Abstract
Background: Hypoglycemia remains an impediment to good glycemic control, with nocturnal hypoglycemia being particularly dangerous. Information on major contributors to nocturnal hypoglycemia remains critical for understanding and mitigating risk.
Materials and Methods: Continuous glucose monitoring (CGM) data for 855 nights were studied, generated by 45 subjects 15–45 years of age with hemoglobin A1c (HbA1c) levels of ≤8.0% who participated in a larger randomized study. Factors assessed for potential association with nocturnal hypoglycemia (CGM measurement of <60 mg/dL for ≥30 min) included bedtime blood glucose (BG), exercise intensity, bedtime snack, insulin on board, day of the week, previous daytime hypoglycemia, age, gender, HbA1c level, diabetes duration, daily basal insulin, and daily insulin dose.
Results: Hypoglycemia occurred during 221 of 885 (25%) nights and was more frequent with younger age (P<0.001), lower HbA1c levels (P=0.006), medium/high-intensity exercise during the preceding day (P=0.003), and the occurrence of antecedent daytime hypoglycemia (P=0.001). There was a trend for lower bedtime BG levels to be associated with more frequent nocturnal hypoglycemia (P=0.10). Bedtime snack, before bedtime insulin bolus, weekend versus weekday, gender, and daily basal and bolus insulin were not associated with nocturnal hypoglycemia.
Conclusions: Awareness that HbA1c level, exercise, bedtime BG level, and daytime hypoglycemia are all modifiable factors associated with nocturnal hypoglycemia may help patients and providers decrease the risk of hypoglycemia at night. Risk for nocturnal hypoglycemia increased in a linear fashion across the range of variables, with no clear-cut thresholds to guide clinicians or patients for any particular night.
Introduction
Both hypoglycemia and the fear of hypoglycemia remain impediments to good glycemic control among patients with type 1 diabetes (T1D).1 A disproportionate share of hypoglycemia occurs at night, making nocturnal hypoglycemia a particularly important issue. In the large continuous glucose monitoring (CGM) JDRF study,2 biochemical hypoglycemia (CGM glucose concentration of ≤60 mg/dL) occurred during 8.5% of nights, with 23% of these events lasting more than 2 h.
Although severe hypoglycemia is associated with adverse events such as seizures and rarely death, less severe nocturnal hypoglycemia is linked to a broad range of adverse consequences,3 both acutely4,5 and long term.6 In a multinational survey of the consequences of nonsevere nocturnal hypoglycemia, Brod et al.7 reported that among the participants who awoke, the average time to return to sleep was over an hour, and some did not return to sleep at all that night. Most respondents reported a moderate to severe impact on ability to function on the following day. A better understanding of the antecedents of nocturnal hypoglycemia could both improve routine clinical diabetes management and help inform the ongoing development of closed-loop insulin delivery systems.
We analyzed data from 885 control nights in a recently completed randomized study8 of a predictive low-glucose suspend (PLGS) system to examine factors associated with nocturnal hypoglycemia.
Research Design and Methods
The study was conducted at three clinical centers. The protocol was approved by each Institutional Review Board, and informed consent and assent were obtained as appropriate. The study assessed the efficacy of a PLGS system as described in a recently published article8 and listed on the ClinicalTrials.gov Web site (clinical trial registration number NCT01591681). Key aspects of the study protocol are described below.
Major eligibility criteria included being 15–45 years of age, having T1D with use of daily insulin therapy for ≥1 year, use of an insulin infusion pump for ≥6 months, hemoglobin A1c (HbA1c) level measured with a point-of-care device of ≤8.0%, at least 1 night with a sensor glucose value of ≤60 mg/dL or at least 3 different nights with a sensor glucose value of ≤70 mg/dL during 10–15 days of CGM preceding the trial, and demonstrated ability to use the PLGS system. Of note is that only three of the 49 subjects enrolled failed to meet these hypoglycemia criteria, and one did not complete a system-use criterion. The PLGS system comprised the Enlite® glucose sensor and MiniMed Paradigm® Veo system (Medtronic Diabetes, Northridge, CA), and the proprietary PLGS algorithm was installed on a laptop computer.
During the randomized trial, the system was used until 42 nights with at least 4 h of sensor glucose data were completed, with half the nights randomly assigned to be intervention nights and half control nights.
Each night, upon startup, the system prompted the subject to enter whether or not a bedtime snack was consumed and the level of exercise intensity for that day (Supplementary Figs S1 and S2; Supplementary Data are available online at www.liebertonline.com/dia). The protocol had no specific instructions about bedtime snacks. Timing and size of insulin boluses were obtained from pump downloads. During the day, the participant used the CGM device and pump as it would be prescribed for usual diabetes management (without the algorithm being active). The threshold-based low-glucose suspend feature of the Veo pump was disabled during the study, so as not to interfere with the PLGS algorithm.
Statistical methods
The data used for this analysis only included control nights (i.e., the PLGS algorithm was inactive) to explore the predictors of nocturnal hypoglycemia. The time period for outcome assessment each night was from bedtime (which the subject indicated in the system) until deactivation the following morning. Only nights with at least 6 h of CGM data were included in the analysis.
Hypoglycemia was defined as having at least 30 min of CGM values <60 mg/dL. Factors assessed that could potentially affect the proportion of nights with hypoglycemia included bedtime blood glucose (BG) level, exercise intensity (see Supplementary Fig. S2 for the assessment tool), bedtime snack, insulin on board, day of the week, previous daytime CGM hypoglycemia, age of subject, gender, HbA1c level at enrollment, diabetes duration, daily percentage basal insulin, and daily insulin dose (in units/kg-day).
CGM measurements from noon to 8 p.m. were used to assess the effect of antecedent daytime hypoglycemia (time <60 mg/dL) on nocturnal hypoglycemia. Sufficient CGM data (at least 6 h) were available for 754 days (85%).
The percentage of nights with hypoglycemia was tabulated for each factor, giving equal weight to each night. A repeated-measures regression model with random subject effects and a spatial power autocorrelation structure was used for univariate and multivariate analysis. A linear relationship was fitted for continuous covariates. Nonlinear and two-way interaction effects were tested but were not significant. A multivariate model was determined using a stepwise approach. Factors with a value of P<0.10 were added to the model, but only values of P<0.01 were considered significant to adjust for multiple hypothesis testing. A 99% confidence interval of the odds ratio and P values were calculated for the factors in the final model. All P values are two tailed. Analyses were performed using SAS version 9.3 software (SAS Institute, Inc., Cary, NC).
Results
The 45 individuals included in the analyses had an age range of 15–45 years (mean age, 30 years); 47% were male, and 93% were white. Median T1D duration was 15 years, and median HbA1c level was 6.8%.
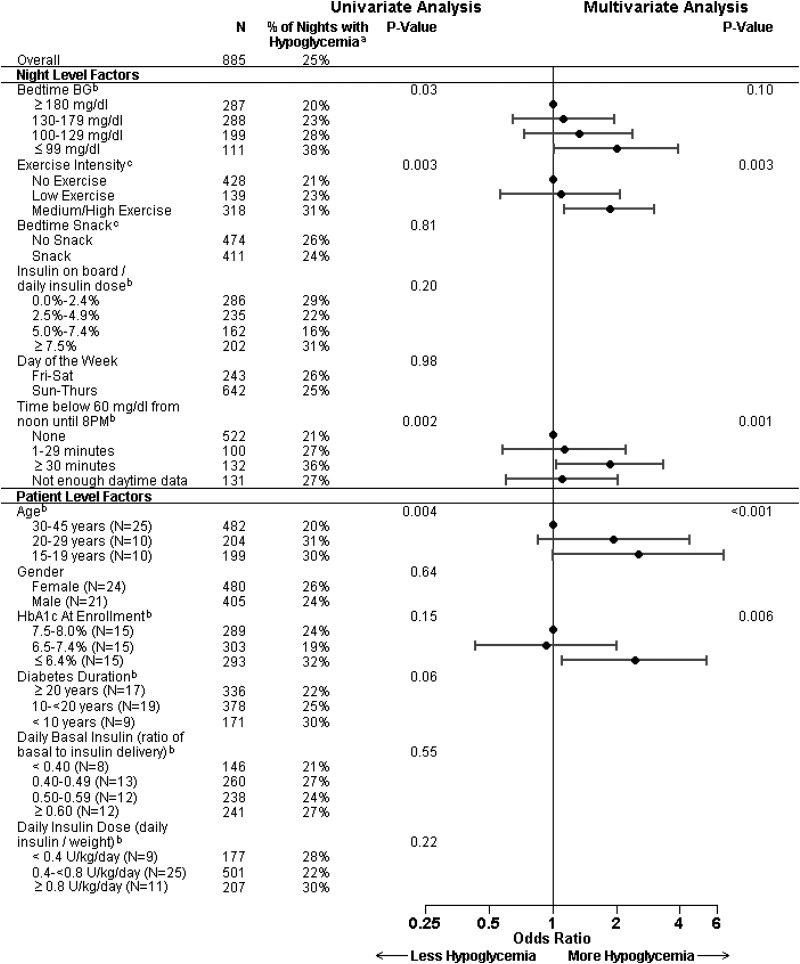
Hypoglycemia, as measured by CGM, occurred during 221 of 885 (25%) control nights. In a multivariate model, nocturnal hypoglycemia was more frequent with younger age (P<0.001), lower HbA1c levels (P=0.006), medium/high-intensity exercise during the preceding day (P=0.003), and the occurrence of biochemical hypoglycemia during the preceding day (P=0.001) (Fig. 1). There was a trend for lower bedtime BG levels to be associated with more frequent nocturnal hypoglycemia that did not meet our criterion for statistical significance (P=0.10). All of these factors had similar odds ratios of about 2 across the clinically relevant ranges.
FIG. 1.
Factors related to overnight hypoglycemia. aNights with hypoglycemia had at least 30 min below 60 mg/dL. bLinear relationship fitted for continuous factor. cExercise intensity and snacks entered by the subjects. BG, blood glucose; HbA1c, hemoglobin A1c.
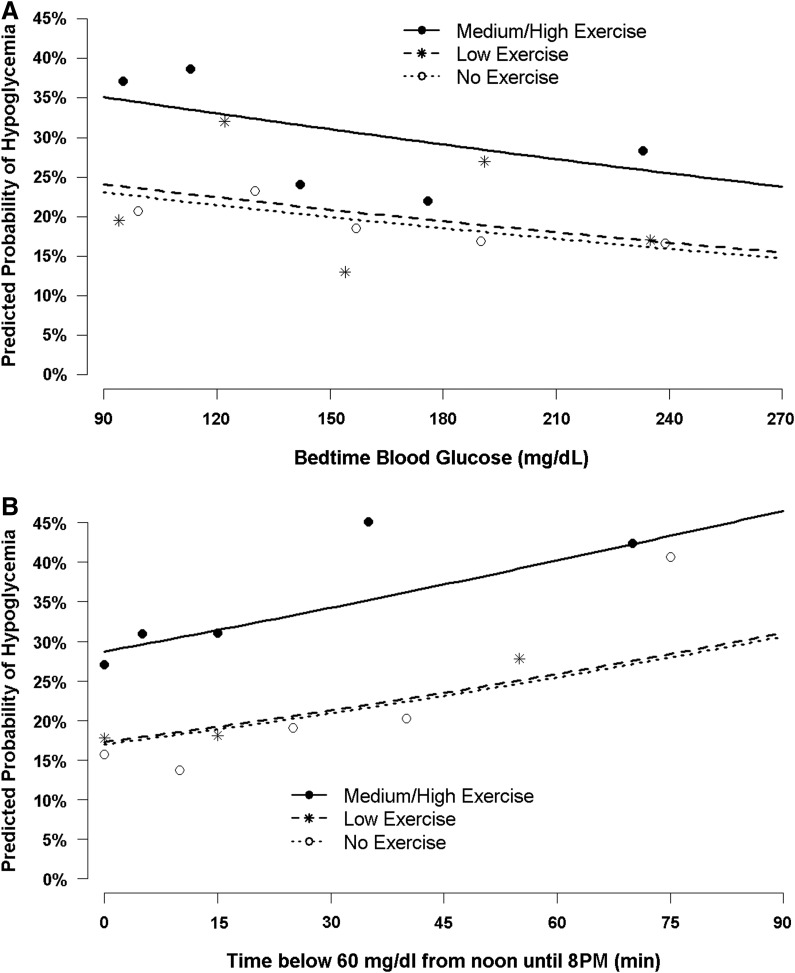
The probability of nocturnal hypoglycemia as a function of time of daytime hypoglycemia, bedtime BG concentrations, and exercise intensity is shown in Figure 2. Bedtime snack, insulin on board, weekend versus weekday, gender, daily basal insulin, and daily bolus dose were not associated with nocturnal hypoglycemia in the univariate or multivariate analyses. It also was not possible to distinguish the separate effects of age and diabetes duration because these were highly correlated.
FIG. 2.
Predicted overnight hypoglycemia by (A) bedtime blood glucose or (B) daytime hypoglycemia and exercise. Lines represent the predicted probability of hypoglycemia based on the repeated-measures regression model. Dots represent the least square means of the observed percentage of hypoglycemia.
Discussion
Nocturnal hypoglycemia occurred during 25% of nights, a frequency somewhat higher than the 15% rate found in the pilot for this trial.9 The increase in hypoglycemia may be due to switching from the Sof-Sensor in the pilot trial over to the Enlite sensor in the current study, which has been reported to be biased low.10 Even if the Enlite sensor used in our study overestimated the frequency of hypoglycemia, this should not have had an impact on the interpretation of the results of analyses assessing the association of factors with nocturnal hypoglycemia.
By design, we required that our subject population have some nocturnal hypoglycemia during the run-in period of this study, which likely selected for increased hypoglycemia during the study period. The JDRF CGM trial, with broader entry criteria, reported that 8.5% of 36,467 nights had at least two consecutive glucose values ≤60 mg/dL within a 20-min period.2 On the control nights of a recent closed-loop study at home using the FreeStyle Navigator® sensor (Abbott Diabetes Care, Alameda, CA), Hovorka et al.11 found that glucose levels fell below 63 mg/dL for at least 20 min on 17% of the nights. Another factor potentially contributing to our higher incidence of nocturnal hypoglycemia was the inability of subjects to use a temporary basal rate when they went to bed (this was not compatible with our system for pump suspensions). It is therefore possible that our high rate of hypoglycemia following moderate to strenuous activity would have been reduced if subjects had access to a temporary reduction in their basal rates on those nights. Subjects were given the option to change their basal rate patterns during the study.
Nocturnal hypoglycemia has been associated with a multitude of antecedent factors.1 Although some factors such as gender, age of diagnosis, and duration of diabetes are not modifiable, knowledge about their association with nocturnal hypoglycemia can still be helpful in setting therapeutic targets or closed-loop algorithm parameters.
In the current study, those with HbA1c values >6.5% had significantly less nocturnal hypoglycemia. Of note is that although the range of HbA1c levels in this study was limited (HbA1c ≤8% was an inclusion criterion), there was no difference in the rate of nocturnal hypoglycemia among those with HbA1c values between 6.5% and 7.4% compared with those with a higher HbA1c level (7.5–8%). Whereas many studies12–14 have found an inverse relationship with HbA1c levels and severe hypoglycemia, recent data support our finding that this relationship is likely waning. Likewise, O'Connell et al.15 and Cooper et al.16 found a sharp decrease in severe hypoglycemia over about a decade in nearly 1,800 children and adolescents. During that time, average HbA1c levels remained roughly the same, and the investigators further found no association with HbA1c level. Among adults over 26 years of age, Weinstock et al.17 reported no consistent relationship between HbA1c level and severe hypoglycemia and reported that the frequency of severe hypoglycemia was lowest in those with HbA1c values between 7.0% and 7.5%. With that said, the results of our study may not apply to those with HbA1c levels of >8%.
Physical exertion during the day is a well-known contributor to subsequent nocturnal hypoglycemia. The current study found that self-reported exercise of moderate to high intensity increased the subsequent rate of nocturnal hypoglycemia. Although nocturnal hypoglycemia occurred across a broad range of bedtime glucose concentrations, there was a trend toward higher rates of nocturnal hypoglycemia following lower bedtime BG levels (Fig. 2A). Following moderate to high exercise intensity, nocturnal hypoglycemia occurred on 37% of nights with a bedtime BG level of ≤130 mg/dL and 27% of nights with a bedtime BG level of >130 mg/dL. The DirecNet group18 studied the effect of a uniform, late-afternoon “heavy” exercise challenge in 50 children with T1D and found nocturnal hypoglycemia (a single central laboratory glucose value of <60 mg/dL) developed in 16 (57%) of 28 subjects whose bedtime BG level was ≤130 mg/dL and in eight (36%) of 22 subjects whose level was >130 mg/dL. Although others have found that a bedtime snack can reduce the incidence of nocturnal hypoglycemia after exercise,19 we did not find an overall effect of a bedtime snack on nocturnal hypoglycemia. In a recent review of bedtime nutritional strategies and the incidence of nocturnal hypoglycemia, Desjardins et al.20 concluded that although current evidence does not support the systematic use of bedtime snacks, their use appears to be prudent in many settings when the likelihood of nocturnal hypoglycemia is high. As demonstrated in Figure 2A, we also did not observe a bedtime threshold glucose at which nocturnal hypoglycemia did not occur, similar to the findings of Kaufman et al.21
Daytime hypoglycemia was significantly correlated with nocturnal hypoglycemia. Garg et al.22 recently reported in the crossover-designed ASPIRE in-clinic study that exercise-induced hypoglycemia was significantly longer in the group that experienced antecedent hypoglycemia, suggesting that “hypoglycemia begets hypoglycemia.” Data from the JDRF CGM trial23 showed that severe hypoglycemia was eight times more likely when 30% of CGM values were <70 mg/dL on the previous day (4.5% vs. 0.5%; P<0.001). Although quite significant, the frequency of severe hypoglycemia was low and less than 5%.
Conclusions
We found that younger age, lower HbA1c levels, exercise during the preceding day, and biochemical hypoglycemia during the preceding day were associated with a greater frequency of nocturnal hypoglycemia. Our study strengths include a large number of nights in the home environment where uniform data were collected electronically. Moreover, the study was designed to enroll subjects with a higher likelihood of nocturnal hypoglycemia. Limitations of the study included enrolling subjects across a wider age range and not formally testing for hypoglycemic unawareness. The very nature of the primary study limited us to subjects who were delivering insulin by infusion pumps and use of self-reported data about exercise and snacks. We evaluated hypoglycemia using CGM, which adds possible misclassification noise.
Knowledge of the potentially modifiable daytime factors (increased exercise, lower bedtime BG, and minutes of daytime hypoglycemia) can be used to advise families to monitor subsequent nocturnal glucose levels more closely. However, no factor was strongly predictive of the occurrence of nocturnal hypoglycemia. Some of the risk factors, such as increased physical exercise24 and reasonable bedtime BG levels, are themselves important in the overall health of patients with diabetes.
Because it is difficult to modify the clinical management of diabetes to avoid nocturnal hypoglycemia, the continued development and commercialization of systems to automatically manage glucose concentrations provide the best hope to decrease nocturnal hypoglycemia while maintaining good overall glycemic control, and significant decreases in nocturnal hypoglycemia and improved glycemic time in range using closed-loop systems have recently been demonstrated.11,25–27
Supplementary Material
Appendix
In Home Closed Loop Study Group
Clinical Centers
Listed with clinical center name, city, and state. Personnel are listed as (PI) for Principal Investigator, (I) for co-Investigator, (C) for Coordinators, and (O) for other personnel:
Division of Pediatric Endocrinology and Diabetes, Stanford University, Stanford, CA: Bruce Buckingham, MD (PI); Darrell M. Wilson, MD (I); Tandy Aye, MD (I); Paula Clinton, RN, CDE (C); Breanne P. Harris, BS (C)
Barbara Davis Center for Childhood Diabetes, University of Colorado, Denver, CO: H. Peter Chase, MD (PI); David M. Maahs, MD, PhD (I); Robert Slover, MD (I); Paul Wadwa, MD (I); Jaime Realsen, BS (C); Laurel Messer, RN, CDE (C)
St. Joseph's Health Care, London, ON, Canada: Irene Hramiak, MD, FRCP (PI); Terri Paul, MD, MSc, FRCPC (I); Sue Tereschyn, RN, CDE, CCRA (C); Marsha Driscoll, BScN, RN, CDE (C)
JDRF Canadian Clinical Trial Network: Olivia Lou, PhD (O)
Rensselaer Polytechnic Institute, Troy, NY: B. Wayne Bequette, PhD (PI); Fraser Cameron, PhD (I)
Coordinating Center
Jaeb Center for Health Research, Tampa, FL: Roy W. Beck, MD, PhD (PI); John Lum, MS; Craig Kollman, PhD; Peter Calhoun, MA; Judy Sibayan, MPH; Nelly M. Njeru; Werner Sauer; Jennifer Lott
Data and Safety Monitoring Board
John C. Pickup, BM, DPhil (Chair), Irl Hirsch, MD; Howard Wolpert, MD
Acknowledgments
We would like to recognize the efforts of the participants and their families and thank them. We also would like to recognize Martin Cantwell, BSc, Medtronic Diabetes, Northridge, CA, and Werner Sauer, BS, Jaeb Center for Health Research, Tampa, FL, for their significant engineering contributions. The project described was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (grant R01DK085591), the JDRF (grant 22-2013-266), and the JDRF Canadian Clinical Trial Network, which is a public–private partnership including JDRF International, JDRF-Canada, and the Federal Economic Development Agency for Southern Ontario and is supported by JDRF grant 80-2010-585. Continuous glucose monitors and sensors were purchased at a bulk discount price from Medtronic MiniMed, Inc. Home glucose meters and test strips as well as ketone meters and test strips were provided to the study by LifeScan, Inc. and Abbott Diabetes Care, Inc.
Author Disclosure Statement
D.M.W. reports research supplies support from LifeScan, Inc., and Medtronic MiniMed, Inc., and a patent Kalman filter-based hypoglycemia prevention algorithm pending. D.M.M. reports grants from American Diabetes Association and Medtronic. H.P.C. reports a patent Kalman filter-based hypoglycemia prevention algorithm pending. B.A.B. reports grants from Medtronic MiniMed, Inc., research supplies support from LifeScan, Inc., and a patent Kalman filter-based hypoglycemia prevention algorithm pending. I.H. reports serving as a board member for Medtronic MiniMed, Inc. C.K. reports consultant fees received from Medtronic MiniMed, Inc. R.W.B. reports grants to his institution from the National Institutes of Health and from JDRF for the conduct of the study and payments to his institution from Animas for statistical consulting outside the submitted work. P.M.C., L.M., T.A., and P.K.C. have nothing to disclose.
The study was designed and conducted by the investigators. The Writing Group collectively wrote the manuscript and vouch for the data. D.M.W. is the guarantor of this work and, as such, had full access to all the data in the study. D.M.W., P.M.C., and L.M. wrote the manuscript, contributed to the discussion, and reviewed/edited the manuscript. D.M.M., H.P.C., B.A.B., T.A., P.K.C., and I.H. researched data, contributed to discussion, and reviewed/edited manuscript. C.K. and R.W.B. contributed to the discussion and reviewed/edited the manuscript.
References
- 1.Ly TT, Maahs DM, Rewers A, Dunger D, Oduwole A, Jones TW: Assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes 2014;15(Suppl 20):180–192 [DOI] [PubMed] [Google Scholar]
- 2.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group: Prolonged nocturnal hypoglycemia is common during 12 months of continuous glucose monitoring in children and adults with type 1 diabetes. Diabetes Care 2010;33:1004–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris S, Mamdani M, Galbo-Jorgensen CB, Bogelund M, Gundgaard J, Groleau D: The effect of hypoglycemia on health-related quality of life: Canadian results from a multinational time trade-off survey. Can J Diabetes 2014;38:45–52 [DOI] [PubMed] [Google Scholar]
- 4.Buckingham B, Wilson DM, Lecher T, Hanas R, Kaiserman K, Cameron F: Duration of nocturnal hypoglycemia before seizures. Diabetes Care 2008;31:2110–2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanenberg RJ, Newton CA, Drake AJ: Confirmation of hypoglycemia in the “dead-in-bed” syndrome, as captured by a retrospective continuous glucose monitoring system. Endocr Pract 2010;16:244–248 [DOI] [PubMed] [Google Scholar]
- 6.McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA: Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care 2012;35:1897–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brod M, Pohlman B, Wolden M, Christensen T: Non-severe nocturnal hypoglycemic events: experience and impacts on patient functioning and well-being. Qual Life Res 2013;22:997–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maahs DM, Calhoun P, Buckingham BA, Chase HP, Hramiak I, Lum J, Cameron F, Bequette BW, Aye T, Paul T, Slover R, Wadwa RP, Wilson DM, Kollman C, Beck RW; In Home Closed Loop Study Group: A randomized trial of a home system to reduce nocturnal hypoglycemia in type 1 diabetes. Diabetes Care 2014;37:1885–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckingham BA, Cameron F, Calhoun P, Maahs DM, Wilson DM, Chase HP, Bequette BW, Lum J, Sibayan J, Beck RW, Kollman C: Outpatient safety assessment of an in-home predictive low-glucose suspend system with type 1 diabetes subjects at elevated risk of nocturnal hypoglycemia. Diabetes Technol Ther 2013;15:622–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calhoun P, Lum J, Beck RW, Kollman C: Performance comparison of the Medtronic Sof-sensor and Enlite glucose sensors in inpatient studies of individuals with type 1 diabetes. Diabetes Technol Ther 2013;15:758–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hovorka R, Elleri D, Thabit H, Allen JM, Leelarathna L, El-Khairi R, Kumareswaran K, Caldwell K, Calhoun P, Kollman C, Murphy HR, Acerini CL, Wilinska ME, Nodale M, Dunger DB: Overnight closed-loop insulin delivery in young people with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care 2014;37:1204–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diabetes Control and Complications Trial Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 13.Diabetes Control and Complications Trial Research Group: Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr 1994;125:177–188 [DOI] [PubMed] [Google Scholar]
- 14.Rewers A, Chase HP, Mackenzie T, Walravens P, Roback M, Rewers M, Hamman RF, Klingensmith G: Predictors of acute complications in children with type 1 diabetes. JAMA 2002;287:2511–2518 [DOI] [PubMed] [Google Scholar]
- 15.O'Connell SM, Cooper MN, Bulsara MK, Davis EA, Jones TW: Reducing rates of severe hypoglycemia in a population-based cohort of children and adolescents with type 1 diabetes over the decade 2000–2009. Diabetes Care 2011;34:2379–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper MN, O'Connell SM, Davis EA, Jones TW: A population-based study of risk factors for severe hypoglycaemia in a contemporary cohort of childhood-onset type 1 diabetes. Diabetologia 2013;56:2164–2170 [DOI] [PubMed] [Google Scholar]
- 17.Weinstock RS, Xing D, Maahs DM, Michels A, Rickels MR, Peters AL, Bergenstal RM, Harris B, Dubose SN, Miller KM, Beck RW; T1D Exchange Clinic Network: Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange clinic registry. J Clin Endocrinol Metab 2013;98:3411–3419 [DOI] [PubMed] [Google Scholar]
- 18.Tsalikian E, Mauras N, Beck RW, Tamborlane WV, Janz KF, Chase HP, Wysocki T, Weinzimer SA, Buckingham BA, Kollman C, Xing D, Ruedy KJ; Diabetes Research in Children Network Direcnet Study Group: Impact of exercise on overnight glycemic control in children with type 1 diabetes mellitus. J Pediatr 2005;147:528–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell MD, Walker M, Trenell MI, Stevenson EJ, Turner D, Bracken RM, Shaw JA, West DJ: A low-glycemic index meal and bedtime snack prevents postprandial hyperglycemia and associated rises in inflammatory markers, providing protection from early but not late nocturnal hypoglycemia following evening exercise in type 1 diabetes. Diabetes Care 2014;37:1845–1853 [DOI] [PubMed] [Google Scholar]
- 20.Desjardins K, Brazeau AS, Strychar I, Rabasa-Lhoret R: Are bedtime nutritional strategies effective in preventing nocturnal hypoglycaemia in patients with type 1 diabetes? Diabetes Obes Metab 2014;16:577–587 [DOI] [PubMed] [Google Scholar]
- 21.Kaufman FR, Austin J, Neinstein A, Jeng L, Halvorson M, Devoe DJ, Pitukcheewanont P: Nocturnal hypoglycemia detected with the Continuous Glucose Monitoring System in pediatric patients with type 1 diabetes. J Pediatr 2002;141:625–630 [DOI] [PubMed] [Google Scholar]
- 22.Garg SK, Brazg RL, Bailey TS, Buckingham BA, Slover RH, Klonoff DC, Shin J, Welsh JB, Kaufman FR: Hypoglycemia begets hypoglycemia: the order effect in the ASPIRE in-clinic study. Diabetes Technol Ther 2014;16:125–130 [DOI] [PubMed] [Google Scholar]
- 23.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Fiallo-Scharer R, Cheng J, Beck RW, Buckingham BA, Chase HP, Kollman C, Laffel L, Lawrence JM, Mauras N, Tamborlane WV, Wilson DM, Wolpert H: Factors predictive of severe hypoglycemia in type 1 diabetes: analysis from the Juvenile Diabetes Research Foundation continuous glucose monitoring randomized control trial dataset. Diabetes Care 2011;34:586–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galassetti P, Riddell MC: Exercise and type 1 diabetes (T1DM). Compr Physiol 2013;3:1309–1336 [DOI] [PubMed] [Google Scholar]
- 25.Ly TT, Breton MD, Keith-Hynes P, De Salvo D, Clinton P, Benassi K, Mize B, Chernavvsky D, Place J, Wilson DM, Kovatchev BP, Buckingham BA: Overnight glucose control with an automated, unified safety system in children and adolescents with type 1 diabetes at diabetes camp. Diabetes Care 2014;37:2310–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nimri R, Muller I, Atlas E, Miller S, Fogel A, Bratina N, Kordonouri O, Battelino T, Danne T, Phillip M: MD-Logic overnight control for 6 weeks of home use in patients with type 1 diabetes: randomized crossover trial. Diabetes Care 2014;37:3025–3032 [DOI] [PubMed] [Google Scholar]
- 27.Thabit H, Lubina-Solomon A, Stadler M, Leelarathna L, Walkinshaw E, Pernet A, Allen JM, Iqbal A, Choudhary P, Kumareswaran K, Nodale M, Nisbet C, Wilinska ME, Barnard KD, Dunger DB, Heller SR, Amiel SA, Evans ML, Hovorka R: Home use of closed-loop insulin delivery for overnight glucose control in adults with type 1 diabetes: a 4-week, multicentre, randomised crossover study. Lancet Diabetes Endocrinol 2014;2:701–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.