from IDEATel were published in the same issue of the Journal of the American Medical Informatics Association in 2002. The first66 described the technical implementation of the project, and the second67 described the research methodology. These will not be included in this analysis because they were descriptive in nature, and the relevant information they contained regarding project design, rationale, and research methods have been repeated in subsequent publications.
This project was conducted in two sites: an urban region in New York City and a rural region in Upstate New York State. In both places, all patients were Medicare beneficiaries residing in medically underserved areas and using a Federally Qualified Health Center. The research was undertaken by a consortium based at Columbia University.
Patients who had type 1 or type 2 diabetes were allocated randomly as clusters to either the telemedicine or usual care group based on their primary care provider. This probably implies that all patients of a given provider were allocated to either the experimental or the control group. Hence, greater variability within the cluster would result in a smaller sampling error—or better representation—and vice versa. Participants in the intervention group received a home telemedicine unit specifically developed for the project, consisting of a Web-enabled modem connected to a telephone line with four components: videoconferencing, a remote glucose monitoring device, a Web portal for patients with access to their own clinical data and to a nurse case manager, and an educational Web site developed by the American Diabetes Association. Nurse managers were trained in diabetes management and information technology. Each intervention subject was assigned a case manager who worked under the supervision of diabetologists. The protocols were based on the Veterans Administration's “VA/DoD Clinical Practice Guideline for the Management of Diabetes Mellitus in Primary Care.”84 Initial plans called for a total sample of 1,500 to provide adequate statistical power for subgroup analysis. This was increased during enrollment to compensate for early differential dropout rates, which were larger in the intervention group (248 out of 1,665: 144 from the intervention group and 104 from the control group). About 90% of all patients were 70 years of age or older.
A synopsis of the main findings from empirical analysis of data that were based on this project is shown in Table 4. The following is a discussion of these findings.
The first set of three research articles was published in 2006. The first article68 reported the results of the intervention on health outcomes after 1 year of observation. The results showed a positive trend in terms of improved glycemic control, blood pressure, and LDL cholesterol among patients in the experimental group. The second article69 had a different focus, and it investigated the relationship between clinical depression and glycemic control. A weak relationship was observed between these two variables. However, the presence of depression did not predict change in glycemic control in either the control or experimental group. The third article70 was concerned with clinical predictors of glycemic control after 2 years from baseline. The researchers discovered “that ambulatory pulse pressure [but not office pulse pressure] improves the prediction of increased urine albumin excretion in older people with type 2 diabetes.”70 The importance of this finding lies in the fact that “ambulatory monitoring appears to provide information above and beyond that provided by office blood pressure measurements.”70
Subsequently, a set of three articles from the project was published in 2007 from the project. The first71 was focused on the effects of the intervention on psychosocial outcomes, after 1 year of follow-up. This analysis was based on the expectation (hypothesis) that a supportive relationship between the patients and knowledgeable providers will likely benefit the patients' emotional well-being. Here, the authors concluded that the intervention “resulted in significantly improving diabetes self-efficacy.…”71 However, it did not improve depression or diabetes distress. Diabetes self-efficacy was not explicitly defined. It probably referred to how patients felt about their diabetes. The second article72 in this set was concerned with the incidence of medically urgent situations, but only on the part of patients in the intervention group. In other words, there was no control group comparison in this analysis. Based on 3 years of follow-up, 67 medically urgent situations were encountered among 338 patients. All were successfully identified and remediated. This success was attributed to improved communication between primary care providers and their patients as well as improved access to diabetes care. The third article73 was a report from a survey (n=116) of the primary care providers who cared for the patients in this project. It was conducted at 12 and 24 months from baseline. The findings from the survey revealed that providers were generally supportive of telemedicine and that they were sanguine on its positive impact on their patients. Nonetheless, these providers were also concerned about excessive paperwork and duplication, as well as conflicting advice and management decisions regarding individual patients.
In 2009, two research articles were published from this project. The first74 reported clinical results based on 5 years of follow-up. However, because of the long time frame, substantial numbers had dropped out (358 from the intervention group and 514 from the control group). The authors used “intention-to-treat” analysis in order to retain the original sample size, by imputing data for those who dropped out. This analysis revealed net improvements in glycemic control, LDL cholesterol, and blood pressure. The second article75 was an evaluation of a remote educational program that was included under the umbrella of this project. According to the authors, this educational tool resulted in “significant improvements in their [patients] ability to perform tasks on their home telemedicine unit.”75 In other words, patients who received this education were better able to use their telemedicine units.
Four articles were published from this project in 2010.
The first article76 in this set was focused on body weight (waist circumference and body mass index). Patients in the intervention group were connected to a dietitian or nurse via their home videoconferencing equipment, and the dietitian or nurse recommended lifestyle and medication changes, as indicated in each case, every 4–6 weeks. It is not surprising that, after 2 years, patients in the intervention group improved their knowledge of diabetes risk factors and their actual body weight. Women fared better than men in this regard.
The second article77 was based on data analysis from Medicare claims over 6 budget years. Medicare claims data were similar in the two groups. However, the authors pointed out that the intervention would not be cost-efficient since “the cost of implementing the telemedicine intervention was high, largely representing specific purpose hardware and software costs required at the time. Lower implementation costs will need to be achieved using lower cost technology in order for telemedicine case management to be more widely used.”77
The third article78 was focused on a very specific issue—namely, why patients may request additional training and their preference of delivery mode of such training. Here, a total of 109 patients from the entire group requested additional training. Of the three options offered to them—in-home visit, unassisted use of user's manual, and telephone training—the majority preferred in-home training.
The fourth article79 (initial online publication was in 2010) investigated the effects of the intervention on physical activity and impairment. The intervention group experienced a lower rate of decline in physical activity and impairment compared with the control group. Hence, the authors suggested that “pedometers may be a helpful inexpensive adjunct to diabetes initiatives delivered remotely with emerging technologies.”79
Two research reports from this study were published in 2011.The first80 was concerned with socioeconomic disparities, particularly ethnicity. It reported that glycemic control improvement was noted among “Hispanics, who had the highest baseline A1c levels.”80 The second81 was an analysis of the differences in cognitive outcome between the intervention and control groups. The authors reported “a slower cognitive decline in the intervention group, mostly as a result of improvement in A1c.”81 This is an interesting finding as it suggests a link between A1c and cognitive decline. Moreover, sicker Hispanic patients paid more attention to their A1c than their counterparts.
In 2013, two reports were published from this study. The first82 investigated whether the intervention had an effect on socioeconomic disparities (as measured by income and education) and ethnicity. The results from this analysis revealed that participants of lower socioeconomic status (SES) “benefited at least as much as higher SES”82 counterparts. However, limited variations in income did not permit an analysis of the full impact of SES. Average annual income in the entire sample was about $15,000. The other analysis83 investigated differential adherence to prescribed regimens, including glucose testing, diet, exercise, and foot care, and also whether adherence mediates glycemic control. In this case, adherence improved glycemic control. Overall, members of minority groups never achieved the same level of self-care as whites.
GDM
GDM is a glucose intolerance that occurs during pregnancy, typically at around week 24. It may or may not antedate pregnancy, and it may not necessarily continue after delivery. In other words, pregnant women without a history of diabetes may develop high blood glucose levels during pregnancy. GDM affects the mother's health as well as that of the baby. It can lead to macrosomia (fat baby), hypoglycemia, or low blood glucose and a higher risk for breathing problems and subsequent type 2 diabetes. Babies with macrosomia face a higher risk for obesity and type 2 diabetes, as well as breathing problems, low blood glucose levels at birth, and damage to their shoulders during the birth process.
Based on diagnostic criteria by the International Association of Diabetes in Pregnancy Study Groups, GDM affects 17.8% of pregnancies.85 This is nearly double the rate (9.2%) at which pregnant women are currently diagnosed as having GDM.86 The difference between the two estimates derives from the diagnostic threshold for defining GDM. The more liberal definition has the benefit of alerting pregnant women to potential problems, and hence it may lead to preventing some cases of shoulder dystocia and birth injury by corrective actions such as weight control. Indeed, excess weight in the offspring of women with GDM can be ameliorated with appropriate therapy.87 Another estimate indicates that approximately 6–7% of pregnancies in the United States are complicated by diabetes and that approximately 85% of these cases represent women with GDM.88
The causes of GDM are not fully known. The prevailing view points to insulin resistance in the placenta. Hormones from the placenta help the baby develop. But, these hormones can also block the action of the mother's insulin, which makes it hard for her body to use insulin. When this happens, the mother's pancreas works overtime to produce insulin, but it would still be unable to lower blood glucose levels. She may need up to three times as much insulin. Although insulin does not cross the placenta, glucose and other nutrients do. The extra glucose going through the placenta gives the baby high glucose levels. This causes the baby's pancreas to produce extra insulin to maintain normal glycemia. Because the baby is getting more energy than he or she needs to grow and develop, the extra energy is stored as fat.
Without adequate insulin, glucose cannot leave the blood and be changed to energy. Glucose builds up in the blood to high levels, or hyperglycemia. The risk of GDM is associated with older age, higher body weight, and family history.89
GDM affects the mother after the baby's body has been formed, but while the baby is growing. Because of this, GDM does not cause the kinds of birth defects sometimes seen in babies whose mothers had diabetes before pregnancy.
GDM is diagnosed using one step (75-g OGTT) or two steps (screening using the 40-g glucose challenge, followed by a 100-g OGTT for those who screen positive). The specific glycemic goals should be specified for each individual, typically aimed at achieving the following levels:
• Before a meal (preprandial): 95 mg/dL or less
• 1 h after a meal (postprandial): 140 mg/dL or less
• 2 h after a meal (postprandial): 120 mg/dL or less
The optimal treatment for GDM includes glucose monitoring, diet and exercise, oral diabetes medications, and initiation and titration of insulin, as indicated, to maintain glucose targets.
The Cost of GDM
A report published in 2009 estimated the national costs associated with GDM for 2007.90 Average expenditures for GDM were estimated at $3,305 per pregnancy and an additional $209 in the newborn's first year of life. About 36% of this cost is covered by public programs (mostly Medicaid), 56% is covered by private insurance, and 8% are self-pay charity.90 However, the authors explained that these estimates are limited to “near-term medical costs, omitting the increased risk for long-term sequelae.”90 Long-term costs are much greater than those of the near term. As an indication of that cost, it may be appreciated that 20% of babies born to mothers with GDM will be overweight at birth and thus also have an increased risk for type 2 diabetes and cardiovascular disease91 (see also Kim et al.92).
Several highlights from a statistical brief from the Agency for Healthcare Research and Quality demonstrated the added hospitalization costs among women with GDM. For instance, in 2008 “one-third (33.9% of hospital stays with pre-existing diabetes complicating pregnancy involved no delivery. …) This compares with 10.3 percent of stays without diabetes. …”88 About two-thirds of women with diabetes complicating pregnancy delivered via cesarean section. The rate for women with GDM was 45.6%. “From 1997 to 2007, there was a 14% increase in the number of [hospital] stays for all deliveries. In contrast, deliveries involving gestational diabetes increased 75%. …”88 The average hospital cost for women without diabetes was $3,800, for women with preexisting diabetes was $5,900, and for women with GDM was $4,500. Overall, “total costs of hospitalization for all diabetes in pregnancy was over $1.4 billion. …”88
An estimate of maternity care and costs in Ireland among 4,372 women, of whom 354 had GDM, indicated a significantly higher level of emergency cesarean section, neonatal admission, and 34% higher costs.93 Similar findings were reported in an RCT (n=848) in 2012 in Finland.94 “The cost of inpatient visits was 44% higher and neonatal intensive care unit use was 49% higher in the GDM group than among women without GDM.”94
The Telemedicine Intervention in GDM
The telemedicine intervention in GDM is the same as that in type 1 and type 2 diabetes. It consists of a structured electronic system for monitoring, communication, and coordination between patients with GDM and their care providers. Despite some variation in technical design and configuration, the system is intended to provide connectivity for pregnant women with GDM from their homes, places of work, or while traveling. It serves four functions: (1) ongoing monitoring of blood glucose and related aspects, (2) clinical instruction, (3) medication titration and compliance, and (4) educational programming. Hence, it includes the measurement, storage, and analysis of relevant data, as well as the use of the information for dosage adjustment of hypoglycemic medications for alerting patients when warranted, and for providing counseling from nurses or dietitians in managing blood glucose, appropriate exercise, and dietary intake. The anticipated benefits are better adherence to medication, better therapy adjustment, improved life style, and ultimately a healthy and uncomplicated delivery.
Feasibility of GDM Telemonitoring
None of the studies that investigated the feasibility of telemonitoring GDM met the sample size criterion for inclusion in this analysis. However, we chose three articles for brief discussion here. All used the RCT design. These will not be considered when discussing the empirical evidence in this domain.
In 2007, a U.S. RCT (n=57) investigated the use of an Internet telemonitoring system to manage GDM among medically underserved women.95 This study produced a modest finding—namely, that “women in the telemedicine group did experience enhanced feelings of diabetes psychosocial self-efficacy.”95 This may be translated roughly to mean that women with GDM in the intervention group felt better about their condition (or perhaps their ability to control it) when compared with their counterparts. However, the feasibility of using an Internet-based approach to manage GDM among poor inner city women was obviously limited. The authors suggested that an interactive voice recognition telephone link may have produced better results.
In 2010, an RCT (n=100) in Spain evaluated the feasibility of a telemonitoring system for GDM based on the Internet and short message service.96 “The system significantly reduces the need for outpatient clinic visits (62%) and achieves similar pregnancy, delivery, and newborn outcomes.”96 Among insulin-treated women, there was an 82% reduction in outpatient visits.
In 2012, a U.S.-based RCT (n=80) demonstrated that adding interactive voice response to a telemonitoring system for pregnant women with multiple medical conditions “increased system utilization and contact between women with GDM and their healthcare providers.”97 However, it had no impact on blood glucose values or infant birth weight.97
GDM and Intermediate Outcomes (Weight, Diet, Exercise)
Two studies meeting the criteria for inclusion in this analysis investigated the intermediate outcomes of telemonitoring GDM, in terms of body weight, diet, and exercise. These were conducted in The Netherlands (2011)98 and United States (2014).99 They are summarized in Table 5.
Table 5.
Tele-Gestational Diabetes Mellitus Outcomes
| INTERMEDIATE OUTCOMES | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| REFERENCE (YEAR) | COUNTRY | RESEARCH DESIGN | SAMPLE SIZE | WEIGHT | HEALTH BEHAVIOR | HEALTH KNOWLEDGE | E-HEALTH ENGAGEMENT | COMMENTS | |
| Kelders et al.98 (2011) | The Netherlands | RCT | 297 | O | O | O | ↑ (64%) | Most respondents were female and highly educated; Web-based intervention for diet and physical activity | |
| Graham et al.99 (2014) | United States | Formative research interviews | 1,689 | ↓ | ↑ | ↑ | ↑ (85%) | E-Moms ROC analyzed e-health usage rather than vital sign change (e.g., A1c, weight). Most appropriate mix of online features and their effectiveness predictors is unknown. | |
| HEALTH OUTCOMES | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| REFERENCE (YEAR) | COUNTRY | RESEARCH DESIGN | SAMPLE SIZE | STRESS | MACROSOMIA | A1C | CESAREAN | POSTPARTUM A1C TESTING | COMMENTS |
| Dalfra et al.100 (2009) | Italy | Sequential assignment | 276 | ↓ | ∼ ↓ | O (type 1);↓(GDM) | ↓ | ↑ metabolic control | Intervention group had less frustration with DM; used CES-D, SF-36, and Stress/Distress measure to evaluate patients; QoL↑; Role Emotional score improved in UC group only. Reduced number of visits to clinic |
| Ferrara et al.101 (2012) | United States | Quasi-experimental | 11,435 | NM | ↓ | NM | NM | ↑ | Referral to GDM telephonic nurse management is effective. |
A downward arrow indicates down or decreased; an upward arrow indicates up or increased.
A1c, glycated hemoglobin A1c; CES-D, Center for Epidemiologic Studies Depression Scale; DM, diabetes mellitus; e-Moms ROC, e-Moms of Rochester; GDM, gestational diabetes mellitus; NM, not measured; O, no difference; QoL, quality of life; RCT, randomized controlled trial; SF-36, 36-item Short Form; UC, usual care.
In 2011, an RCT (n=297)98 was conducted in The Netherlands that investigated the predicted usage of a Web-based intervention aimed at promoting healthy diet and physical activity. In total, 297 respondents were recruited from various public sources, including advertisements in local newspapers, postings in supermarkets, and a health-related Web site. The sole inclusion criterion was BMI indicating slight overweight (18.5–28.0 kg/m2), in addition to pregnancy. The majority of those who agreed to participate were highly educated women. These were randomly allocated in blocks of four to either the intervention (n=147) or control (n=150) group. Both groups filled out online questionnaires at the start of the project and 12 weeks from baseline. The intervention group received a Web-based lifestyle application developed by The Netherlands Nutrition Center, whereas the control group did not. The study produced several methodologically relevant findings, but none substantive in nature: (1) Of the 269 (in both experimental and control groups) who completed the baseline questionnaire, only 159 filled out the posttest questionnaire (overall response rate of 59%). The response rate was significantly lower in the intervention group (51% versus 66%). Over one-third (or 36%) of respondents in the intervention group did not use the application at all or as intended. (2) Nonetheless, as expected, the users tended to be healthier and more knowledgeable than the nonusers. Hence, those who had room for improvement in terms of their diet and exercise were less likely to use the application compared with their counterparts.
In 2014, a multistep formative evaluation was conducted to ascertain the effectiveness of an e-intervention in preventing excessive gestational weight gain.99 The technology consisted of a Web site and mobile phones. It was designed to maximize and sustain the use of the system by the intended users. However, the development of the clinical trial protocols followed an elaborate formative process, including short interviews (n=110), in-depth interviews (n=24), and focus groups (n=26), all this to inform the study design. The authors wanted to establish a theoretical foundation for linking intervention features to observed outcomes that may result. In total, 1,689 participants were randomized to an intervention group (n=1,126) and a control group (n=563), at the rate of 2:1. Participants in both groups had access to blogs, local resources, articles, and an information guide to “frequently asked questions.” But, the intervention group also had a weight gain tracker as well as diet and physical activity goal-setting tools. In addition, they received customized reminders, tailored content, and access to local community features. Use of the Internet among the participants was “comparable to those in other weight studies of young adults and higher than reported in a published study with pregnant women.”99 The weight gain tracker was used by the majority of the participants in the intervention group (n=705), but only 39% of this group actually set either diet or physical activity goals.
GDM and Health Outcomes
Results from two studies (in Italy100 and United States,101 published in 2009 and 2012, respectively) investigated the effects of GDM telemonitoring on intermediate health outcomes, including metabolic control, cesarean section, macrosomia, and referrals, shown in Table 5.
The first was an Italian study (using sequential assignment to intervention and control groups; n=276) that investigated the effects of a telemonitoring system on metabolic and fetal outcomes among women with diabetes.100 During enrollment in the study, women were sequentially assigned to the intervention or control group, but it was not clear whether the selection process was randomized or not. The intervention group consisted of 88 women with GDM. Of these, 17 had type 1 diabetes. The control group consisted of 115 women with GDM; 15 of them had type 1 diabetes. Women in the intervention group were trained to use a home glucose meter and were asked to submit their blood glucose measures every week and more often when necessary. They were also given a medical examination once a month. Women in the control group received a medical examination every 2 weeks but no glucose meters. Overall, clinical values were comparable between the two groups. However, those in the intervention group had better glycemic control in the third trimester of pregnancy, fewer cesarean sections, and lower rates of macrosomic infants, compared with those in the control group. In addition, they expressed lower levels of frustration with their condition.
The second study was a quasi-experiment published in 2012. It was based on a large sample (n=11,435) at Kaiser Permanente of Northern California, an integrated health system that provides care to over 3 million members in 14 counties.101 The study population was limited to pregnant women with GDM, and the intervention consisted of telephone counseling 7 days a week on glucose monitoring and control, diet, and physical activity by registered nurses and dietitians. Referred women received one or two counseling calls per week. They could also initiate the calls themselves when they had questions or concerns. The outcome measures (body weight, infant birth weight, and use of insulin) were extracted from the medical records. The study design allowed an assessment of the association between referral to this program and the outcomes. The results suggest that “receiving care at the centers with the higher referral frequency to telephonic nurse management for [GDM] was associated with decreased risk of macrosomic infants and increased postpartum glucose testing.”101
DR
DR is the result of damage to the blood vessels in the retina—the image-processing layer of tissue in the back of the inner eye. The main function of the retina is to convert the images into electric signals and send them to the optic nerve of the brain.103 A healthy retina is necessary for clear vision.
At an early stage, DR may not have any obvious symptoms. However, diabetes starts to damage the small blood vessels, especially those inside the retina, during this stage. The damaged blood vessels can leak between the retinal layers, causing further damage, which can be manifest in the form of blood spots, macular edema (fluid leaking into the central portion of the retina), which causes swelling and hence blurring vision, or abnormal new blood vessel growth. As it progresses to an advanced stage, the typical symptoms include blurry vision, flashing lights, dark floaters, or limited peripheral vision.
Type 1 and type 2 diabetes are also associated with other eye diseases, including cataracts (clouding of the lens of the eye), dry eyes, and glaucoma (pressure build-up in the eye that damages the optic nerve).
DR usually affects both eyes. If left untreated, DR can develop in stages marked by different degrees of small blood vessel damage in the retina. The severity of the disease is classified into four stages: mild, moderate, and severe nonproliferative retinopathy, with the fourth stage as proliferative retinopathy. Each of the first three stages, labeled as “nonproliferative,” involves increasing damage in the retinal blood vessels, ranging from (1) microaneurysms, (2) some blocked blood vessels, and (3) cotton-wool spots (damage to retinal nerves) to (4) several blocked retina blood vessels. The resulting lack of oxygenation of the retinal tissue alerts the body to grow new blood vessels for nourishment. The new blood vessels represent the most advanced fourth stage, labeled “proliferative.” It begins with the growth of new but abnormal and fragile blood vessels along the retina and the clear vitreous gel that fills the eye. The rupture of these thin fragile vessel walls can result in bleeding, which can lead to severe vision loss or blindness. These new abnormal blood vessels can also cause traction on the retina resulting in retinal detachment. Symptoms can range from none to dark areas in the vision to specks of dark or “floaters.” In 50% of persons with proliferative DR (PDR), fluid can leak into the center of the retina, or macula where visual acuity is greatest, and result in central retina swelling leading to blurred vision. This condition is called diabetic macular edema.104
DR is the leading cause of adult blindness in the United States and the most common diabetic eye disease. It can cause vision loss in three ways: (1) rupture of blood vessels that occur in proliferative retinopathy, causing intra-eye bleeding, (2) retinal detachment, which occurs in proliferative retinopathy, or (3) fluid leaking into the center of the macula, which occurs in about 50% of proliferative retinopathy. At the end stage of the disease, new blood vessels can form in other parts of the eye, leading to advanced glaucoma. Individuals with type 1 and type 2 diabetes are advised to have an annual comprehensive dilated pupil (mydriatic) eye examination, which also includes visual acuity and tonometry (pressure) tests. The ophthalmologist is able to detect blood vessel changes or leaking, retinal swelling, pale or fatty deposits on the retina, and nerve tissue damage. Macular edema treatment may require a photograph of the retina or dye-based fluorescein angiogram to identify leaking vessels and aid in creating a treatment plan. Glycemic control is associated with the progression, if not prevention, of retinopathy.105 Maintaining good blood pressure control is also important, as is controlling lipid levels and kidney disease (if present). Treatment for DR in stage 4, or PDR, involves the use of a scatter laser procedure around the retina away from the macula to shrink blood vessels.104
More recently, medications injected into the eye that selectively target the stimulus of new blood vessels (anti–vascular endothelial growth factor medications or control inflammation steroids) have been used to impede proliferative disease or macular edema.106 Side effects can include some loss of peripheral, color, or night vision. In some cases, a surgical procedure (vitrectomy) may be required to remove the blood accumulation within the eye or to repair retinal detachments and release traction from the retina. Diabetic macular edema is also treated with lasers, but a limited amount of burns (typically less than 200) is performed near the macula to slow fluid leakage and resulting in 50% less vision loss.104
DR Epidemiology
The major risk factors for DR include prolonged diabetes, uncontrolled hyperglycemia, high blood pressure, and LDL cholesterol, as well as genetic factors. In addition to genetic factors, duration and level of diabetes tend to increase the incidence of DR. The prevalence of DR is higher in type 1 compared with type 2 diabetes. “Sight-threatening retinopathy is 2.5 times more common in type 1.”107 However, the prevalence and incidence of DR in type 1 diabetes have been declining in industrialized countries, probably “the result of improved glycemic control and possibly greater access to health care.”107 Still, DR is the leading cause of new cases of blindness in the United States, and it may soon become the leading cause globally.107 The percentages of those with diabetes who are legally blind are 3.6% of those with type 1 and 1.6% of those with type 2. The META-EYE study found varying rates of DR among African Americans (49.6%) and Asians (19%), as well as PDR variation of whites (12%) and South Asians (1.29%).107
As in diabetes generally, regular screening and early diagnosis and treatment of DR can be instrumental in saving vision, improving quality of life, and perhaps saving money by diminishing the need for costly treatment to restore vision.
Telemonitoring/Telescreening for DR
Annual examination monitoring for DR and other eye diseases is recommended for all patients with type 1 and type 2 diabetes.108 These examinations are typically performed by ophthalmologists in their clinics. The reasons for DR telemonitoring/telescreening (teleDR) are the same as those for other forms of telediabetes—namely, early detection, prompt treatment, and prevention of exacerbations. The teleDR tools enable patients to have these services in their home communities or closer to where they live and work, thereby avoiding unnecessary trips to the ophthalmologists. They also free ophthalmologists to see patients who need treatment and avoid long waiting times for appointments. TeleDR involves the use of cameras to photograph the retina, often by lesser trained professionals or technicians, but with reliable results.
Although there are several fundus imaging options (computed tomography, magnetic resonance imaging, ultrasound, infrared thermography, hyperspectral imaging, color Doppler imaging, photo-acoustic ophthalmoscopy, blood flow magnetic resonance imaging, fundus photography, fluorescein angiography, slit lamp), digital photography offers the advantages of low-cost and ready access to images, duplication, transmission, and archiving. Photography is the primary tool in teleDR.107 There are two types of fundus images: mydriatic (with pupil dilation) and nonmydriatic (without dilation). Most programs use nonmydriatic photography so that patients' eyes would not have to be dilated for the examination. “Cameras that can capture images through small, non-mydriatic pupils are tailored for physiological dilation that occurs in a darkened room.”109 This makes them suitable for remote monitoring/screening programs, whereas mydriatic cameras typically provide better fundus images. A nondilated pupil is smaller and allows less light into the eye chamber, thereby limiting the field angle and the working distance from the eye surface and increasing exposure to artifacts. In addition, there are several variables to be considered, including the number of field images, image field angle, observational and photographic light sources, working distance from the eye, regular pupil size, frame resolution, filters used, and whether stereoscopic images were captured.
A 2012 comparison of the advantages versus disadvantages of nonmydriatic fundus cameras is provided by Meszaros,110 making a case for why nonmydriatic cameras will not replace dilated fundus examinations. Nonmydriatic camera advantages include modest cost, a variety of filters for image enhancement, quick, easy to use, wide angle of the retina, little training required, and well tolerated by patients. However, disadvantages include artifacts from over-/underexposure, inability to detect abnormalities outside the photographic field, poor quality secondary to media opacity, and no stereoscopic capability. The lack of stereoscopic capability has been obviated by recent advances in technology, and nonmydriatic photography avoids the risk of dilation causing acute glaucoma and the 20-min wait time for dilation.
The most important goal of teleDR is to differentiate patients with no DR (not needing additional screening) from those with some degree of DR requiring referral and a more advanced level of monitoring and care. To determine the level of telescreening capability of a program, validation categories (1–4) were developed by the American Telemedicine Association111 as shown in Table 6. Screening levels are described in terms of Early Treatment Diabetic Retinopathy Study and International Classification Levels of DR. Category 1 includes those with an absence or very mild non-PDR (NPDR) from those requiring additional screening to differentiate the level and severity of DR. The highest category (4) is a screening system that equals or exceeds any clinical or research DR screening capability.
Table 6.
Tele-ophthalmology Programs and Systems
| TELE-OPHTHALMOLOGY PROGRAM VALIDATION CATEGORY LEVELS | |||||
|---|---|---|---|---|---|
| INTERNATIONAL CLASSIFICATION LEVEL OF DR | ETDRS LEVEL OF DR | CATEGORY 1 SYSTEMS IDENTIFY | CATEGORY 2 SYSTEMS IDENTIFY | CATEGORY 3 SYSTEMS IDENTIFY | CATEGORY 4 SYSTEMS IDENTIFY |
| No apparent DR | DR absent | No or minimal DR | Patients without sight-threatening DR or DME | No DR, NPDR, early and high-risk DR, and DME for follow-up and treatment strategies | All levels of DR or DME; photographs match, exceed, or can replace ETDRS photographs in any clinical or research program. |
| Mild DR | Very mild NPDR | ||||
| Moderate NPDR | Moderate NPDR | More than minimal DR—but patients require additional screening | |||
| Severe NPDR | Severe NPDR, very severe NPDR | Patients with sight-threatening DR, NPDR, and PDR | |||
| PDR | PDR, High Risk PDR, Very Severe or Advanced PDR | ||||
DME, diabetic macular edema; DR, diabetic retinopathy; ETDRS, Early Treatment Diabetic Retinopathy Study (30°, stereo seven-standard field, color, 35-mm slides as reference); NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy.
The most important limitation of teleDR is missing other ocular diseases that may require the attention of an ophthalmologist. Similar to research on telediabetes in type 1 and type 2 and GDM screening, the research findings on teleDR are organized into three sets—namely, (1) feasibility and effectiveness, (2) intermediate outcomes, including patient adherence to testing, resource use, outreach, and time to care, and (3) health outcomes.
The most important function of teleDR is the early detection of abnormalities or pathology. Hence, as a screening tool, the two most important measures of its success are “sensitivity” and “specificity.” These are normally presented as percentages. Sensitivity, or the true-positive rate, is the percentage of people with pathology who are correctly identified as such. It is also complementary to the false-negative rate. Specificity, or the true-negative rate, is the percentage of people without pathology who are identified as such. It is also complementary to the false-positive rate. Typically, there is a trade-off between the two measures. In instances where discovery of pathology is critical, a high sensitivity rate is desirable to ensure that all cases of pathology are promptly identified and treated. The trade-off can be represented graphically as a “receiver operating characteristic” curve.
Finally, it may be noted that the evidence in several studies did not fit into a single category. In those instances, the study is reported only once and typically under the most significant category.
Feasibility of TeleDR
A retrospective chart review of a very large mobile telescreening program was conducted in several rural villages in India between April 2009 and September 2010.112 The team consisted of trained social workers (for organizing the program and registering the patients) and optometrists (for comprehensive clinical examination), sometimes augmented by an information technology team (for electronic medical registration). The initial examinations used nonmydriatic fundus cameras. Abnormal findings were referred to ophthalmologists for further evaluation. In total, 54,751 patients received eye examinations/screening. Of these, 58% had some form of visual impairment: 59% uncorrected refractive error, 30.3% cataract, and 3.3% DR with visual impairment. The authors concluded that their mass screening model proved to be “efficient in delivering comprehensive eye care to the rural population of India.”112
A retrospective chart review of 643 patients over a period of nearly 7 years was conducted to describe the experience of using teleDR among patients with diabetes and hypertension at a community health center in rural West Virginia (published in 2012).113 Screening was performed by a trained registered nurse using a nonmydriatic retinal camera. About 5% required pupil dilation to obtain adequate imaging. Ophthalmologists interpreted the images and followed up, when indicated. The program detected unknown eye pathology in 44.5% of the patients. Thirty-three percent of the patients were recommended for prompt follow-up. According to the authors, this experience “demonstrates the actual benefits of telemedicine in the effective screening of diabetic and hypertensive patients for eye pathology.”113
DR Screening Intermediate Outcomes: Screening, Adherence, Resource Use, and Cost
Obviously, teleDR by itself is an investigational tool. Hence, the primary criteria for judging its quality would be sensitivity and specificity. Nonetheless, some studies tried to ascertain whether screening can also have spillover effects on diabetes-related health outcomes, such as glycemic control, improvement in LDL, and arterial hypertension. We decided to include both types of studies here, presented in historical order.
We identified 23 such studies.114–136 Of these, nine were conducted in the United States, five in France, three in Canada, two in Spain, and one each in Mexico and Peru. The vast majority of the studies used retrospective record reviews, and the samples varied from 158 to 38,595. These are summarized in Table 7.
Table 7.
Diabetic Retinopathy Telemonitoring/Telescreening
| INTERMEDIATE OUTCOMES | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCREENING OUTCOMES | RESOURCE USE | |||||||||||
| REFERENCE (YEAR) | COUNTRY | RESEARCH DESIGN | SAMPLE SIZE | PHOTOGRAPHY | GRADABLE IMAGES | ADHERENCE | SPECIFICITY | SENSITIVITY | OUTREACH | USE/REFERRAL | COST | COMMENTS |
| Conlin et al.114 (2006) | United States | RCT | 448 | Non-Myd | 64% | ↑ 10% | 61% for NPDR 100% for PDR |
96% NPDR 99% PDR |
↑ | ↑ | NM | Improves assessment rates; gradability related to cataracts and small pupil size. Patients highly satisfied |
| Ahmed et al.115 (2006) | United States | Retrospective review | 243 | Non-Myd | 65% | 48–93% | 89% | 98% | ↑ | ↑ | NM | |
| Fonda et al.116 (2007) | United States | Retrospective review | 13,752 | Non-Myd | NM | ↑ | NM | NM | ↑ 16.1% | ↑ 1.71 relative risk of standardd eye care | NM | Using tele-ophthalmology associated with ↓in A1c and LDL; used JVN |
| Taylor et al.117 (2007) | United States | Retrospective cohort | 293 | Non-Myd | 99.5% | 40.6% Tx 31.3% standard care |
NM | NM | ↑ 36.2% | ↑ | NM | Telescreening: ↑screening rates, access ↑, specialty care for medically indigent ↑ |
| Massin et al.118 (2008) | France | Retrospective review | 15,307 | Non-Myd | 87.8% - 92% | NM | NM | NM | ↑ | ↑ (25.2%) | NM | Outreach to hospitals, primary healthcare centers, and prisons |
| Boucher et al.119 (2008) | Canada | Cohort study | 3,505 | Non-Myd | 99.3% | NM | 96.9% | 99% | ↑ | 17.9% | $100/exam | Category 3 Program; identified severe NPDR and PDR 100%; 33% received mild dilation; 85.6% avoided ophthalmologist examination |
| Lemmetty et al.120 (2009) | Finland | Survey | 17,471 | Non-Myd | NM | ↑ | NM | NM | ↑ | ↑ | NM | Early-stage DR diagnosed |
| Tran et al.121 (2009) | France | Retrospective review | 1,147 | Non-Myd | 86% | ↑ | NM | NM | ↑ | ↑ | NM | Collaboration required between screening site and follow-up site |
| Chabouis et al.122 (2009) | France | Retrospective review | 500 | 85% Non-Myd, 15% ophthalmoscope | NM | NM | NM | NM | ↑ | ↑ 22% | NM | Photography time is 15 min versus 30 min (dilated); DR exam time reduced from 0.90 to 0.32 half-day |
| Perol et al.123 (2012) | France | Retrospective review | 254 | Non-Myd | >90% | ↑ | 92–99% | 92–99% | ↑ | ↑ | NM | |
| Schulze-Döbold et al.124 (2012) | France | Retrospective review | 38,596 | Non-Myd | 90.1% | ↑14% | 0–92% | 0–92% | ↑ | ↑ | NM | 5-year OPHDIATt Project |
| Nathoo et al.125 (2010) | Canada | Descriptive | 394 | Non-Myd | 99.4% | 76.8% | NM | NM | ↑ | 87.30% | NM | |
| Andonegui et al.126 (2010) | Spain | Prospective review | 1,223 | Non-Myd | 98% | 100% | 93% | 91.7% NPDR 99.2% PDR |
↑ | ↑ | NM | GP DR treatable lesion sensitivity=99.2% (detection=90.9%); GPs DR specificity=83%; GPs can screen for DR. |
| Vargas-Sánchez et al.127 (2011) | Mexico (in Spanish) | Cross-sectional observational | 676 | Myd | NM | NM | 97% | 80% | ↑ | NM | NM | GP versus ophthalmologists; GP 97% specificity, 80% specificity, 33% positive predictive value, 100% negative predictive value, 4.88 positive likelihood ratio, and 0.04 negative likelihood ratio |
| Villena et al.128 (2011) | Peru | Prospective observational | 1,311 | Non-Myd | 93.20% | NM | NM | NM | ↑ | ↑ | NM | Cost-free evaluation; type 2 DM only |
| Rudnisky et al.129 (2012) | Canada | Retrospective cohort study | 980 | Stereoscopic Myd | 100% (selected) | 100% (selected) | NM | NM | ↑ | ↑ | NM | Suburban, rural, and remote First Nations patients; DR progression correlated with A1c and BP levels |
| Shahid et al.130 (2012) | United States | Cohort study | 341 | Non-Myd | 96% | NM | NM | NM | ↑ | ↑ | $37.50 / person | Focus on urban homeless |
| Martínez Rubio et al.131 (2012) | Spain | Descriptive cross-sectional | 2,435 | Non-Myd (with 5.22% mild dilation) | 98.31% | NM | NM | NM | ↑ | ↑ | NM | Benefits: early screening, diagnosis, treatment, and improved communication between GPs and specialists |
| Cavallerano et al.132 (2012) | United States | Prospective | 158 | Non-Myd | 88% | ↑ | 98% | 100% | ↑ | ↑ | NM | JVN, Category 3 program |
| Hautala et al.133 (2014) | Finland | Retrospective review | 14,866 | Non-Myd | 98%(Grd 1–3) | ↑ (100% w/ letter invite) | NM | NM | ↑ 24% | ↑ | NM | Efficient, timely mobile eye screening (Eye-Mo) |
| Richardson et al.134 (2012) | United States | Cost savings analysis | 659 | Non-Myd | NM | 100% | NM | NM | ↑ | ↑ | ↓ $153/Pt | Rural Appalachia |
| Li et al.135 (2012) | United States | Retrospective review | 611 | Non-Myd | NM | NM | NM | NM | ↑ | ↑ | ↓ $27.85/Pt | Used Medicaid reimbursement $ |
| Kirkizlar et al.136 (2013) | United States | Retrospective chart review | 900 | Not specified | NM | NM | NM | NM | ↑ | NM | See comments | Teleretinal screenings are cost-effective for pool sizes >3,500 and less than 80 years of age. QALYs ↑ |
BP, blood pressure; DM, diabetes mellitus; DR, diabetic retinopathy; GP, general practitioner; Hybrid, mydriatic and nonmydriatic camera options in one camera; JVN, Joslin Vision Network; LDL, low-density lipoprotein; Myd, mydriatic camera; NM, not mentioned or not measured; Non-Myd, nonmydriatic camera; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; Pt, patient; QALYs, quality-adjusted life years; RCT, randomized controlled trial.
We start with an initial set of six studies conducted between 2006 and 2008 (four in the United States and one each in France and Canada).
The first, an RCT (n=448), was conducted in the United States in 2006.114 All patients had diabetes and received their care from the VHA. Patients in the intervention group received nonmydriatic (without pupil dilation) digital retinal imaging with remote interpretation in an ambulatory care setting, whereas patients in the control group received their care from primary care providers in regularly scheduled appointments. Patient adherence was measured by documented evidence of a dilated eye examination in the ensuing 12 months from the start of the study. Twelve months after randomization, patients in the teleDR intervention (n=223) were more adherent than those did not have the imaging (87% versus 77%). Nearly two-thirds, or 64%, of the images were gradable. The remainder had cataract, other diseases, or a small pupil. Teleretinal imaging tended to “over identify the presence of DR.”114 In other words, they had a higher false-positive rate compared with those who had their eye examinations in-person at the clinic. However, nearly two-thirds of these false-positive, or 63%, for DR suggested other ocular pathologies. The study concluded that “non-mydriatic teleretinal imaging in the ambulatory care setting may improve screening rates for DR.”114
Also in 2006, a retrospective review of 243 patients (482 eyes) was conducted to determine sensitivity and specificity of nonmydriatic stereoscopic retinal imaging in detecting DR among diabetic patients. The project was conducted at four locations in the metropolitan Washington, DC area.115 The system failed to transmit 4 out of 482 images, and 35% of the images were not gradable. Retinal thickness could not be assessed in 21% of images. However, when the images were gradable, “the overall sensitivity was 98%, and the specificity was 100% for retinopathy within one grade of dilated funduscopic examination.”115
A retrospective analysis of a large dataset of electronic medical records (n=13,752) was conducted at the Joslin Diabetes Center over a period of 2 years (2004 and 2005). The results were published in 2007.116 The patients were classified into four sets: “no eye care, eye care outside the clinic, standard eye care at the clinic, and participants in the Joslin Vision Network telemedicine program.”116 The overall prevalence rate of DR was 23.1%, with a vast majority of NPDR (77% of cases). DR frequency peaked at the sixth and seventh decades of age and also increased with duration of diabetes. The results indicated significant benefits accrued from participation in the telescreening eye care program, especially improvement in both A1c and LDL. The authors concluded that “such programs can address the many aspects of care necessary to reduce risk of vision loss due to diabetic retinopathy and other diabetes-related health outcomes.”116 Based on their experience, they recommend the adoption of “a national screening DR program. …”116
Another retrospective study (n=495) was conducted between 2003 and 2004, and also published in 2007.117 The subjects were 18 years of age or older who had diabetes and were using a nurse-managed primary care clinic in the inner city. Nearly 90% were on Medicaid. The participants were offered a choice between a telemedicine-based mydriatic digital retinal imaging system or referral to an ophthalmology clinic at the next available appointment date (typically within 12 weeks). In total, 201 patients chose digital screening during their primary care visit, whereas 294 chose referral to the ophthalmology clinic. All patients in the telemedicine group were screened during the year, whereas only 31.3% of those selecting the deferred appointment actually followed up with an in-person examination. Inter-rater reliability was assessed in a subsample of 25 records, and it revealed a failure rate of 0.5% of digital image screening. The authors concluded that digital image screening of diabetic patients is “an efficient strategy to overcome traditional barriers to diabetic eye care.”117
A large-scale (n=15,307) investigation of the effectiveness of nonmydriatic fundus photography for detecting DR and adherence to annual evaluation was conducted in a regional network in France in 2008.118 The project was labeled OPHDIAT (Ophthalmologie-Diabete- Telemedicine). Fundus photographs were taken by trained orthoptists (health professionals who evaluate and manage eye movement abnormalities) or nurses in 16 screening centers (located in general practices, hospitals, prisons, and primary care centers) in the Île-de-France region. All locations were linked to a telemedicine center where ophthalmologists graded the images. Each screening lasted about 15 min. Patients with a previously diagnosed DR were excluded from the analysis. After 28 months, in total, 15,307 screening examinations were completed. Diabetic retinopathy was detected in 23.4% of the cases, and 9.7% of the images in at least one eye could not be graded, mostly because of lens opacity or small pupil. After the screening, 25.2% were referred to an ophthalmologist for DR, cataract, or nongradable images. The results of the study confirmed the importance of annual DR screening for people with diabetes, especially in view of the fact that DR incidence has been increasing (estimated at the rate of 4.8% in 5 years, and projected to increase by 50% by 2025.
A Canadian descriptive study (n=3,505), conducted from July 2003 to December 2005, was based on screening patients with diabetes in 182 pharmacies located in urban communities in five provinces: Quebec, British Columbia, Alberta, Saskatchewan, and Manitoba.119 The results were published in 2008. A photographer used mobile phones to capture the images with the assistance of a nurse, who performed mild dilation when necessary for quality photographs. High-resolution (1280×980 pixels) images were forwarded to ophthalmologists for interpretation and timely referral, as indicated. The service was offered free of charge. For 38% of the patients, this was their first eye examination, and an additional 30% had not received an eye examination in over 2 years. The results of the screening revealed a 22.5% prevalence rate of DR pathology, 2.4% requiring urgent referral for various reasons, and 10.1% requiring nonurgent referrals. A poor-quality image was observed in only 0.7% of the entire group. The cost estimate for the telescreening exam was around $100, similar to the cost of conventional screening. The authors highlighted the benefits of telescreening in terms of lowering barriers to screening for a diabetic population in need of such services, while “maximizing the use of limited ophthalmologic resources.”119
A retrospective analysis of data from a large DR screening program in a rural county in Finland was conducted on 17,471 patients (with type 1 or type 2 diabetes) from 1999 to 2006. The report was published in 2009. The purpose of the study was to ascertain the effects of the program on outreach, coverage, and referral.120 Screening was performed initially by trained nurses using a mobile digital nonmydriatic fundus camera. Ophthalmologists did the final scoring of the images. Images showing clear abnormalities were referred to the eye clinic for follow-up. Prior to this program, only type 1 diabetic patients were screened at the regional hospital. This program enabled screening for 85% of all patients with diabetes in the region. Telescreening was especially effective in diagnosing nonproliferative retinopathy, which enabled early treatment. It was also effective in having fewer cases referred for follow-up, thereby decreasing the workload of ophthalmologists at the regional hospital. A survey of patients revealed high satisfaction levels with the screening.
Another large observational study (n=1,147; 90% with diabetes type 2) evaluated the feasibility of DR telescreening, as well as its effectiveness in estimating DR prevalence and in outreach. It was conducted in France in 2009.121 The patients were screened consecutively upon recruitment over a period of 18 months, using a nonmydriatic fundus camera with three 45° digital images per eye. The images were subsequently transmitted to an ophthalmology department in a referral hospital for grading. Of the total study population, 45% never had a fundus examination before this project. About 30% of them required referral to an ophthalmologist or had unreadable photographs. Findings from this study demonstrated the usefulness of telescreening for DR in terms of identifying patients requiring complete eye examinations.
Three separate articles were published from a regional network of telescreening centers in the Île-de-France in France, OPHDIAT. The network was established in 2004 and continues to be operational. Its primary objective is to provide comprehensive telescreening fundus examinations for diabetic patients on an annual basis. The centers are linked to a central server, the OPHDIAT Reading Centre, at reference hospitals. Each screening center is staffed by trained nurses and orthoptists and equipped with a digital, nonmydriatic camera. All images are read and graded by ophthalmologists.
The first report, published in 2009,122 was based on a retrospective study of 500 case reports before and after the implementation of OPHDIAT in five reference hospitals. At each hospital, 100 case reports (50 before and 50 after selected at random) were assessed to determine the average proportion of people screened per site (as an indication of successful outreach), the duration of fundus examinations, and their effectiveness in terms of human resource use. The results demonstrated an improvement in DR screening outreach (50.4% before and 72.4% after), whereas the prevalence of DR was 11.1% before and 12.7% after (although the difference is not statistically significant), and a shorter time for the ophthalmologist to make a diagnosis (0.9 half-day before and 0.32 half-day after), suggesting greater efficiency in telescreening compared with in-person examination.
Two other articles were published in 2012 from the OPHDIAT program. The first was a report on clinical and biological risk factors associated with DR.123 It was based on an analysis of 254 patients who were studied in detail in order to determine the staging or progression of DR. At the initial screening, 236 showed no signs of DR, whereas 18 patients did. Excluding the 18 patients having preexisting DR, the 3-year DR follow-up focused on the remaining 236 who were free of DR at baseline. Of this latter group, 33 developed DR (29 with mild NPDR and 4 with moderate NPDR). None of these had proliferative DR at the end of 3 years. The general conclusion from this study suggests that diabetes can be controlled and that telescreening for DR is effective in identifying early stages of DR.
The second publication in 2012 described the results of the 5-year experience of this program.124 Between June 2004 and December 2009, in total, 38,596 patients with diabetes were screened at 17 hospitals, 11 primary healthcare centers, and 2 prisons. Around 73% had either mild or no DR, 26.6% were referred to an ophthalmologist, and 9.9% had nongradable photographs. This failure rate declined over the 5 years (from 10% to 8.2%). Nearly 94% of the photographs were interpreted on the day they were taken. Overall, the total prevalence of DR in this region was 24.3%. Based on the 5-year experience, the authors concluded that DR telescreening (they referred to it as tele-ophthalmology) is a reliable DR screening tool, is well accepted by patients and by ophthalmologists, saves time by not needing to dilate patients' eyes, is convenient because it can be done outside of office visits, and is an effective tool to offset the increase in the population with diabetes and the limited availability of ophthalmologists to serve them.
A retrospective analysis of consecutive cases (n=394) of DR telescreening from 2005 to 2007 in rural Alberta was published in 2010.125 Images were captured by mydriatic, seven-field digital retinal photography and three-dimensional software. These were subsequently transferred to Edmonton for evaluation. Results indicated a 24.9% prevalence rate of NPDR and 2.3% with PDR. Because the screening program was conducted in rural areas, it was credited with saving the patients 450 trips, 1,900 h of travel time, and 180,000 km over a 3-year period. However, a large majority of patients (76.8%) did not follow up or attend referred appointments in a timely manner. Eventually, 87.3% did so.
Another retrospective study (n=1,223) evaluated the effectiveness of telescreening for DR in a primary care setting in Spain over a 12-month period (published in 2010).126 The providers were general practitioners specially trained to evaluate nonmydriatic images for grading DR using the International Classification for Diabetic Retinopathy. The results were assessed for specificity and sensitivity. Overall, 24% were found to have DR, and 2% of the images were unreadable. Subsequent evaluation by ophthalmologists on a subset sample determined that “the sensitivity [true positive rate] of GPs [general practitioners] for detecting diabetic retinopathy was 90.9%; the sensitivity for detecting treatable lesions was 99.2%.”126
An observational study (n=676) conducted in Mexico in 2011 was aimed at measuring coverage of DR screening in primary care settings and agreement between ophthalmologists and family physicians.127 Mydriatic photography was conducted among diabetic patients in three urban primary care health centers. The study revealed substantial variations in coverage of DR screening between the clinics. However, the rate of pathological findings detected from retinal photographs by family physicians was 27%, but only 9% by ophthalmologists. These rates improved in year 2. There was an overall sensitivity of 97% and specificity of 80%.
A prospective analysis of data from a large screening program from 2007 to 2010 (n=1,311) was conducted in Peru.128 The results were published in 2011. Screening was conducted by a nurse using a nonmydriatic fundus camera. Patients were placed in a dark room to allow natural pupil dilation. The purpose of the study was to estimate the prevalence rate of DR among patients with type 2 diabetes. More than one-half, or 58.3%, of the participants never had an ophthalmological evaluation before. To ensure image quality, the Vanderbilt Ophthalmic Imaging Center reviewed images and grading and observed an 86% concurrence (or matching) rate between centers during the first year. DR was detected in 23.1% of patients (20.3% NPDR and 2.8% PDR). Prevalence rates increased with duration of diabetes, arterial hypertension, neuropathy, or renal complication. Also, DR impairment peaked in the 51–70-year age group. However, DR prevalence did not vary by gender. In those with DR, blindness was twice as common and low vision was more prevalent, compared with their counterparts. The analysis demonstrated that retinal telescreening was feasible and efficient, allowing specialists to intervene promptly among those with the most severe diseases requiring vitrectomy surgery and laser photocoagulation.
A retrospective cohort study (n=980) tracked the predictors and progression of DR among Alberta First Nations communities over a 10-year period, from 1999 to 2009.129 Results were published in 2012. Eligibility for inclusion in the study was based on the availability of gradable retinal photographs, two or more photographic records, and an initial screening. Study subjects received a minimum of two screenings during the study period, which included serial laboratory testing and stereoscopic mydriatic photography of the retina. At baseline 20.7% had DR: 18.3% NPDR and 2.5% PDR. A small minority of the study population experienced progression of DR over time, which occurred at a median of 7.6 years, and it was associated with poor glycemic control and hypertension. Ironically, these two factors are controllable. The authors concluded that “targeted individualized care to reduce blood pressure and control blood sugars could reduce the progression of diabetic retinopathy. …”129
The effectiveness of telescreening in identifying and referring undetected vision-disease in a homeless population was investigated in a U.S. descriptive study (n=341) in 2012.130 The study collected screening data on visual acuity, blood pressure, pulse/oxygen saturation, BMI, and intraocular pressure among participants at soup kitchens and community shelters. Apparently, the researchers did not use a probability sampling scheme for representing the target population. Hence, there is no way of telling the extent to which this sample represents the larger population of homeless people. At any rate, this at-risk population, with limited access to standard ophthalmic care, was screened using a nonmydriatic retinal camera for image capture and transfer to an offsite clinic for evaluation. Ocular disease was detected in 105 patients (or 30.8%), including glaucoma (n=34), cataracts (n=22), DR (n=5), optic atrophy (n=1), age-related macular degeneration (n=1), and other retinal disease (n=43) with visual impairment (10%). These estimates were about 2.5 times higher for this population than the national average in other studies. Poor image quality was encountered in 13 examinations because of small pupil size and ocular opacity, which prevented a diagnosis and required patient referral for a regular office examination. The average screening cost was about $37.50 per patient, which demonstrated a priori the cost-effectiveness of this modality of screening for those with limited medical access and no financial resources.
A descriptive cross-sectional study (n=2,435 patients with diabetes: 2,376 with type 1 and 59 with type 2) was conducted from 2006 to 2009 in Spain and published in 2012.131 This study evaluated the benefits of telescreening in terms of early diagnosis, treatment, and communication between primary care providers and ophthalmologists. A trained nurse used a nonmydriatic camera to take three 45° retinal photographs, and two ophthalmologists evaluated the images at a viewing station at the reference hospital. An overall DR prevalence rate of 17.9% was observed, of whom 80.7% had mild to moderate NPDR, 12.2% had severe NPDR, and 2.3% had PDR. In addition, there was a general incidence of diabetic maculopathy of 4.8% across all levels of DR over the study period. Most of the screenings were performed without dilation, except for 127 patients who required a drop of tropicamide in order to obtain acceptable images from 86 patients. The remaining 41 with unacceptable image quality were referred to a specialty center. The two primary benefits realized from telescreening were (1) avoidance of more expensive conventional screening and (2) the timely detection, diagnosis, and treatment for DR. The turnaround time was 15 days from photography to treatment, whereas conventional assessments using (mydriasis, slit lamp, and 78 D lens) had a 3-month waiting list, followed by 2–3 months of additional waiting for treatment.
A prospective evaluation (n=158) of retinal imaging by trained certified retinal imagers was conducted in the Joslin Vision Network in 2012.132 It was aimed at evaluating the ability of certified retinal imagers to detect and grade DR. Their grading was compared with that of optometrists. Sight-threatening DR (stDR) images of 316 eyes were taken and immediately graded by imagers, resulting in 48 (15%) images being classified as stDR. “The sensitivity and specificity of identifying stDR at the time of imaging by a certified imager is 1.00 [100%] and 0.97 [97%] respectively.”132 There was 100% agreement between imagers and reads regarding ungradable images, which accounted for 12% of all cases. Imagers identified 48 images with stDR, whereas optometrist readers reclassified 6 as mild NPDR, yielding 88% accuracy in identifying stDR. Of note in this study is that imagers had BA degrees, had no previous medical experience, and had received an intensive 3-day training program involving camera and software usage, diabetes, ocular anatomy, DR, review of (non-)diseased eyes, and common eye disorders. The primary conclusion here is that lower-cost imagers can perform well and thus free ophthalmologists for more complex interventions within their domain.
A retrospective analysis of a large electronic database (n=14,866) from the Finnish Register of Visual Impairment was conducted in 2013 and published in 2014.133 It compared mobile eye screening with traditional models. In Finland, all patients with diabetes are offered free access to DR screening via fundus photography. Imaging technicians record the images using a fundus mydriatic camera and transfer them to a central server, where nurses prescreen the images. When DR or other abnormalities are detected, the images are forwarded to an ophthalmologist for follow-up. Results from 5 years of telescreening of patients with either type 1 or type 2 diabetes revealed that patients with DR were being screened annually and that patients who needed further treatment were also referred to the reference hospital. Evaluations resulted in no DR detected (43%), mild DR (23%), moderate or severe DR (31%), and PDR (3%). Those requiring treatment for PDR or macular edema decreased from 5% in 2007 to 3% in 2011. Nurse-read examinations increased from 46% in 2007 up to 74% in 2011, freeing ophthalmologists for more complex interventions and reducing screening costs. Greater nurse readings still yielded 95–100% accuracy, when compared with those of ophthalmologists. Time delays from photography to hospital treatment decreased from an average of 127 days for conventional screening down to 75 days for telescreening. Overall DR impairment decreased 86% (1.8 to 0.25/100,000 patients) with the intervention versus only 35% (2.3 to 1.5/100,000 patients) with conventional screening. This analysis demonstrated faster treatment, higher-quality images, reduced DR impairment, and use of non-MD clinicians for screening.
DR Screening: Cost
Three studies investigated the economic effects of DR screening in terms of cost savings, all U.S.-based and published in 2012–2013.
A cost savings analysis of DR telescreening was conducted on a program in a small, remote, rural, mountainous, and economically depressed community in West Virginia from 2003 to 2009.134 The results were published in 2012. Of the 937 residents using the Tug Regional Medical Center for routine visits, 659 were in need of ophthalmic screening. Fundus photographs were taken by a nurse during routine clinical visits using a nonmydriatic camera and the images were forwarded to an offsite ophthalmologist for interpretation. Of the 659 images, 288 (or 43.7%) readings were abnormal, but only 195 required follow-up with an ophthalmologist located an hour away. Cost savings estimates considered travel, missed work, and the Medicare payment for binocular screening. Total cost savings over the 7-year period were calculated at $71,189.28 (after subtracting $23,940.00 for the fundus camera and additional expenses), resulting in an average cost savings of $153.43 per patient. Although a cost savings was gained, many variables were not addressed, such as false-positives and -negatives (specificity and sensitivity) and patient compliance. Nonetheless, using a minimum wage of $7.25 for residents in this community may yield a very different cost benefit in settings where wages are higher.
The second was a cost analysis (n=611) that compared a “telemedicine-based” retinal imaging evaluation with conventional ophthalmic fundus examination of diabetic patients.135 All patients were users of a Federally Qualified Community Health Center. A nurse took the images using a nonmydriatic fundus camera and saved them on a network server. An offsite ophthalmologist read the images. The costs for standard care were based on 2009 Medicaid reimbursement rates, whereas the cost of the telemedicine systems included per patient cost for medical assistant, ophthalmologist, capital cost (equipment and training), maintenance, and transportation. The cost of the traditional method of DR screening was $77.80 per patient, whereas the telescreening cost was $40.40.
The third was a cost-effectiveness analysis of telescreening for DR in a VHA population based on a simulation model of the experience of 900 patients with type 1 or type 2 diabetes.136 The model simulated the progress of disease and testing decisions involved in “no screening, teleretinal screening, or ophthalmologist screening,” whereas the treatment is a form of laser surgery. Based on this model, “telescreening was cost-effective under most conditions.”136 It may be noted that a much earlier (2000) cost-utility analysis suggested a more tailored timing for screening intervals among patients with type 2 diabetes to fit individual circumstances would be warranted.137
Summary and Conclusions
Our review of the telemedicine intervention in all manifestations of diabetes (telediabetes) revealed a closed loop system of care composed of a number of stages, from patient to provider and return, as shown in Figure 1. In Stage 1, patients collect their own diabetes-related data (including insulin dosage, glucose levels, body weight, level of physical activity, etc.). They may use glucose meters and short questionnaires. One variant of the entry point to this telediabetes circuit involves nurse-initiated telephone calls to assess, monitor, and motivate patients. In Stage 2, patients transmit the data electronically to a call center or clinic, staffed by trained nurses or a nurse-lead team. In Stage 3, these data are stored, collated, and processed by various algorithms, display time trends, and trigger alarms when significant deviations in values are exceeded. Patients are alerted to these situations and given explicit guidance and assistance. Nurses may communicate with physicians, as indicated by the data, who will provide medical assistance or directions to receive in-person care. In addition, nurses may provide individualized education/motivational information directed toward encouraging patients to adopt healthy life styles. In DR, the focus is on screening underserved patient populations in their respective communities and periodic monitoring of patients with diabetes after their initial visit with an ophthalmologist for early detection of complications and prompt treatment. In brief, the core functions in telediabetes, teleGDM, and teleDR include the collection, storage, analysis, and retrieval of clinically relevant information and the attendant actions pursuant to the findings from these data.
Fig. 1.
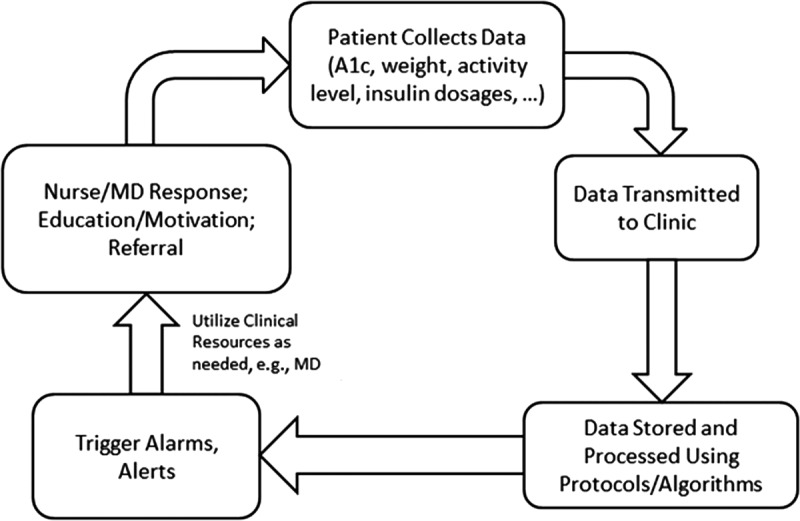
The telediabetes closed loop system. A1c, glycated hemoglobin A1c.
Our analysis of the scientific evidence aimed at identifying and explaining the merit of telediabetes was predicated on two coterminous assumptions—namely, (1) credibility of empirical findings derives from the application of sound and robust research designs and methodologies and (2) the relevance of these findings for policy development and clinical decision-making derives from their specificity in terms of the various parameters of the applications that were assessed as well as their context. Hence, we selected for our review and analysis only robust/rigorous studies, defined operationally as RCTs or other research designs approximating an RCT and sample sizes of 150 or more cases. Few exceptions were warranted vis-à-vis sample size when the research was deemed particularly significant or innovative. On the other hand, surveys and retrospective record reviews are the methodologies of choice for estimating population-based values such as prevalence and incidence rates. Finally, because of the rapid obsolescence of the underlying technology, we limited our time purview to materials published from January 2005 to December 2013.
Working within publication constraints, we excerpted and included information on the contexts, target populations, methodologies, and intervention details for each study sufficient to explicate the logical and empirical links between specific inputs and outputs. This approach was deemed appropriate because of our observation that the effects of the telemedicine intervention in chronic disease management, and perhaps more generally, cannot be fully understood from overarching reviews or even meta-analyses that are based on categorical conceptions of telemedicine (e.g., stated simply as telemedicine versus in-person care) or collective nuanced conclusions. The search for policy-relevant evidence in telemedicine research should not be aimed at making blanket conclusions on the merits, or lack thereof, resulting from inputting multiple studies of various types into a “statistical black box” from which conclusions emerge. Indeed, the literature should not be organized to draw conclusions simply as a voting scheme or statistical process to produce categorical or nuanced findings or outputs in one of three columns—positive, negative, or neutral—in response to a single hypothesis.
As with studies of the telemedicine interventions in other chronic diseases, there were variations in the definition of telediabetes from study to study in terms of setting, technology, staffing, duration, frequency, and target population. The empirical studies investigated the effects of various configurations of these variables on various combinations of outcome measures. In addition to feasibility, measures of effects included intermediate outcomes (use of service, compliance) and health outcomes (glycemic and lipid controls, weight, diet and exercise). We used the latter variables as surrogate outcome measures because of their correlation with diabetes control and functional performance.
Neither the telediabetes intervention nor its outcomes should be viewed as unidimensional in nature. Hence, the most meaningful way to interpret the empirical evidence from this research is to consider the findings from each study in the context of a complex matrix of inputs and outputs. Viewed this way, the results from specific studies provide partial answers to our understanding of the complex sets of relationship between inputs and outputs in telemedicine research. This is not a puzzle in a classical sense. Instead, it constitutes an emerging pattern of cause-and-effect relationships in a multidimensional space. From policy and programmatic perspectives, this approach would enable us to identify optimal configurations of technology, human resources, and other structural attributes of telemedicine as they pertain to costs and benefits.
In terms of technology, the telephone was the predominant tool for connectivity in studies of telediabetes, whereas the Internet was the prevailing mode in connectivity for teleretinopathy. Telephone connectivity entailed various types and levels of sophistication, including interactive voice recognition and automated capture and transmission of data, as well as smartphones and applications on mobile phones. Some studies used a combination of telephones and Internet for connectivity. Data-gathering technology is a critical component in telemonitoring/telescreening patients with diabetes or its retinopathy. The glucose meter is the usual tool for glucose monitoring, as is nonmydriatic photography in retinopathy. In terms of personnel, the vast majority of studies in telediabetes used nurse practitioners, individually or in teams, as the lead providers who received the data from the patients and also provided coaching and educational materials. Few studies used dietitians and educators in combination with nurses. Nurses also conducted teleDR photography, as did orthoptists and trained photographers. The duration of the study periods varied from 1 month to 5 years.
• The telephone was the method of choice for connectivity in telediabetes, sometimes in combination with the Internet.
• The typical providers are nurses, sometimes in teams with other specially trained personnel.
Initially, we organized the findings by diabetes type in the study population (i.e., those with type 1 and/or type diabetes, GDM, and DR). We did not differentiate the studies any further than this classification, even though some studies selected patients on the basis of severity, complications, age, and SES. Finally, we organized the empirical findings on the basis of two sets: intermediate outcomes and clinical outcomes. On the other hand, the merit of teleDR is assessed primarily in terms of effectiveness as a screening/monitoring tool. Hence its outcomes pertain mostly to sensitivity and specificity.
The ultimate aims of telediabetes, teleGDM, and teleDR are (1) glycemic control through various means, including glucose monitoring, compliance with prescribed medication regimen, and the adoption of healthy life styles and (2) early detection of disorders and prompt treatment. Changes in life style are critical for overweight/obese individuals and for those who lead a sedentary life style. Because of their higher risk for DR and its associated complications, it is also important for patients with diabetes to have periodic retinopathy screening and monitoring for early detection of complications and prompt treatment of problems.
We reported the results of four studies that focused on intermediate outcomes in telediabetes for type 1 and/or type 2 diabetes. The empirical findings from these studies are remarkably consistent in that all reported positive results, albeit based on only four studies.35–38 The effects were measured in terms of reduction in use of service (decrease of 44% in rehospitalization among VHA patients receiving comprehensive case management).37 In addition, there was evidence of increased adherence to periodic assessment35 and average annual savings of $2,816 among patients using a Federally Qualified Health Center.38
• Cost savings among users of a Federally Qualified Health Center of $2,816
• Participation in telediabetes increased adherence to prescribed testing.
In total, 16 studies that met the inclusion criteria for this analysis investigated the effects of telediabetes on diabetes-related outcomes, defined here as control over glycemic level, blood pressure, LDL, and body weight. These studies were conducted in 10 countries (six in the United States, two in France, and one each in Finland, Spain, Italy, the United Kingdom, The Netherlands, India, Korea, and Taiwan). Eight were based on RCT design, three case-control, and two record review, and the remaining three were observational studies. Sample size varied from 146 to 1,797. In addition, this set included 9 VHA studies and 16 separate reports from the IDEATel project.
With minor exceptions, conclusions from studies pertaining to the effects of telediabetes on related clinical and behavioral measures are remarkably consistent and positive. Outcomes were measured in terms of glycemic control (A1c), blood pressure, body weight, and LDL. It is interesting that a small percentage difference in A1c (for example, a decrease from 6.5% to 5.6%) means an actual change in diagnosis from diabetes to normal. An A1c level of 4.5–5.6% is considered normal, whereas 5.7–6.4% is prediabetes, and ≥6.5% is diagnosed as diabetes.
The data summarized in Table 2 reveal a near-universal trend of A1c decline as a result of patient active participation in a nurse-led regular telediabetes program based on telephonic contacts with patients to help them monitor their glucose levels, titrate their medication, and adhere to an appropriate diet and exercise regimen. There were two exceptions. One study of the effects of a supplemental telephonic disease management program among an underserved, predominantly Hispanic and African American low-income (Medicaid) population reported no significant effects of telediabetes on clinical or behavioral outcomes.42 Depression was highly prevalent in both the intervention and control groups. A study from The Netherlands (that also included patients with depression) focused on Web-based cognitive therapy for only 1 month. As in the study above, similar neutral findings regarding the effect on A1c were reported.46 The findings from both studies are open to different interpretations. Of course, the 1-month duration of the study in The Netherlands is far too short to expect any reliable results. But, the findings from the U.S. study may be explained differently. One plausible explanation may relate to a hierarchy of need, that is, patients suffering from depression may be more in need of dealing with their depression than their diabetes.
• With minor exceptions, telediabetes resulted in improved diabetes-related outcomes.
• Weight monitors are effective in weight control, especially among the young.
• Patients with depression may have higher priorities and may not participate actively in telemonitoring.
In addition to these studies, there were 9 VHA studies and 16 reports from the IDEATel project.
The findings from the VHA studies were positive in terms of A1c and other clinical indicators. In addition, researchers reported several other kinds of effects, including decline in mortality,64 improvement in physical and social functioning,56 enhanced patient perceptions/knowledge,63 and reduced use of service.57,58
• Participation in the VHA home monitoring program reduced hospital re-admissions in the VHA by 44%.
• VHA studies reported improved adherence to glycemic testing, improved physical and social functioning, a decline in mortality, and reduced use of service.
The 5-year IDEATel project produced 16 articles encompassed several substudies, both short term (1 year) and long term (3 and 5 years). Empirical findings from this project show consistent improvements in glucose levels, blood pressure, and LDL on the part of the participants. These effects tended to increase with time (i.e., the longer patients remained in the program, the more appreciable the effects). In addition to clinical improvements, special topic analysis of data from the project indicated improvements in psychosocial outcomes,71 preference for automated and in-home learning by participants,78 and improved task performance and slowing of physical decline79 and cognitive decline,81 as well as reduced comorbidities.82 On the other hand, the intervention did not prove to be cost-effective, which was explained by the authors as being the result of customized expensive equipment.
• Participation in the IDEATel project among low-income and minority Medicare beneficiaries resulted in a decline in diabetes-related clinical measures.
• Expensive technology may reduce, if not eliminate, cost-effectiveness of telediabetes.
Only three GDM studies met the inclusion criteria. A “weight gain tracker” linked to a Web site and mobile phones proved to be useful but did not result in any definitive findings.99 However, another study reported better glycemic control among women receiving a glucose meter and reporting values every week. The same group experienced a lower rate of cesarean section as well as fewer macrosomic infants.100 The latter finding were also confirmed in another study.101
• TeleGDM proved to be effective in glycemic control as well as reduced cesarean section deliveries and macrosomic babies.
Interest in regular screening for DR via photography is both strong and widespread. Its appeal derives from two sources: (1) The risk of DR and potential vision loss increase with the duration and severity of diabetes. Hence, early detection and prompt medical intervention are critical to diminishing these risks. (2) The requisite photography is relatively inexpensive and is easy to implement in community settings, away from clinics and private offices. The evidence from several very large screening programs confirms the reliability of nonmydriatic photography as a tool for community screening of DR. The percentage of gradable images varied from a low of 65% to a high of 100%. Both false-positive and false-negative rates were low, and the effectiveness of this mode of screening in outreach has been universally confirmed. Additional benefits were accrued in terms of expediting the referral to ophthalmologists when indicated.
• Nonmydriatic photography is easy to administer by trained nurses, orthoptists, and photographers, is relatively inexpensive, is fast, and is reliable.
• TeleDR improves outreach, early detection, and prompt treatment of DR.
The evidence presented and conclusions pertaining to telediabetes derive from a targeted selection of studies published over the last decade. These studies met our criteria in terms of scientific rigor and statistical power. The majority of these studies focused on type 1 and type 2 diabetes and retinopathy. Considerably fewer studies focused on GDM. The studies included here were conducted in a wide variety of settings based on a variety of interventions for a wide spectrum of conditions and populations. Notable also is the global distribution of pertinent telediabetes research, reflecting the global concern with diabetes and its sequelae. The extensive variety of institutional and geographic settings, populations, interventions, and the like, although perhaps not providing the depth required by some readers to draw more definitive conclusions regarding the benefits and costs of telediabetes, as well as the breadth of the research dimensions, leads us to conclude that telediabetes warrants a prominent place in the medical armamentarium that must be marshaled to face and perhaps change the future path of diabetes.
Acknowledgments
The research leading to this publication was supported by the National Library of Medicine, which is gratefully acknowledged. M.A.W. is the recipient of grant NEI 1K23EY023596-01 from the National Institutes of Health.
Disclosure Statement
R.L.B. serves on an advisory board for Voluntis. G.W.S., B.R.S., and M.A.W. declare no competing financial interests exist.
References
- 1.Bashshur RL, Shannon GW, Smith B, et al. The empirical foundations of telemedicine intervention for chronic disease management. Telemed J E Health 2014;20:769–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adeghate E. Diabetes mellitus—Multifactorial in aetiology and global in prevalence. Arch Physiol Biochem 2001;109:197–199 [DOI] [PubMed] [Google Scholar]
- 3.Ahmed AM. History of diabetes mellitus. Saudi Med J 2002;23:373–378 [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. National diabetes fact sheet. 2011. Available at www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf (last accessed February25, 2015)
- 5.Alberti KGMM. Screening and diagnosis of prediabetes: Where are we headed? Diabetes Obes Metab 2007;9:12–16 [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care 2015;38(Suppl 1):S8–S16 [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Prediabetes. Available at www.cdc.gov/diabetes/basics/prediabetes.html (last accessed January6, 2015)
- 8.Davis JE, Mcdonald JM, Jarett L. A high-performance liquid chromatography method for hemoglobin A1c. Diabetes 1978;27:102–107 [DOI] [PubMed] [Google Scholar]
- 9.National Diabetes Statistics Report. 2014. Available at www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf (last accessed January6, 2015)
- 10.Aguiree F, Brown A, Cho NH, Dahlquist G, Dodd S, Dunning T, Hirst M, Hwang C, Magliano D, Patterson C, Scott C, Shaw J, Soltesz G, Usher-Smith J, Whiting D. IDF diabetes atlas, 6th ed. Brussels: International Diabetes Federation, 2013 [Google Scholar]
- 11.American Diabetes Association. Statistics about diabetes. Available at www.diabetes.org/diabetes-basics/statistics/ (last accessed December24, 2014)
- 12.Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP) description of lifestyle intervention. Diabetes Care 2002;25:2165–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Diabetes Association. The cost of diabetes. Available at www.diabetes.org/advocacy/news-events/cost-of-diabetes.html (last accessed December24, 2014)
- 14.World Health Organization. Diabetes: The cost of diabetes. Available at www.who.int/mediacentre/factsheets/fs236/en/ (last accessed December24, 2014)
- 15.Woolliscroft J, Koelling TM. Redesigning the management of chronic illness. Telemed J E Health 2004;10:118–121 [DOI] [PubMed] [Google Scholar]
- 16.Daaleman TP. The medical home: Locus of physician formation. J Am Board Fam Med 2008;21;451–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenthal TC. The medical home: Growing evidence to support a new approach to primary care. J Am Board Fam Med 2008;21:427–440 [DOI] [PubMed] [Google Scholar]
- 18.Miller WL. Patient-centered medical home (PCMH) recognition: A time for promoting innovation, not measuring standards. J Am Board Fam Med 2014;27:309–311 [DOI] [PubMed] [Google Scholar]
- 19.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: What does it mean? (or it takes at least two to tango). Soc Sci Med 1997;44:681–692 [DOI] [PubMed] [Google Scholar]
- 20.Peters A. Overcoming challenges and improving outcomes in the management of type 2 diabetes. J Managed Care Med 2012;15:52–55 [Google Scholar]
- 21.Worswick J, Wayne SC, Bennett R, et al. Improving quality of care for persons with diabetes: An overview of systematic reviews—What does the evidence tell us? Syst Rev 2013;2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nunn E, King B, Smart C, Anderson D. A randomized controlled trial of telephone calls to young patients with poorly controlled type 1 diabetes. Pediatr Diabetes 2006;7:254–259 [DOI] [PubMed] [Google Scholar]
- 23.Mollon B, Holbrook AM, Keshavjee K, et al. Automated telephone reminder messages can assist electronic diabetes care. J Telemed Telecare 2008;14:32–36 [DOI] [PubMed] [Google Scholar]
- 24.Sarkar U, Handley MA, Gupta R, et al. Use of an interactive, telephone-based self-management support program to identify adverse events among ambulatory diabetes patients. J Gen Intern Med 2008;23:459–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Istepanian RS, Zitouni K, Harry D, et al. Evaluation of a mobile phone telemonitoring system for glycaemic control in patients with diabetes. J Telemed Telecare 2009;15:125–128 [DOI] [PubMed] [Google Scholar]
- 26.Salzsieder E, Augstein P. The Karlsburg Diabetes Management System: Translation from research to eHealth application. J Diabetes Sci Technol 2011;5:13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klug C, Bonin K, Bultemeier N, et al. Integrating telehealth technology into a clinical pharmacy telephonic diabetes management program. J Diabetes Sci Technol 2011;5:1238–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciemins E, Coon P, Peck R, et al. Using telehealth to provide diabetes care to patients in rural Montana: Findings from the Promoting Realistic Individual Self-Management Program. Telemed J E Health 2011;17:596–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams ED, Bird D, Forbes AW, et al. Randomised controlled trial of an automated, interactive telephone intervention (TLC Diabetes) to improve type 2 diabetes management: Baseline findings and six-month outcomes. BMC Public Health 2012;12:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiel R, Kaps A, Bieber G. Electronic health technology for the assessment of physical activity and eating habits in children and adolescents with overweight and obesity IDA. Appetite 2012;58:432–437 [DOI] [PubMed] [Google Scholar]
- 31.Leichter SB, Bowman K, Adkins RA, Jelsovsky Z. Impact of remote management of diabetes via computer: The 360 Study—A proof-of-concept randomized trial. Diabetes Technol Ther 2013;15:434–438 [DOI] [PubMed] [Google Scholar]
- 32.Rossi MC, Nicolucci A, Lucisano G, et al. Impact of the “Diabetes Interactive Diary” telemedicine system on metabolic control, risk of hypoglycemia, and quality of life: A randomized clinical trial in type 1 diabetes. Diabetes Technol Ther 2013;15:670–679 [DOI] [PubMed] [Google Scholar]
- 33.Rossi MC, Perozzi C, Consorti C, et al. An interactive diary for diet management (DAI): A new telemedicine system able to promote body weight reduction, nutritional education, and consumption of fresh local produce. Diabetes Technol Ther 2010;12:641–647 [DOI] [PubMed] [Google Scholar]
- 34.Rossi MC, Nicolucci A, Di Bartolo P, et al. Diabetes Interactive Diary: A new telemedicine system enabling flexible diet and insulin therapy while improving quality of life: An open-label, international, multicenter, randomized study. Diabetes Care 2010;33:109–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orr PM, McGinnis MA, Hudson , et al. A focused telephonic nursing intervention delivers improved adherence to A1c testing. Dis Manage 2006;9:277–283 [DOI] [PubMed] [Google Scholar]
- 36.Jia H, Feng H, Wang X, Wu SS, Chumbler N. A longitudinal study of health service utilization for diabetes patients in a care coordination home-telehealth programme. J Telemed Telecare 2011;17:123–126 [DOI] [PubMed] [Google Scholar]
- 37.Graham J, Tomcavage J, Salek D, et al. Postdischarge monitoring using interactive voice response system reduces 30-day readmission rates in a case-managed Medicare population. Med Care 2012;50:50–57 [DOI] [PubMed] [Google Scholar]
- 38.Fischer HH, Eisert SL, Everhart RM, et al. Nurse-run, telephone-based outreach to improve lipids in people with diabetes. Am J Managed Care 2012;18:77–84 [PubMed] [Google Scholar]
- 39.Harno K, Kauppinen-Mäkelin R, Syrjäläinen J. Managing diabetes care using an integrated regional e-health approach. J Telemed Telecare 2006;12(Suppl 1):13–15 [DOI] [PubMed] [Google Scholar]
- 40.Rodríguez-Idígoras MI, Sepúlveda-Muñoz J, Sánchez-Garrido-Escudero R, et al. Telemedicine influence on the follow-up of type 2 diabetes patients. Diabetes Technol Ther 2009;11:431–437 [DOI] [PubMed] [Google Scholar]
- 41.Berg GD, Wadhwa S. Diabetes disease management results in Hispanic Medicaid patients. J Health Care Poor Underserved 2009;20:432–443 [DOI] [PubMed] [Google Scholar]
- 42.Anderson DR, Christison-Lagay J, Villagra V, et al. Managing the space between visits: A randomized trial of disease management for diabetes in a community health center. J Gen Intern Med 2010;25:1116–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis RM, Hitch AD, Salaam MM, et al. TeleHealth improves diabetes self-management in an underserved community: Diabetes TeleCare. Diabetes Care 2010;33:1712–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jordan RE, Lancashire RJ, Adab P. An evaluation of Birmingham Own Health® telephone care management service among patients with poorly controlled diabetes. A retrospective comparison with the General Practice Research Database. BMC Public Health 2011;11:707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Musacchio N, Lovagnini Scher A, Giancaterini A, et al. Impact of a chronic care model based on patient empowerment on the management of type 2 diabetes: Effects of the SINERGIA programme. Diabet Med 2011;28:724–730 [DOI] [PubMed] [Google Scholar]
- 46.van Bastelaar KM, Pouwer F, Cuijpers P, et al. Web-based depression treatment for type 1 and type 2 diabetic patients: A randomized, controlled trial. Diabetes Care 2011;34:320–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Charpentier G, Benhamou PY, Dardari D, et al. The Diabeo software enabling individualized insulin dose adjustments combined with telemedicine support improves HbA1c in poorly controlled type 1 diabetic patients: A 6-month, randomized, open-label, parallel-group, multicenter trial (TeleDiab 1 Study). Diabetes Care 2011;34:533–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Franc S, Borot S, Ronsin O, et al. Telemedicine and type 1 diabetes: Is technology per se sufficient to improve glycaemic control? Diabetes Metab 2014;40:61–66 [DOI] [PubMed] [Google Scholar]
- 49.Stamp K, Allen N, Lehrer S, et al. Telehealth program for Medicaid patients with type 2 diabetes lowers hemoglobin A1C. J Managed Care Med 2012;15:38–49 [Google Scholar]
- 50.Tang PC, Overhage JM, Chan AS, et al. Online disease management of diabetes: Engaging and Motivating Patients Online With Enhanced Resources-Diabetes (EMPOWER-D), a randomized controlled trial. J Am Med Inform Assoc 2013;20:526–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ellis DA, Naar-King S, Chen X, et al. Multisystemic therapy compared to telephone support for youth with poorly controlled diabetes: Findings from a randomized controlled trial. Ann Behav Med 2012;44:207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kesavadev J, Shankar A, Pillai PBS, et al. Cost-effective use of telemedicine and self-monitoring of blood glucose via Diabetes Tele Management System (DTMS) to achieve target glycosylated hemoglobin values without serious symptomatic hypoglycemia in 1,000 subjects with type 2 diabetes mellitus—A retrospective study. Diabetes Technol Ther 2012;14:772–776 [DOI] [PubMed] [Google Scholar]
- 53.Chen L, Chuang LM, Chang CH, Wang CS, et al. Evaluating self-management behaviors of diabetic patients in a telehealthcare program: Longitudinal study over 18 months. J Med Internet Res 2013;15:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown-Guion SY, Youngerman SM, Hernandez-Tejada MA, et al. Racial/ethnic, regional, and rural/urban differences in receipt of diabetes education. Diabetes Educ 2013;39:327–334 [DOI] [PubMed] [Google Scholar]
- 55.Lee BJ, Kim KH, Ku B, et al. Prediction of body mass index status from voice signals based on machine learning for automated medical applications. Artif Intell Med 2013;58:51–61 [DOI] [PubMed] [Google Scholar]
- 56.Chumbler NR, Neugaard B, Kobb R, et al. Evaluation of a care coordination/home-telehealth program for veterans with diabetes health services utilization and health-related quality of life. Eval Health Prof 2005;28:464–478 [DOI] [PubMed] [Google Scholar]
- 57.Chumbler NR, Vogel WB, Garel M, et al. Health services utilization of a care coordination/home-telehealth program for veterans with diabetes: A matched-cohort study. J Ambul Care Manage 2005;28:230–240 [DOI] [PubMed] [Google Scholar]
- 58.Barnett TE, Chumbler NR, Vogel WB, et al. The effectiveness of a care coordination home telehealth program for veterans with diabetes mellitus: A 2-year follow-up. Am J Managed Care 2006;12:467. [PubMed] [Google Scholar]
- 59.Chumbler NR, Neugaard B, Kobb R, et al. An observational study of veterans with diabetes receiving weekly or daily home telehealth monitoring. J Telemed Telecare 2005;11:150–156 [DOI] [PubMed] [Google Scholar]
- 60.Chumbler NR, Mann WC, Wu S, et al. The association of home-telehealth use and care coordination with improvement of functional and cognitive functioning in frail elderly men. Telemed J E Health 2004;10:129–137 [DOI] [PubMed] [Google Scholar]
- 61.Stone RA, Rao RH, Sevick MA, et al. Active care management supported by home telemonitoring in veterans with type 2 diabetes: The DiaTel randomized controlled trial. Diabetes Care 2010;33:478–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wakefield BJ, Holman JE, Ray A, et al. Effectiveness of home telehealth in comorbid diabetes and hypertension: A randomized, controlled trial. Telemed J E Health 2011;17:254–261 [DOI] [PubMed] [Google Scholar]
- 63.Wakefield BJ, Holman JE, Ray A, et al. Outcomes of a home telehealth intervention for patients with diabetes and hypertension. Telemed J E Health 2012;18:575–579 [DOI] [PubMed] [Google Scholar]
- 64.Chumbler NR, Chuang HC, Wu SS, et al. Mortality risk for diabetes patients in a care coordination, home-telehealth programme. J Telemed Telecare 2009;15:98–101 [DOI] [PubMed] [Google Scholar]
- 65.Powers BJ, Olsen MK, Oddone EZ, Bosworth HB. The effect of a hypertension self-management intervention on diabetes and cholesterol control. Am J Med 2009;122:639–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Starren J, Hripcsak G, Sengupta S, et al. Columbia University's Informatics for Diabetes Education and Telemedicine (IDEATel) project: Technical implementation. J Am Med Inform Assoc 2002;9:25–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shea S, Starren J, Weinstock RS, et al. Columbia University's Informatics for Diabetes Education and Telemedicine (IDEATel) project: Rationale and design. J Am Med Inform Assoc 2002;9:49–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shea S, Weinstock RS, Starren J, et al. A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus. J Am Med Inform Assoc 2006;13:40–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trief PM, Morin PC, Izquierdo R, et al. Depression and glycemic control in elderly ethnically diverse patients with diabetes: The IDEATel project. Diabetes Care 2006;29:830–835 [DOI] [PubMed] [Google Scholar]
- 70.Palmas W, Moran A, Pickering T, et al. Ambulatory pulse pressure and progression of urinary albumin excretion in older patients with type 2 diabetes mellitus. Hypertension 2006;48:301–308 [DOI] [PubMed] [Google Scholar]
- 71.Trief PM, Teresi JA, Izquierdo R, et al. Psychosocial outcomes of telemedicine case management for elderly patients with diabetes: The randomized IDEATel trial. Diabetes Care 2007;30:1266–1268 [DOI] [PubMed] [Google Scholar]
- 72.Izquierdo R, Meyer S, Starren J, et al. Detection and remediation of medically urgent situations using telemedicine case management for older patients with diabetes mellitus. Ther Clin Risk Manage 2007;3:485. [PMC free article] [PubMed] [Google Scholar]
- 73.Tudiver F, Wolff LT, Morin PC, et al. Primary care providers' perceptions of home diabetes telemedicine care in the IDEATel project. J Rural Health 2007;23:55–61 [DOI] [PubMed] [Google Scholar]
- 74.Shea S, Weinstock RS, Teresi JA, et al. A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus: 5 year results of the IDEATel study. J Am Med Inform Assoc 2009;16:446–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lai AM, Kaufman DR, Starren J, Shea S. Evaluation of a remote training approach for teaching seniors to use a telehealth system. Int J Med Inform 2009;78:732–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Izquierdo R, Lagua CT, Meyer S, et al. Telemedicine intervention effects on waist circumference and body mass index in the IDEATel project. Diabetes Technol Ther 2010;12:213–220 [DOI] [PubMed] [Google Scholar]
- 77.Palmas W, Shea S, Starren J, et al. Medicare payments, healthcare service use, and telemedicine implementation costs in a randomized trial comparing telemedicine case management with usual care in medically underserved participants with diabetes mellitus (IDEATel). J Am Med Inform Assoc 2010;17:196–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robinson KS, Morin PC, Shupe JAC, et al. Use of three computer training methods in elderly underserved rural patients enrolled in a diabetes telemedicine program. Comput Inform Nurs 2010;28:172–177 [DOI] [PubMed] [Google Scholar]
- 79.Weinstock RS, Brooks G, Palmas W, et al. Lessened decline in physical activity and impairment of older adults with diabetes with telemedicine and pedometer use: Results from the IDEATel study. Age Ageing 2011;40:98–105 [DOI] [PubMed] [Google Scholar]
- 80.Weinstock RS, Teresi JA, Goland R, et al. Glycemic control and health disparities in older ethnically diverse underserved adults with diabetes: Five-year results from the Informatics for Diabetes Education and Telemedicine (IDEATel) study. Diabetes Care 2011;34:274–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luchsinger JA, Palmas W, Teresi JA, et al. Improved diabetes control in the elderly delays global cognitive decline. J Nutr Health Aging 2011;15:445–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shea S, Kothari D, Teresi JA, et al. Social impact analysis of the effects of a telemedicine intervention to improve diabetes outcomes in an ethnically diverse, medically underserved population: Findings from the IDEATel study. Am J Public Health 2013;103:1888–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Trief PM, Izquierdo R, Eimicke JP, et al. Adherence to diabetes self care for white, African-American and Hispanic American telemedicine participants: 5 year results from the IDEATel project. Ethn Health 2013;18:83–96 [DOI] [PubMed] [Google Scholar]
- 84.Veterans Health Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of diabetes mellitus in primary care. Completion date: March2003. Release Date: October 2003. Version 3.0. Available at www.healthquality.va.gov/diabetes/dm_fulltext.pdf (last accessed January7, 2015)
- 85.Ryan EA. Diagnosing gestational diabetes. Diabetologia 2011;54:480–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.American Diabetes Association. What is gestational diabetes. Available at www.diabetes.org/diabetes-basics/gestational/?loc=db-slabnav (last accessed January7, 2015)
- 87.Caro JJ, Getsios D, Caro I, et al. Economic evaluation of therapeutic interventions to prevent type 2 diabetes in Canada. Diabet Med 2004;21:1229–1236 [DOI] [PubMed] [Google Scholar]
- 88.Wier LM, Witt E, Burgess J, Elixhauser A. Hospitalizations related to diabetes in pregnancy, 2008. Statistical Brief #102, December2010. In: Healthcare Cost and Utilization Project (HCUP) statistical briefs. Rockville, MD: Agency for Health Care Policy and Research, 2006. Available at www.ncbi.nlm.nih.gov/books/NBK52649/ (last accessed February25, 2015) [PubMed] [Google Scholar]
- 89.Gillespie P, Cullinan J, O'Neill C, Dunne F. Modeling the independent effects of gestational diabetes mellitus on maternity care and costs. Diabetes Care 2013;36:1111–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Y, Quick WW, Yang W, et al. Cost of gestational diabetes mellitus in the United States in 2007. Popul Health Manage 2009;12:165–174 [DOI] [PubMed] [Google Scholar]
- 91.Landon MB, Mele L, Spong CY, Carpenter SM, Casey B, et al. The relationship between maternal glycemia and perinatal outcome. Obstet Gynecol 2011;117:218–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim SY, England L, Wilson G, Bish C, Satten A, Dietz P. Percentage of gestatational diabetes mellitus attributable to overweight and obesity. Am J Public Health 2010;100:1047–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gillespie P, Cullinan J, O'Neukk G, Dunne F. Modeling the independent effects of gestational diabetes on maternity care and costs. Diabetes Care 2012;36:1111–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kolu P, Raitanen J, Rissanen P, Luoto R. Health care costs associated with gestational diabetes mellitus among high-risk women—Results from a randomised trial. BMC Pregnancy Childbirth 2012;12:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Homko CJ, Santamore WP, Whiteman V, et al. Use of an internet-based telemedicine system to manage underserved women with gestational diabetes mellitus. Diabetes Technol Ther 2007;9:297–306 [DOI] [PubMed] [Google Scholar]
- 96.Pérez-Ferre N, Galindo M, Fernández MD, Velasco V, Runkle I, de la Cruz MJ, et al. The outcomes of gestational diabetes mellitus after a telecare approach are not inferior to traditional outpatient clinic visits. Int J Endocrinol 2010;2010:386941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Homko CJ, Deeb LC, Rohrbacher K, et al. Impact of a telemedicine system with automated reminders on outcomes in women with gestational diabetes mellitus. Diabetes Technol Ther 2012;14:624–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kelders SM, Van Gemert-Pijnen JE, Werkman A, Nijland N, Seydel ER. Effectiveness of a Web-based intervention aimed at healthy dietary and physical activity behavior: A randomized controlled trial about users and usage. J Med Internet Res 2011;13:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Graham ML, Uesugi KH, Niederdeppe J, Gay GK, Olson CM. The theory, development, and implementation of an e-intervention to prevent excessive gestational weight gain: e-Moms Roc. Telemed J E Health 2014;20:1135–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dalfrà MG, Nicolucci A, Lapolla A. The effect of telemedicine on outcome and quality of life in pregnant women with diabetes. J Telemed Telecare 2009;15:238–242 [DOI] [PubMed] [Google Scholar]
- 101.Ferrara A, Hedderson MM, Ching J, Kim C, Peng T, Crites YM. Referral to telephonic nurse management improves outcomes in women with gestational diabetes. Am J Obstet Gynecol 2012;206:491..e1–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thangaratinam S, Rogozińska E, Jolly K, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: Meta-analysis of randomised evidence. BMJ 2012;344:e208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.National Institutes of Health. Diabetic eye disease. 2015. Available at www.nlm.nih.gov/medlineplus/diabeticeyeproblems.html (last accessed February16, 2015)
- 104.National Eye Institute/National Institutes of Health. Facts about diabetic eye disease. 2014. Available at https://www.nei.nih.gov/health/diabetic/retinopathy (last accessed November30, 2014)
- 105.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 106.Virgili G, Parravano M, Menchini F, Evans JR. Anti-vascular endothelial growth factor for diabetic macular oedema. Cochrane Database Syst Rev 2014;10:CD007419. [DOI] [PubMed] [Google Scholar]
- 107.Bhagat N, Zarbin M. Epidemiology, risk factors, and pathophysiology of diabetic retinopathy. In: Bandello F, Zarbin MA, Lantanzio R, Zucchiatti I, eds. Clinical strategies in the management of diabetic retinopathy, a step-by-step guide for ophthalmologists. Heidelberg: Springer, 2014:1–18 [Google Scholar]
- 108.American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care 2015;38(Suppl 1):S1–S94 [PubMed] [Google Scholar]
- 109.Bernardes R, Serranho P, Lobo C. Digital ocular fundus imaging: A review. Ophthalmologica 2011;226:161–181 [DOI] [PubMed] [Google Scholar]
- 110.Meszaros L. Why non-mydriatic cameras will not replace dilated fundus exams. June1, 2012. Available at http://optometrytimes.modernmedicine.com/optometrytimes/news/modernmedicine/modern-medicine-feature-articles/why-non-mydriatic-cameras-will-n?page=full (last accessed January15, 2015)
- 111.Li HK, Horton M, Bursell SE, Cavallerano J, Zimmer-Galler I, Tennant M, et al. Telehealth practice recommendations for diabetic retinopathy. Telemed J E Health 2011;17:814–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.John S, Sengupta S, Reddy SJ, Prabhu P, Kirubanandan K, Badrinath SS. The Sankara Nethralaya mobile teleophthalmology model for comprehensive eye care delivery in rural India. Telemed J E Health 2012;18:382–387 [DOI] [PubMed] [Google Scholar]
- 113.Ahmed R, Petrany S, Fry R, Krasnow M. Screening diabetic and hypertensive patients for ocular pathology using telemedicine technology in rural West Virginia: A retrospective chart review. W V Med J 2012;109:6–10 [PubMed] [Google Scholar]
- 114.Conlin PR, Fisch BM, Cavallerano AA, Cavallerano JD, Bursell S, Aiello LM. Nonmydriatic teleretinal imaging improves adherence to annual eye examinations in patients with diabetes. J Rehabil Res Dev 2006;43:733. [DOI] [PubMed] [Google Scholar]
- 115.Ahmed J, Ward TP, Bursell SE, Aiello LM, Cavallerano JD, Vigersky RA. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Diabetes Care 2006;29:2205–2209 [DOI] [PubMed] [Google Scholar]
- 116.Fonda SJ, Bursell SE, Lewis DG, Garren J, Hock K, Cavallerano J. The relationship of a diabetes telehealth eye care program to standard eye care and change in diabetes health outcomes. Telemed J E Health 2007;13:635–644 [DOI] [PubMed] [Google Scholar]
- 117.Taylor CR, Merin LM, Salunga AM, Hepworth JT, Crutcher TD, O'Day DM, Pilon BA. Improving diabetic retinopathy screening ratios using telemedicine-based digital retinal imaging technology: The Vine Hill Study. Diabetes Care 2007;30:574–578 [DOI] [PubMed] [Google Scholar]
- 118.Massin P, Chabouis A, Erginay A, Viens-Bitker C, Lecleire-Collet A, Meas T, et al. OPHDIAT©: A telemedical network screening system for diabetic retinopathy in the Île-de-France. Diabetes Metab 2008;34:227–234 [DOI] [PubMed] [Google Scholar]
- 119.Boucher MC, Desroches G, Garcia-Salinas R, et al. Teleophthalmology screening for diabetic retinopathy through mobile imaging units within Canada. Can J Ophthalmol 2008;43:658–668 [DOI] [PubMed] [Google Scholar]
- 120.Lemmetty R, Mäkelä K. Mobile digital fundus screening of type 2 diabetes patients in the Finnish county of South-Ostrobothnia. J Telemed Telecare 2009;15:68–72 [DOI] [PubMed] [Google Scholar]
- 121.Tran TH, Rahmoun J, Hui Bon Hoa AA, Denimal F, Delecourt F, Jean JE, Forzy G. [Screening for diabetic retinopathy using a three-field digital nonmydriatic fundus camera in the North of France]. J Fr Ophtalmol 2009;32:735–741 [DOI] [PubMed] [Google Scholar]
- 122.Chabouis A, Berdugo M, Meas T, Erginay A, Laloi-Michelin M, et al. Benefits of Ophdiat®, a telemedical network to screen for diabetic retinopathy: A retrospective study in five reference hospital centres. Diabetes Metab 2009;35:228–232 [DOI] [PubMed] [Google Scholar]
- 123.Perol J, Balkau B, Guillausseau PJ, Massin P. A study of the 3-year incidence of diabetic retinopathy in a French diabetic population seen at Lariboisière Hospital, Paris. Diabetes Metab 2012;38:225–229 [DOI] [PubMed] [Google Scholar]
- 124.Schulze-Döbold C, Erginay A, Robert N, Chabouis A, Massin P. Ophdiat®: Five-year experience of a telemedical screening programme for diabetic retinopathy in Paris and the surrounding area. Diabetes Metab 2012;38:450–457 [DOI] [PubMed] [Google Scholar]
- 125.Nathoo N, Ng M, Rudnisky CJ, Tennant MT. The prevalence of diabetic retinopathy as identified by teleophthalmology in rural Alberta. Can J Ophthalmol 2010;45:28–32 [DOI] [PubMed] [Google Scholar]
- 126.Andonegui J, Serrano L, Eguzkiza A, Berástegui L, Jiménez-Lasanta L, Aliseda D, Gaminde I. Diabetic retinopathy screening using tele-ophthalmology in a primary care setting. J Telemed Telecare 2010;16:429–432 [DOI] [PubMed] [Google Scholar]
- 127.Vargas-Sánchez C, Maldonado-Valenzuela JJ, Pérez-Durillo FT, González-Calvo J, Pérez-Milena A. Cribado de retinopatía diabética mediante retinografía midriática en atención primaria. Salud Publica Mexico 2011;53:212–219 [PubMed] [Google Scholar]
- 128.Villena JE, Yoshiyama CA, Sánchez JE, Hilario NL, Merin LM. Prevalence of diabetic retinopathy in Peruvian patients with type 2 diabetes: Results of a hospital-based retinal telescreening program. Rev Panam Salud Publica 2011;30:408–414 [PubMed] [Google Scholar]
- 129.Rudnisky CJ, Wong BK, Virani H, Tennant MT. Risk factors for progression of diabetic retinopathy in Alberta First Nations communities. Can J Ophthalmol 2012;47:365–375 [DOI] [PubMed] [Google Scholar]
- 130.Shahid K, Kolomeyer AM, Nayak NV, et al. Ocular telehealth screenings in an urban community. Telemed J E Health 2012;18:95–100 [DOI] [PubMed] [Google Scholar]
- 131.Martínez Rubio M, Moya Moya M, Bellot Bernabé A, Belmonte Martínez J. Diabetic retinopathy screening and teleophthalmology [in Spanish]. Arch Soc Esp Oftalmol 2012;87:392–395 [DOI] [PubMed] [Google Scholar]
- 132.Cavallerano JD, Silva PS, Tolson AM, et al. Imager evaluation of diabetic retinopathy at the time of imaging in a telemedicine program. Diabetes Care 2012;35:482–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hautala N, Aikkila R, Korpelainen J, Keskitalo A, Kurikka A, et al. Marked reductions in visual impairment due to diabetic retinopathy achieved by efficient screening and timely treatment. Acta Ophthalmol 2014;92:582–587 [DOI] [PubMed] [Google Scholar]
- 134.Richardson DR, Fry RL, Krasnow M. Cost-savings analysis of telemedicine use for ophthalmic screening in a rural Appalachian health clinic. W V Med J 2012;109:52–55 [PubMed] [Google Scholar]
- 135.Li Z, Wu C, Olayiwola JN, Hilaire DS, Huang JJ. Telemedicine-based digital retinal imaging vs standard ophthalmologic evaluation for the assessment of diabetic retinopathy. Conn Med 2012;76:85–90 [PubMed] [Google Scholar]
- 136.Kirkizlar E, Serban N, Sisson JA, Swann JL, Barnes CS, Williams MD. Evaluation of telemedicine for screening of diabetic retinopathy in the Veterans Health Administration. Ophthalmology 2013;120:2604–2610 [DOI] [PubMed] [Google Scholar]
- 137.Vijan S, Hofer TP, Hayward RA. Cost-utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA 2000;283:889–896 [DOI] [PubMed] [Google Scholar]


