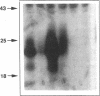
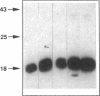
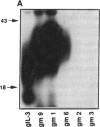
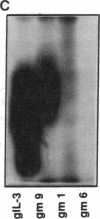
Abstract
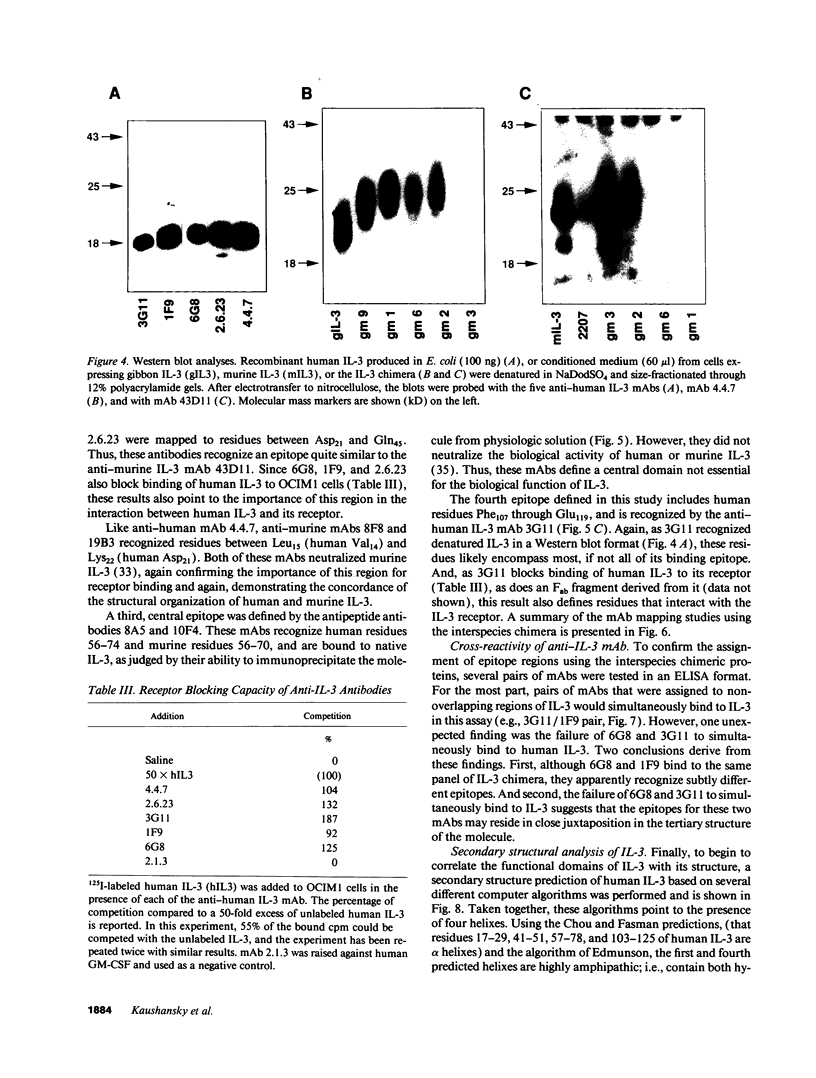
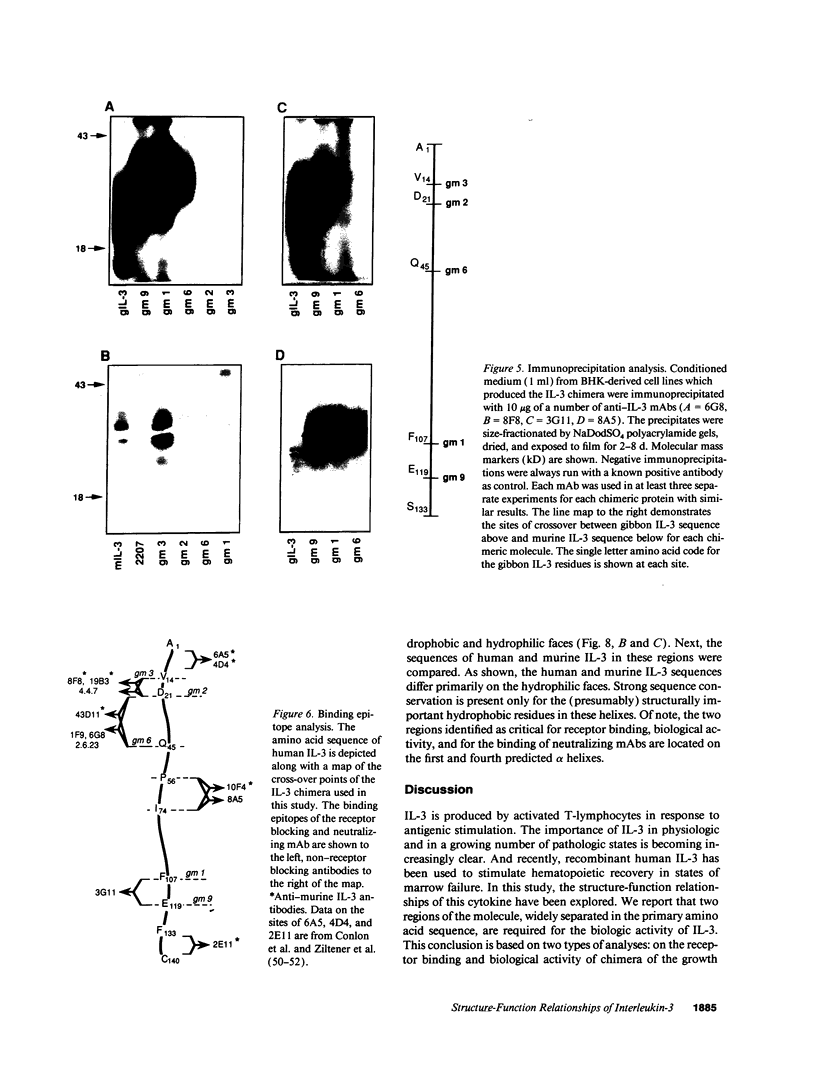
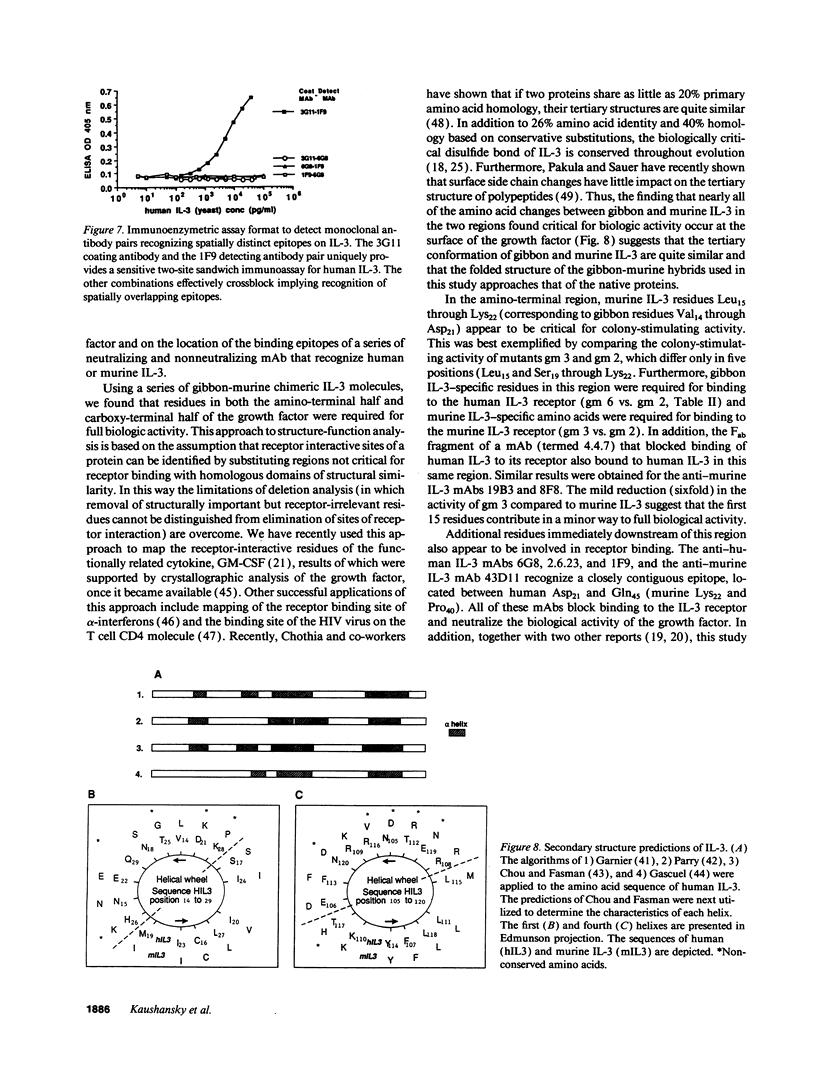
IL-3 is a glycoprotein cytokine involved in the hematopoietic response to infectious, immunologic, and inflammatory stimuli. In addition, clinical administration of recombinant IL-3 augments recovery in states of natural and treatment-related marrow failure. IL-3 acts by binding to high affinity cell surface receptors present on hematopoietic cells. To determine the site(s) at which IL-3 binds to it receptor, we analyzed a series of interspecies chimera of the growth factor for species-specific receptor binding and biological activity. The results suggest that IL-3 binds to its receptor and triggers a proliferative stimulus through two noncontiguous helical domains located near the amino terminus and the carboxy terminus of the molecule. To corroborate these findings, we have also mapped the binding epitopes of 10 mAb of human or murine IL-3, and have defined four distinct epitopes. Two of these epitopes comprise the amino-terminal receptor binding domain. A third epitope corresponds to the carboxy-terminal receptor interactive domain, and the fourth epitope, apparently not involved in the interaction of IL-3 and its receptor, lies between these sites. And on the basis of sandwich immunoassays using pairs of these mAbs, the two receptor interactive regions appear to reside in close juxtaposition in the tertiary structure of the molecule. These results provide a correlation of the structure-function relationships of IL-3 that should prove useful in evaluating the details of IL-3-IL-3 receptor interaction and in the rational design of clinically useful derivatives of this growth factor.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams J. S., Pearce M. K. Development of rat anti-mouse interleukin 3 monoclonal antibodies which neutralize bioactivity in vitro. J Immunol. 1988 Jan 1;140(1):131–137. [PubMed] [Google Scholar]
- Bazan J. F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkner K., Busby S., Davie E., Hart C., Insley M., Kisiel W., Kumar A., Murray M., O'Hara P., Woodbury R. Isolation and expression of cDNAs encoding human factor VII. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):531–541. doi: 10.1101/sqb.1986.051.01.065. [DOI] [PubMed] [Google Scholar]
- Bot F. J., van Eijk L., Schipper P., Löwenberg B. Effects of human interleukin-3 on granulocytic colony-forming cells in human bone marrow. Blood. 1989 Apr;73(5):1157–1160. [PubMed] [Google Scholar]
- Brakenhoff J. P., Hart M., Aarden L. A. Analysis of human IL-6 mutants expressed in Escherichia coli. Biologic activities are not affected by deletion of amino acids 1-28. J Immunol. 1989 Aug 15;143(4):1175–1182. [PubMed] [Google Scholar]
- Brandhuber B. J., Boone T., Kenney W. C., McKay D. B. Three-dimensional structure of interleukin-2. Science. 1987 Dec 18;238(4834):1707–1709. doi: 10.1126/science.3500515. [DOI] [PubMed] [Google Scholar]
- Broudy V. C., Lin N., Egrie J., de Haën C., Weiss T., Papayannopoulou T., Adamson J. W. Identification of the receptor for erythropoietin on human and murine erythroleukemia cells and modulation by phorbol ester and dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6513–6517. doi: 10.1073/pnas.85.17.6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. B., Hart C. E., Curtis D. M., Bailey M. C., Kaushansky K. Two neutralizing monoclonal antibodies against human granulocyte-macrophage colony-stimulating factor recognize the receptor binding domain of the molecule. J Immunol. 1990 Mar 15;144(6):2184–2189. [PubMed] [Google Scholar]
- Budel L. M., Elbaz O., Hoogerbrugge H., Delwel R., Mahmoud L. A., Löwenberg B., Touw I. P. Common binding structure for granulocyte macrophage colony-stimulating factor and interleukin-3 on human acute myeloid leukemia cells and monocytes. Blood. 1990 Apr 1;75(7):1439–1445. [PubMed] [Google Scholar]
- Calvo J. C., Radicella J. P., Charreau E. H. Measurement of specific radioactivities in labelled hormones by self-displacement analysis. Biochem J. 1983 May 15;212(2):259–264. doi: 10.1042/bj2120259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M. The relation between the divergence of sequence and structure in proteins. EMBO J. 1986 Apr;5(4):823–826. doi: 10.1002/j.1460-2075.1986.tb04288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Chrétien I., Van Kimmenade A., Pearce M. K., Banchereau J., Abrams J. S. Development of polyclonal and monoclonal antibodies for immunoassay and neutralization of human interleukin-4. J Immunol Methods. 1989 Feb 8;117(1):67–81. doi: 10.1016/0022-1759(89)90120-8. [DOI] [PubMed] [Google Scholar]
- Clark-Lewis I., Aebersold R., Ziltener H., Schrader J. W., Hood L. E., Kent S. B. Automated chemical synthesis of a protein growth factor for hemopoietic cells, interleukin-3. Science. 1986 Jan 10;231(4734):134–139. doi: 10.1126/science.3079915. [DOI] [PubMed] [Google Scholar]
- Clark-Lewis I., Hood L. E., Kent S. B. Role of disulfide bridges in determining the biological activity of interleukin 3. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7897–7901. doi: 10.1073/pnas.85.21.7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon P. J., Luk K. H., Park L. S., March C. J., Hopp T. P., Urdal D. L. Generation of anti-peptide monoclonal antibodies which recognize mature CSF-2 alpha (IL 3) protein. J Immunol. 1985 Jul;135(1):328–332. [PubMed] [Google Scholar]
- Diederichs K., Boone T., Karplus P. A. Novel fold and putative receptor binding site of granulocyte-macrophage colony-stimulating factor. Science. 1991 Dec 20;254(5039):1779–1782. doi: 10.1126/science.1837174. [DOI] [PubMed] [Google Scholar]
- Dorssers L. C., Mostert M. C., Burger H., Janssen C., Lemson P. J., van Lambalgen R., Wagemaker G., van Leen R. W. Receptor and antibody interactions of human interleukin-3 characterized by mutational analysis. J Biol Chem. 1991 Nov 5;266(31):21310–21317. [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gascuel O., Golmard J. L. A simple method for predicting the secondary structure of globular proteins: implications and accuracy. Comput Appl Biosci. 1988 Aug;4(3):357–365. doi: 10.1093/bioinformatics/4.3.357. [DOI] [PubMed] [Google Scholar]
- Grimaldi J. C., Meeker T. C. The t(5;14) chromosomal translocation in a case of acute lymphocytic leukemia joins the interleukin-3 gene to the immunoglobulin heavy chain gene. Blood. 1989 Jun;73(8):2081–2085. [PubMed] [Google Scholar]
- Hapel A. J., Warren H. S., Hume D. A. Different colony-stimulating factors are detected by the "interleukin-3"-dependent cell lines FDC-Pl and 32D cl-23. Blood. 1984 Oct;64(4):786–790. [PubMed] [Google Scholar]
- Hayashida K., Kitamura T., Gorman D. M., Arai K., Yokota T., Miyajima A. Molecular cloning of a second subunit of the receptor for human granulocyte-macrophage colony-stimulating factor (GM-CSF): reconstitution of a high-affinity GM-CSF receptor. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9655–9659. doi: 10.1073/pnas.87.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida N., Sakurai S., Hosaka T., Hosoi K., Kunitomo T., Shimazu T., Maruyama T., Matsuura Y., Kohase M. Establishment of strongly neutralizing monoclonal antibody to human interleukin-6 and its epitope analysis. Biochem Biophys Res Commun. 1989 Dec 15;165(2):728–734. doi: 10.1016/s0006-291x(89)80027-0. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Morishita K., Parker D. S., Bartholomew C., Askew D., Buchberg A., Jenkins N. A., Copeland N., Weinstein Y. Mechanisms in the transformation of IL3-dependent hematopoietic stem cells. Curr Top Microbiol Immunol. 1989;149:59–69. doi: 10.1007/978-3-642-74623-9_5. [DOI] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Schreurs J., Gorman D. M., Maruyama K., Ishii A., Yahara I., Arai K., Miyajima A. Cloning of an interleukin-3 receptor gene: a member of a distinct receptor gene family. Science. 1990 Jan 19;247(4940):324–327. doi: 10.1126/science.2404337. [DOI] [PubMed] [Google Scholar]
- Kaushansky K., O'Hara P. J., Hart C. E., Forstrom J. W., Hagen F. S. Role of carbohydrate in the function of human granulocyte-macrophage colony-stimulating factor. Biochemistry. 1987 Jul 28;26(15):4861–4867. doi: 10.1021/bi00389a038. [DOI] [PubMed] [Google Scholar]
- Kaushansky K., Shoemaker S. G., Alfaro S., Brown C. Hematopoietic activity of granulocyte/macrophage colony-stimulating factor is dependent upon two distinct regions of the molecule: functional analysis based upon the activities of interspecies hybrid growth factors. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1213–1217. doi: 10.1073/pnas.86.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H., Ohnishi T., Okubo Y., Weiler D., Abrams J. S., Gleich G. J. Granulocyte/macrophage colony-stimulating factor and interleukin 3 release from human peripheral blood eosinophils and neutrophils. J Exp Med. 1991 Sep 1;174(3):745–748. doi: 10.1084/jem.174.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T., Sato N., Arai K., Miyajima A. Expression cloning of the human IL-3 receptor cDNA reveals a shared beta subunit for the human IL-3 and GM-CSF receptors. Cell. 1991 Sep 20;66(6):1165–1174. doi: 10.1016/0092-8674(91)90039-2. [DOI] [PubMed] [Google Scholar]
- Kitamura T., Tange T., Terasawa T., Chiba S., Kuwaki T., Miyagawa K., Piao Y. F., Miyazono K., Urabe A., Takaku F. Establishment and characterization of a unique human cell line that proliferates dependently on GM-CSF, IL-3, or erythropoietin. J Cell Physiol. 1989 Aug;140(2):323–334. doi: 10.1002/jcp.1041400219. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Van Leeuwen B. H., Elsbury S., Martinson M. E., Young I. G., Hapel A. J. Interleukin-3 is significantly more effective than other colony-stimulating factors in long-term maintenance of human bone marrow-derived colony-forming cells in vitro. Blood. 1989 May 15;73(7):1836–1841. [PubMed] [Google Scholar]
- Landau N. R., Warton M., Littman D. R. The envelope glycoprotein of the human immunodeficiency virus binds to the immunoglobulin-like domain of CD4. Nature. 1988 Jul 14;334(6178):159–162. doi: 10.1038/334159a0. [DOI] [PubMed] [Google Scholar]
- Lokker N. A., Strittmatter U., Steiner C., Fagg B., Graff P., Kocher H. P., Zenke G. Mapping the epitopes of neutralizing anti-human IL-3 monoclonal antibodies. Implications for structure-activity relationship. J Immunol. 1991 Feb 1;146(3):893–898. [PubMed] [Google Scholar]
- Lopez A. F., Eglinton J. M., Gillis D., Park L. S., Clark S., Vadas M. A. Reciprocal inhibition of binding between interleukin 3 and granulocyte-macrophage colony-stimulating factor to human eosinophils. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7022–7026. doi: 10.1073/pnas.86.18.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Murthy S. C., Sorensen P. H., Mui A. L., Krystal G. Interleukin-3 down-regulates its own receptor. Blood. 1989 Apr;73(5):1180–1187. [PubMed] [Google Scholar]
- Otsuka T., Miyajima A., Brown N., Otsu K., Abrams J., Saeland S., Caux C., de Waal Malefijt R., de Vries J., Meyerson P. Isolation and characterization of an expressible cDNA encoding human IL-3. Induction of IL-3 mRNA in human T cell clones. J Immunol. 1988 Apr 1;140(7):2288–2295. [PubMed] [Google Scholar]
- Pakula A. A., Sauer R. T. Genetic analysis of protein stability and function. Annu Rev Genet. 1989;23:289–310. doi: 10.1146/annurev.ge.23.120189.001445. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T., Nakamoto B., Kurachi S., Tweeddale M., Messner H. Surface antigenic profile and globin phenotype of two new human erythroleukemia lines: characterization and interpretations. Blood. 1988 Sep;72(3):1029–1038. [PubMed] [Google Scholar]
- Park L. S., Friend D., Price V., Anderson D., Singer J., Prickett K. S., Urdal D. L. Heterogeneity in human interleukin-3 receptors. A subclass that binds human granulocyte/macrophage colony stimulating factor. J Biol Chem. 1989 Apr 5;264(10):5420–5427. [PubMed] [Google Scholar]
- Park L. S., Waldron P. E., Friend D., Sassenfeld H. M., Price V., Anderson D., Cosman D., Andrews R. G., Bernstein I. D., Urdal D. L. Interleukin-3, GM-CSF, and G-CSF receptor expression on cell lines and primary leukemia cells: receptor heterogeneity and relationship to growth factor responsiveness. Blood. 1989 Jul;74(1):56–65. [PubMed] [Google Scholar]
- Parry D. A., Minasian E., Leach S. J. Conformational homologies among cytokines: interleukins and colony stimulating factors. J Mol Recognit. 1988 Jun;1(3):107–110. doi: 10.1002/jmr.300010302. [DOI] [PubMed] [Google Scholar]
- Raj N. B., Israeli R., Kelley K. A., Leach S. J., Minasian E., Sikaris K., Parry D. A., Pitha P. M. Synthesis, antiviral activity, and conformational characterization of mouse-human alpha-interferon hybrids. J Biol Chem. 1988 Jun 25;263(18):8943–8952. [PubMed] [Google Scholar]
- Rector E. S., Schwenk R. J., Tse K. S., Sehon A. H. A method for the preparation of protein-protein conjugates of predetermined composition. J Immunol Methods. 1978;24(3-4):321–336. doi: 10.1016/0022-1759(78)90135-7. [DOI] [PubMed] [Google Scholar]
- Reichlin M., Nisonoff A., Margoliash E. Immunological activity of cytochrome c. 3. Enhancement of antibody detection and immune response initiation by cytochrome c polymers. J Biol Chem. 1970 Mar 10;245(5):947–954. [PubMed] [Google Scholar]
- Rothenberg M. E., Owen W. F., Jr, Silberstein D. S., Woods J., Soberman R. J., Austen K. F., Stevens R. L. Human eosinophils have prolonged survival, enhanced functional properties, and become hypodense when exposed to human interleukin 3. J Clin Invest. 1988 Jun;81(6):1986–1992. doi: 10.1172/JCI113547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketazu F., Chiba S., Shibuya K., Kuwaki T., Tsumura H., Miyazono K., Miyagawa K., Takaku F. IL-3 specifically inhibits GM-CSF binding to the higher affinity receptor. J Cell Physiol. 1991 Feb;146(2):251–257. doi: 10.1002/jcp.1041460209. [DOI] [PubMed] [Google Scholar]
- Valent P., Geissler K., Sillaber C., Lechner K., Bettelheim P. Why clinicians should be interested in interleukin-3. Blut. 1990 Dec;61(6):338–345. doi: 10.1007/BF01738546. [DOI] [PubMed] [Google Scholar]
- Ziltener H. J., Clark-Lewis I., Fazekas de St Groth B., Hood L. E., Kent S. B., Schrader J. W. Antipeptide antibodies define the NH2-terminal structure of the pan-specific hemopoietin interleukin 3. J Immunol. 1987 Feb 15;138(4):1105–1108. [PubMed] [Google Scholar]
- Ziltener H. J., Clark-Lewis I., Fazekas de St Groth B., Orban P. C., Hood L. E., Kent S. B., Schrader J. W. Monoclonal antipeptide antibodies recognize IL-3 and neutralize its bioactivity in vivo. J Immunol. 1988 Feb 15;140(4):1182–1187. [PubMed] [Google Scholar]