Abstract
Background: Telehealth interventions for patients with chronic obstructive pulmonary disease (COPD) have focused primarily on stable outpatients. Telehealth designed to handle the acute exacerbation that normally requires hospitalization could also be of interest. The aim of this study was to compare the effect of home-based telehealth hospitalization with conventional hospitalization for exacerbation in severe COPD. Materials and Methods: A two-center, noninferiority, randomized, controlled effectiveness trial was conducted between June 2010 and December 2011. Patients with severe COPD admitted because of exacerbation were randomized 1:1 either to home-based telehealth hospitalization or to continue standard treatment and care at the hospital. The primary outcome was treatment failure defined as re-admission due to exacerbation in COPD within 30 days after initial discharge. The noninferiority margin was set at 20% of the control group's risk of re-admission. Secondary outcomes were mortality, need for manual or mechanical ventilation or noninvasive ventilation, length of hospitalization, physiological parameters, health-related quality of life, user satisfaction, healthcare costs, and adverse events. Results: In total, 57 patients were randomized: 29 participants in the telehealth group and 28 participants in the control group. Testing the incidence of re-admission within 30 days after discharge could not confirm noninferiority (lower 95% confidence limit [CL], −24.8%; p=0.35). Results were also nonsignificant at 90 days (lower 95% CL, −16.2%; p=0.33) and 180 days (lower 95% CL, −16.6%; p =0.33) after discharge. Superiority testing on secondary outcomes showed nonsignificant differences between groups. Healthcare costs have not yet been evaluated. Conclusions: Whether home-based telehealth hospitalization is noninferior to conventional hospitalization requires further investigation. The results indicate that a subgroup of patients with severe COPD can be treated for acute exacerbation at home using telehealth, without the physical presence of health professionals and with a proper organizational “back-up.”
Key words: : home health monitoring, telehealth, telemedicine, telenursing
Introduction
Patients suffering from severe chronic obstructive pulmonary disease (COPD) are frequently hospitalized with a high re-admission rate approaching 28.5% within 1 month in Denmark.1 The combination of an increasingly aging population and the ensuing awareness of healthcare costs of chronic diseases2 has stimulated research and innovation in the telehealth area.3
The evidence of the effect of telehealth for patients with COPD is sparse.4 Systematic reviews reveal only a few randomized trials with conflicting results and low power.5–8 Furthermore, systematic reviews and meta-analyses have been criticized for their methodological quality,9 making it difficult to draw any conclusions.
Two larger studies including patients with COPD have emerged.10,11 Nevertheless, focus has remained primarily on interventions for the newly discharged patient or the stable outpatient. Interventions designed for handling an exacerbation that normally requires hospitalization could also be of interest.
Using telehealth for treating exacerbations in COPD may lead to a reorganization that allows one hospital to take over the care of COPD patients from other hospitals, thereby sharing the supervision duty and serving of patients.12 Furthermore, telehealth may help patients manage exacerbations better by providing tools for self-monitoring and encourage better self-management of health problems, thereby empowering the patients.13 Patients with COPD fear being hospitalized,14 and studies have shown that patients with COPD prefer homecare treatment of exacerbation.15,16
In the current study, the objective was to compare the effect of home-based telehealth hospitalization with conventional hospitalization for exacerbation in COPD. We hypothesized that home-based telehealth hospitalization was noninferior to conventional hospitalization measured on treatment failure, defined as re-admission due to COPD within 30 days after discharge.
A noninferiority design was chosen because the “new treatment”—home-based telehealth hospitalization—with respect to the “reference” treatment—standard hospitalization—consisted of the same medical treatment and thus was expected to have the same clinical effect on the exacerbation but is still of interest on the premise that the “new” treatment—telehealth hospitalization—might have other advantages.
Noninferiority was decided if the telehealth group had a treatment failure rate less than 20% higher than that of patients assigned to the control group. Reporting of this trial is done in accordance with the CONSORT statement's extension for equivalence and noninferiority trials.17
Materials and Methods
Study Design
We conducted a two-center, noninferiority, randomized effectiveness trial at two university hospitals in the Copenhagen, Denmark, area, which recruited participants between June 2010 and December 2011. The last follow-up visit took place in May 2012.
The study was approved by the Danish Regional Committee on Scientific Ethics (protocol number H-2-2010-021) and the Danish Data Protection Agency (journal number 2010-41-4684.
Patients were externally randomized 1:1 in fixed blocks of 4. The allocation sequence was hidden in sequentially numbered, sealed, opaque envelopes that were delivered to the hospitals in batches of 10. The patients were informed in spoken and in written form about the study and were allowed 1 h to decide whether they wanted to participate or not.
The sealed envelope was not opened by the patient until after the patient had signed a written consent form. The allocation concealment mechanism was monitored closely by the investigators to ensure that envelopes were never resealed and to ensure patients were entered correctly in the study no matter what allocation the envelope revealed.
A detailed methodology article has been published previously.12
Participants
Patients admitted with acute exacerbations were treated according to a strict hospital protocol for exacerbations in COPD.18
We included patients ≥45 years of age, with severe to very severe COPD according to the Global Initiative for Chronic Obstructive Lung Disease,19 who had an acute exacerbation of COPD as defined by Anthonisen et al.,20 who were compliant, and who had an expected hospitalization of more than 2 days. We excluded people with need of noninvasive ventilation (NIV) or manual or mechanical ventilation or of intravenous antibiotics, who had a pH value of <7.35, who had unstable heart disease, malignancy, or poorly regulated diabetes, who were unable to give informed consent, or who had participated in another trial.
Interventions
Experimental group
Participants in the telehealth group were transported home within the first 24 h of hospital admission. The equipment consisted of a touch screen with a Webcam, pulse oximeter, spirometer, thermometer, nebulizer for aerosolized inhalation medication, oxygen compressor, and a medicine box containing antibiotics, prednisone, sedative, beta2 agonists, and anticholinergics. Data were transmitted via wireless broadband. All patients kept the equipment until discharge. The patient was prepared for daily ward rounds using the touch screen at appointed hours. Unscheduled and acute contacts could always be effectuated 24/7 by the patient pressing the “call hospital” button on the touch screen. Hospital personnel were instructed to treat the telehealth participants exactly the same way as they would treat them had they been present at the hospital except from the physical contact, which was not possible.
Apart from the daily virtual ward rounds where the remote exchange of patient data took place, patients were able to, but not asked to, perform any particular self-monitoring tasks or regular observations.
Control group
The patients allocated to the control group were hospitalized as usual, receiving standard hospital treatment for an exacerbation.
A list of the standard medical treatment used in both groups appears in the previous methodology article.12
Discharge Criteria
Patients in both groups were discharged by the attending doctor if they fulfilled the following five criteria: (1) slept >4 h without awakening from respiratory symptoms, (2) forced expiratory volume in 1 s not decreasing, (3) clinically stable, (4) condition improved during admission, and (5) oxygen saturation (SpO2) >90% without supplemental oxygen or with the regular oxygen supply if they were long-term oxygen users.
Primary Outcome
The primary outcome was treatment failure, defined as re-admission due to COPD within 30 days after the intervention had ended (discharge date).
Secondary Outcomes
Secondary outcomes were mortality, need of manual or mechanical ventilation or NIV, physiological measures, length of hospitalization, health-related quality of life, user satisfaction (patient and health professional), adverse events, and healthcare costs.
Healthcare costs will be evaluated in separately elsewhere.
Two substudies have evaluated the patients' coping and self-efficacy21,22 and cognitive status.23
Data Collection
Research staff and nurses working at the two acute emergency departments collected data at baseline, during the intervention, and at follow-up visits 30, 90, and 180 days after discharge.
Data on admissions were extracted from the Danish hospital records at the end of the trial and by asking the patients at the three follow-up visits. Data concerning the need of manual or mechanical ventilation or NIV within the first 30 days after discharge and data concerning mortality were obtained by searching the hospital records after the trial had ended. Data concerning health-related quality of life were obtained at baseline, at discharge, and at the three follow-up visits. The validated health-related quality of life questionnaires used were the St. George Respiratory Questionnaire (SGRQ), the Clinical COPD Questionnaire (CCQ), and the EQ-5D questionnaire, a standardized instrument developed by the Euroqol group. Data concerning physiological parameters (i.e., forced expiratory volume in 1 s, forced vital capacity, heart rate, respiratory rate, and SpO2) were obtained at baseline, during the intervention, and at the three follow-up visits.
A nonvalidated user satisfaction questionnaire was handed out to patients in the telehealth group at discharge. The questionnaire consisted of 29 questions: 24 questions were presented in a 5-point Likert-scale (from 1=strongly disagree through 5=strongly agree), and 5 questions were close-ended (yes/no). The questionnaire covered the patient's experience in different settings such as the daily ward round, acute calls, the equipment use, and the patient's general experience of the home-based telehealth hospitalization.
A similar questionnaire was available to nurses operating the telehealth solution consisting of 21 questions: 20 questions in a 5-point Likert-scale (from 1=strongly disagree through 5=strongly agree) and 1 close-ended question (yes/no). The questionnaire covered the health professional's experience of treating patients via the telehealth solution in regard to quality of equipment, usability, and the health professional's general experience of treating COPD patients by means of telehealth.
Sample Size
Sample size calculation assuming noninferiority with a predefined margin of 20% or less, with 5% alpha error and 80% power, showed that a patient number of 70 in each group was needed. As the estimated re-admission rate was set to 23.1% based on prior studies,24,25 we would accept the alternative hypothesis, that the experimental treatment is as good as the standard hospital treatment or only slightly worse by no more than the predefined margin.
Statistical Analysis
All analyses were done using R.26 Baseline characteristics were summarized using means (standard deviation) and frequencies or median (range) as appropriate. The Kaplan–Meier method and the log-rank test were used to test the differences in overall survival within 3 years after randomization.27 Re-admission-free survival probabilities (combined end point) and re-admission probabilities (accounting for death as a competing risk) were assessed within the 180 days that all patients were followed by crude rates.28 Note that no patients were lost to follow-up within the 180 days. Noninferiority was tested with one-sided confidence limits for the re-admission-free survival probabilities (combined end point) after 30 days, 90 days, and 180 days, respectively, using Eq. 4 from Tunes da Silva et al.29 All other outcomes were superiority tested. Cox regression was used to compare the re-admission rates within 180 days after randomization between the intervention and the control group.30 Reported are the hazard ratio and the expected number of re-admissions, both with 95% confidence intervals based on robust standard errors to account for patients who underwent multiple re-admissions.
For the secondary outcomes, including physiological parameters, we report mean changes (standard deviation) between randomization and 30 days after discharge. Individual patient changes in the health-related quality of life questionnaire scores were graphically evaluated. Average differences in changes of questionnaire scores between randomization and 30 days after randomization were assessed by paired t tests. Because of death of patients it was not possible to assess and compare average questionnaire scores or mean changes in physiological parameters at 90 and 180 days of follow-up by reasonably simple statistical tools (see Kurland et al.31). Changes in the methods used for statistical analysis were made to account for the fact that some patients died without prior re-admission and prior to quality of life evaluation.
Results
Recruitment
The mean time from admission to hospital and recruitment into the study was 16.3 h (standard deviation 6.6 h). Average monthly recruitment for the trial was 3.2 patients.
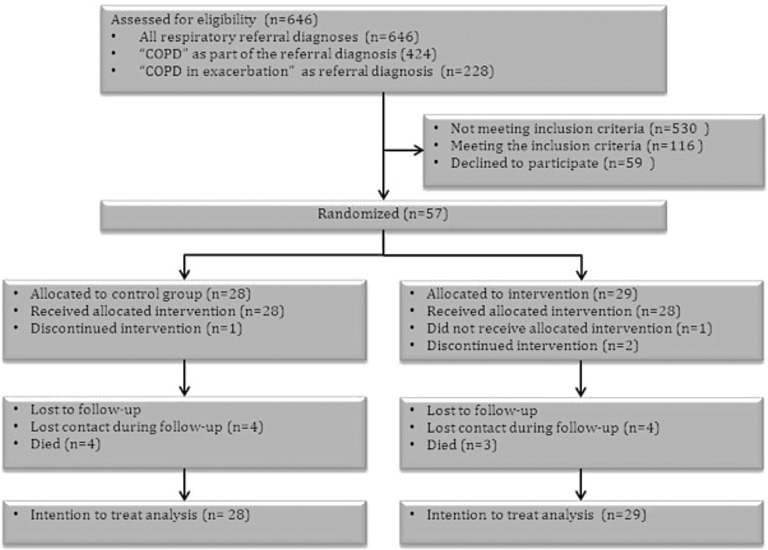
Figure 1 shows the participant flow diagram by group throughout the trial. Referral diagnoses such as “breathing difficulty,” “dyspnea,” or simply “COPD” were vague and required further assessment to assess whether a patient was suffering from an exacerbation in COPD. Consequently, all patients with respiratory referral diagnoses were screened in order to not miss out on eligible candidates. Half of the eligible candidates did not wish to participate in the study.
Fig. 1.
Flow of participants through the trial. COPD, chronic obstructive pulmonary disease.
Losses and Exclusions
As shown in Figure 1 similar numbers of patients in each group were lost to follow-up (i.e., we were not able to get in contact prior to a follow-up visit in the patient's home where questionnaires and measurements were done). Two patients in the intervention group discontinued the intervention: 1 due to hyponatremia and 1 due to severe dyspnea and nebulizer failure (the patient separated the device by accident). Furthermore, 1 patient in the intervention group never received the allocated intervention because of technology failure at the home address and thus returned to the hospital. All patients in the control group received the allocated treatment. One patient in the control group was discontinued owing to suspicion of malignancy.
No patients were excluded from the final intention-to-treat analysis.
Barriers to Recruitment
During the recruitment period there were high rates of nonparticipation. Table 1 presents clinical characteristics obtained for eligible nonparticipants compared with study participants and showed similar age and lung function. There were more women represented in the group of nonparticipants. The reasons given for not wanting to participate were as follow:
• Feel too tired/exhausted/ill/anxious to go home the same day of admittance (n=21)
• No reason given (n=18)
• Do not want to participate in studies for different reasons (n=7)
• Feel stigmatized by the idea of getting an oxygen container at home (n=4) (the same person was asked four times on four different admissions due to COPD)
• Do not wish to participate because of family matters (n=3)
• Need help with dinner/shower/get dressed, etc. (n=3)
• Do not want to have anything to do with technology (n=2)
• Do not feel ill enough to get equipment (n=1)
Table 1.
Clinical Characteristics of Eligible Nonparticipants Compared with Eligible Participants
| VARIABLE, SUBGROUP | NONPARTICIPANTS (N=59) | PARTICIPANTS (N=57) |
|---|---|---|
| Sex [n (%)] | ||
| Female | 48 (81.4) | 35 (61.4) |
| Male | 11 (18.6) | 22 (38.6) |
| Age (years) [n (%)] | ||
| <60 | 8 (13.6) | 10 (17.5) |
| 60–70 | 23 (39.0) | 16 (28.1) |
| 70–80 | 22 (37.3) | 19 (33.3) |
| >80 | 6 (10.2) | 12 (21.1) |
| FEV1 (L) | ||
| Median (range) | 0.7 (0.31–1.2) | 0.7 (0.38–2.07) |
| Missing | 24 | 0 |
| FVC (L) | ||
| Median (range) | 1.5 (0.53–2.79) | 1.6 (0.5–3.42) |
| Missing | 25 | 0 |
Data are median (range) or number (%) as indicated.
FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
Baseline Data on Study Participants
Table 2 shows that the two groups were similar in health at baseline. More patients were living alone in the control group.
Table 2.
Baseline Characteristics of Trial Participants
| VARIABLE, SUBGROUP | CONTROL (N=28) | INTERVENTION (N=29) |
|---|---|---|
| Age (years) [n (%)] | ||
| <60 | 5 (17.9) | 5 (17.2) |
| 60–70 | 8 (28.6) | 8 (27.6) |
| 70–80 | 9 (32.1) | 10 (34.5) |
| >80 | 6 (21.4) | 6 (20.7) |
| Sex [n (%)] | ||
| Female | 17 (60.7) | 18 (62.1) |
| Male | 11 (39.3) | 11 (37.9) |
| Spouse [n (%)] | ||
| Living alone | 20 (71.4) | 15 (51.7) |
| Living with spouse | 8 (28.6) | 14 (48.3) |
| Smoking status [n (%)] | ||
| Former smoker | 14 (50.0) | 12 (41.4) |
| Never smoker | 0 (0.0) | 1 (3.4) |
| Smoker | 14 (50.0) | 16 (55.2) |
| Number of COPD re-admissions 6 months prior to trial [n (%)] | ||
| 0 | 16 (57.1) | 17 (58.6) |
| 1 | 8 (28.6) | 6 (20.7) |
| >1 | 4 (14.3) | 6 (20.7) |
| FEV1 (L) [median (range)] | 0.7 (0.4–1.8) | 0.7 (0.4–2.1) |
| FVC (L) [median (range)] | 1.6 (0.7–3.4) | 1.5 (0.5–3.4) |
| Respiratory frequency (breaths per minute) | ||
| Median (range) | 20.0 (12–36) | 20.0 (16–25) |
| Missing | 3 | 8 |
| SpO2 (%) [median (range)] | 93.0 (89–99) | 93.0 (86–98) |
| Heart rate (beats per minute) [median (range)] | 88.5 (49–126) | 91.0 (59–130) |
| LTOT long-term oxygen user [n (%)] | ||
| No | 26 (92.9) | 28 (96.6) |
| Yes | 2 (7.1) | 1 (3.4) |
| SGRQ total score | ||
| Median (range) | 65.3 (34.1–85.7) | 74.4 (31.9–84.0) |
| Missing | 1 | 4 |
| CCQ total score | ||
| Median (range) | 3.3 (1.6–5.1) | 4.1 (1.3–5.4) |
| Missing | 2 | 5 |
| EQ-5D summary index | ||
| Median (range) | 0.7 (0.077–1) | 0.7 (0.197–1) |
| Missing | 0 | 3 |
| EQ VAS | ||
| Median (range) | 50.0 (24–89) | 42.5 (10–80) |
| Missing | 1 | 3 |
Data are median (range) or number (%).
CCQ, Chronic COPD Questionnaire; COPD, chronic obstructive pulmonary disease; EQ VAS, EQ visual analog scale; LTOT, long-term oxygen therapy; SpO2, oxygen saturation; SGRQ, St. George Respiratory Questionnaire.
Re-Admissions and Mortality
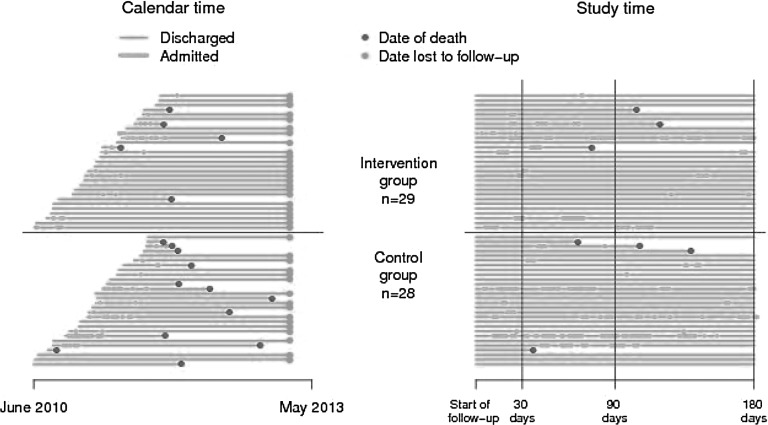
Figure 2 shows the individual follow-up periods until death or date of statistical analysis indicating re-admissions and deaths in both calendar and study time.
Fig. 2.
Individual patient follow-up periods until death or date of statistical analysis indicating re-admissions and deaths in both (left panel) calendar and (right panel) study time.
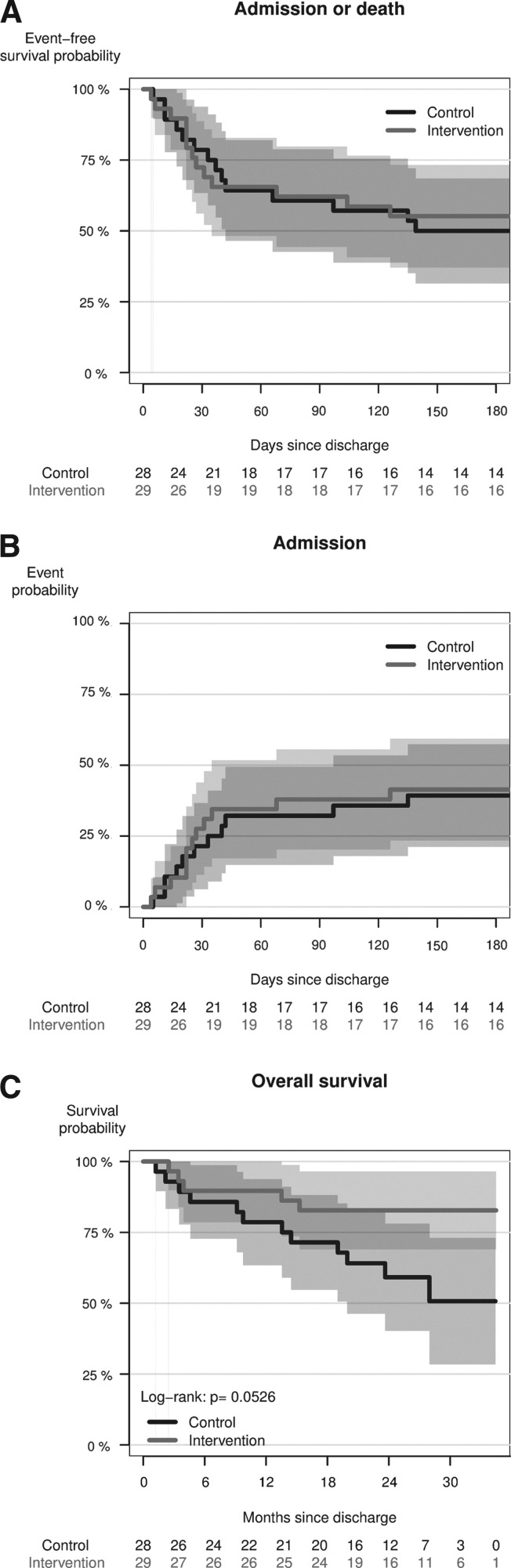
Testing the incidence of re-admission within 30, 90, and 180 days after discharge could not confirm noninferiority (Table 3). Survival outcomes and the cumulative incidence of any re-admission are shown in Figure 3.
Table 3.
Noninferiority Tests on Re-admission-Free Survival Probabilities at 30, 90, and 180 Days After Discharge
| RE-ADMISSION-FREE SURVIVAL PROBABILITY (%) | |||||
|---|---|---|---|---|---|
| DAY | CONTROL GROUP (N=28) | INTERVENTION GROUP (N=29) | DIFFERENCE (%) | LOWER 95% CONFIDENCE LIMIT BOUND FOR DIFFERENCE (%) | NONINFERIORITY P VALUE |
| 30 | 78.6 | 72.4 | −6.2 | −24.8 | 0.35 |
| 90 | 60.7 | 65.5 | 4.8 | −16.2 | 0.33 |
| 180 | 50.0 | 55.2 | 5.2 | −16.6 | 0.33 |
Fig. 3.
(A) Re-admission-free survival probability (combined end point, Kaplan–Meier method) within the first 180 days after randomization. (B) Cumulative incidence of any re-admission due to chronic obstructive pulmonary disease within the first 180 days after randomization here treating death as a competing risk (Aalen–Johansen method). (C) Overall survival probability within 3 years after randomization (Kaplan–Meier method).
Cox regression showed no significant difference between the re-admission rates (hazard ratio=2.01; 95% confidence interval, 0.71–5.71). The expected number of re-admissions within 180 days was estimated as 2.39 (0.37–4.41) in the control group and as 1.08 (0.39–1.77) in the intervention group.
No participants died within 30 days after discharge. In the control group four participants died during 6 months of follow-up compared with three participants in the intervention group. Overall survival was evaluated based on registry data for June 2013. This showed a survival probability 2 years after randomization of 59.2% (40.2–78.1%) in the control group and of 82.8% (69.0–96.5%) in the intervention group (by log-rank test, p=0.053) (Fig. 3C).
Need for NIV and/or Mechanical Ventilation
The need for NIV or mechanical ventilation was assessed for the first 30 days after discharge. Four participants (3 patients in the intervention group and 1 patient in the control group) received NIV and/or mechanical ventilation during a re-admission to hospital. In 1 case (intervention group) it was after surgery.
Length of Hospitalization
In the control group 8 patients (28.6%) were hospitalized for more than 5 days, and in the telehealth group 5 patients (17.2%) were hospitalized at home for more than 5 days (p=0.48).
Changes in Lung Function, Heart Rate, Respiratory Rate, and SpO2
Changes in physiological measurements measured 1 month after discharge were not significantly different between groups (Table 4).
Table 4.
Changes in Physiological Measurements 30 Days After Discharge
| CHANGE AFTER 30 DAYS, SUBGROUP | CONTROL (N=28) | INTERVENTION (N=29) | TOTALS (N=57) | P VALUE |
|---|---|---|---|---|
| FEV1 (L) | ||||
| Mean (95% CI) | 0.2 (0.03–0.3) | 0.2 (0.05–0.3) | 0.2 (0.09–0.3) | 0.9715 |
| Missing | 2 | 6 | 8 | |
| SpO2 (%) | ||||
| Mean (95% CI) | 2.3 (0.9–3.7) | 1.6 (0.4–2.7) | 1.9 (1.1–2.8) | 0.3936 |
| Missing | 2 | 4 | 6 | |
| Heart rate (beats per minute) | ||||
| Mean (95% CI) | −5.1 (−13.4 to 3.3) | −3.0 (−9.2 to 3.1) | −4.1 (−9.1 to 0.9) | 0.6867 |
| Missing | 2 | 4 | 6 | |
| Respiratory rate (breaths per minute) | ||||
| Mean (95% CI) | 1.4 (−2.5 to 5.3) | 0.5 (−2.9 to 3.8) | 1.0 (−1.5 to 3.4) | 0.6978 |
| Missing | 11 | 14 | 25 | |
| FVC (L) | ||||
| Mean (95% CI) | −0.0 (−0.2 to 0.2) | 0.0 (−0.2 to 0.2) | −0.0 (−0.1 to 0.1) | 0.8199 |
| Missing | 2 | 6 | ||
95% CI, 95% confidence interval; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; SpO2, oxygen saturation.
Improvement in the Health-Related Quality of Life Questionnaires CCQ, SGRQ, and EQ-5D
Individual patient changes in questionnaire scores were graphically evaluated for the whole period (Fig. 4).
Fig. 4.
Individual patient changes in health-related quality of life questionnaire scores: (A) Chronic Chronic Obstructive Pulmonary Disease Questionnaire (CCQ), (B) EQ-5D, (C) EQ visual analog scale (VAS), and St. George Respiratory Questionnaire (SGRQ).
The scores of CCQ, SGRQ and EQ-5D improved in both groups over time within the first 30 days after discharge, but the improvement was not significantly different between groups (Table 5).
Table 5.
Analysis of the Health-Related Quality of Life Questionnaires 30 Days After Discharge
| CHANGE AFTER 30 DAYS, SUBGROUP | CONTROL (N=28) | INTERVENTION (N=29) | TOTALS (N=57) | P VALUE |
|---|---|---|---|---|
| SGRQ total score | ||||
| Mean (95% CI) | −14.7 (−22.4 to −7.0) | −8.5 (−15.3 to −1.7) | −11.7 (−16.7 to −6.6) | 0.2105 |
| Missing | 6 | 8 | 14 | |
| CCQ total score | ||||
| Mean (95% CI) | −1.4 (−2.1 to −0.7) | −1.7 (−2.3 to −1.1) | −1.6 (−2.0 to −1.1) | 0.4800 |
| Missing | 5 | 7 | 12 | |
| EQ-5D summary index | ||||
| Mean (95% CI) | 0.1 (0.002–0.3) | 0.1 (0.01–0.1) | 0.1 (0.03–0.2) | 0.4838 |
| Missing | 4 | 8 | 12 | |
| EQ VAS | ||||
| Mean (95% CI) | 7.6 (−2.9 to 18.0) | 14.5 (6.7–22.2) | 10.9 (4.6–17.3) | 0.2740 |
| Missing | 5 | 7 | 12 | |
95% CI, 95% confidence interval; CCQ, Chronic Chronic Obstructive Pulmonary Disease Questionnaire; EQ VAS, EQ visual analog scale; SGRQ, St. George Respiratory Questionnaire.
Adverse Events
Three patients returned to the hospital: 1 patient returned owing to technical failure of wireless broadband technology in the home, 1 patient developed hyponatremia, and 1 patient returned owing to severe dyspnea and nebulizer failure (the patient separated the device by accident).
Patient Satisfaction
Twenty patients in the telehealth group filled out a user satisfaction questionnaire immediately after being discharged. All respondents agreed or strongly agreed that it was easy to see the doctor or nurse on the screen, that it was easy to understand the information given, and that they felt their problems were understood during ward rounds. Furthermore, they all agreed that it was easy to use the medicine box and that the written instructions given on how to use the equipment were easy to understand. Most important is that all respondents agreed that they felt confident using the equipment. Four of the 20 respondents reported making an acute call outside the planned contacts.
Health Professional Satisfaction
Eight nurses responded to a user satisfaction questionnaire after having used the telehealth solution. The answers within the group of nurses were more diverse than within the group of patients. Seven of the eight respondents agreed it was easy to see the patient and to understand the patient's problems and that they experienced that the patients felt confident using the equipment. Six of the eight nurses agreed feeling confident using the equipment. Four of the eight nurses partly agreed they would have felt more confident had the patient been physically present. On the other hand, five of the eight nurses agreed it was easy to work with the telehealth solution, and four nurses found it easier and less time consuming compared with the conventional treatment and care of COPD patients.
Discussion
Whether treating acute exacerbation of severe COPD at home using telehealth is noninferior to standard hospitalization requires further investigation. Replacing standard hospitalization with home-based telehealth hospitalization did not have a significant benefit on the incidence of re-admission, length of hospitalization, physiological parameters, or health-related quality of life when both intervention and control groups had access to the same medical care. In a subgroup of participants with severe to very severe COPD and acute exacerbation, however, it was possible to be treated by means of telehealth without physical presence of health personnel. However, the high rates of nonparticipation and barriers to recruitment must be taken into consideration.
The strengths of our study include its randomized design, the strict treatment protocol used in the two groups along with predefined discharge criteria, and also a multicenter design including two university hospital units in the Copenhagen area, so it can be generalized to like settings. Our subject demographics are typical of those with severe to very severe COPD. Our telehealth solution was simple and readily deployed. We used hospital records for the primary outcome, and data were available on all participants. We used more than one measure of quality of life. We based the analysis on an intention-to-treat method, which compares patients according to their assigned intervention or control group. The main weakness of the study was its small sample size. As the study is underpowered we cannot rule out the chance especially of type 2 statistical errors, which raise questions about the clinical relevance of these results, and the study must be interpreted as rather preliminary. It was not possible to blind patients or health professionals, but the statistician analyzing the data was blinded to allocation. We designed the trial to minimize the possibility of selection bias, but as the patients were randomized in blocks of 4, the recruiters may have guessed the allocation. Furthermore, in this study we included only patients with severe to very severe COPD as they have an increased risk of hospitalization for an exacerbation, but patients with mild or moderate COPD may also benefit from telehealth solutions. The satisfaction questionnaires used for this trial were not validated.
The focus on treating acute exacerbations that normally requires hospitalization by means of telehealth is novel and allowed us to examine the applicability in patients with severe COPD, who are often hospitalized and have a very high mortality during admission.32 It is therefore central to examine the possible problems and limitations that can arise in this type of setting. A recent review examining the effect of hospital-at-home programs for patients with acute exacerbations of COPD found that approximately 21–37% of patients with acute exacerbations of COPD who present to the emergency department may be eligible for hospital-at-home care.33 This is consistent with our findings where 116 of the 424 referrals (27.5%) with the word “COPD” as part of the referral diagnosis were eligible for home-based treatment with telehealth technology. Half of our eligible candidates refused to participate, and the main reason given was because they were too afraid/tired/exhausted to go home the same day of admittance, highlighting the challenge in physically moving the patients back and forth on the same day.
Eligibility and willingness to participate may be increased depending on the design of the home-based telehealth solution used, such as adding other home monitoring devices to be able to handle comorbidities or by implementing a permanent telehealth solution before the patient presents at the emergency department so that the technology can act proactively and be activated during an exacerbation. However, a recent study by Pinnock et al.11 involving 256 participants with COPD of all severity grades examined the effectiveness of telemonitoring over a 1-year period and found no significant difference in number of days to admission and concluded that long-term telemonitoring is unlikely to reduce admissions unless it is a means of enhancing clinical services. In contrast, a recent evidence-based analysis on home telehealth for patients with COPD exploring the effectiveness and safety of home telemonitoring compared with usual care found a trend toward significant increase in time free of hospitalization with home telemonitoring.5 This was based on five smaller trials with low quality evidence according to GRADE.34
In the Whole System Demonstrator Trial, a telehealth study involving 3,230 people with chronic diseases of whom 1,525 were patients with COPD, the largest study reported so far, intervention patients were significantly less likely to die within 12 months compared with control patients.10 Furthermore, they found that a smaller proportion in the telehealth group (n=1,570, of whom 739 had COPD) was admitted during 12 months of follow-up. They concluded that the mechanisms behind these findings are not yet clear but could be that telehealth helps the patients manage their conditions better. The Whole System Demonstrator Trial has highlighted the problem with high rates of nonparticipation in telehealth studies in a qualitative study.35 We found it equally important to uncover reasons for nonparticipation and found that most nonparticipants were not afraid of the technology but simply found it too burdensome to be moved back and forth the same day, indicating that implementation of telehealth before hospitalization might be preferred by the patients.
The Whole System Demonstrator Trial36 and the study by Pinnock et al.11 also assessed health-related quality of life and found no significant differences, which is in accordance with our findings.
Earlier studies have shown great patient satisfaction among patients with chronic obstructive lung disease treated by the means of videoconferencing and virtual visits by health professionals,37 which is also consistent with our own findings.
Conclusions
Owing to small sample size we were unable to answer the research question whether home-based telehealth hospitalization is noninferior to conventional hospitalization. Consequently, further research is needed. In particular, equivalence and noninferiority trials would be of great interest when it concerns interventions replacing conventional hospitalization in order to determine whether it could be an effective alternative for patients who do not wish to be hospitalized. The results suggest that it is possible to treat a subgroup of patients with acute exacerbation and severe COPD via a telehealth solution without the physical presence of healthcare professionals.
Acknowledgments
We wish to thank the patients who participated in The Virtual Hospital Trial. A special thanks to project nurse Steffen Hogg Christensen for all his work with the telehealth solution and with the patients. We thank Laila Barkani, Helle-Marina Oxfeldt, Hanne Pilemand, and Espen Skalle for assisting in screening patients and collecting data. We wish to thank Frederiksberg & Bispebjerg University Hospital and Herlev University Hospital for the use of hospital facilities and organizational support. Thanks to the 20 nurses working at the acute emergency ward at Frederiksberg University Hospital who participated in the trial. Thanks to Pallas Informatik and Viewcare A/S for supporting and assisting in delivering the telehealth technology solution used in this project. We are very grateful for the grants given to the project from The Philanthropic Foundation TrygFonden (grant 7561-08), The Health Insurance Foundation (grant 2011B003), The Danish Lung Association, The Toyota Foundation (grant OH/BG 7003), The Frederiksberg Foundation (grant 2010-88), and a Lykfeldt's grant.
Disclosure Statement
No competing financial interests exist. The companies Pallas Informatik and Viewcare A/S have received no payment or funding, and there was no interference with the study with regard to design, methods, data collection, analyses, manuscript submission, etc. There was also no interference by the institutions providing grant support with the study with regard to design, methods, data collection, analyses, manuscript submission, etc.
References
- 1.Sundhedsstyrelsen, Monitorering & Medicinsk Teknologivurdering. Genindlæggelser af ældre i Danmark 2008—Nye tal fra Sundhedsstyrelsen. 2009. Available at www.sst.dk/publ/Publ2009/DOKU/nye_tal/Genindlaeggelser2008_final_uden_bilag.pdf (last accessed February24, 2014)
- 2.Teknologirådet. Sundhedsydelser med IT—Pervasive HealthCare i den danske sundhedssektor. Vurderinger og anbefalinger fra en arbejdsgruppe under Teknologirådet. 2006. Available at www.tekno.dk/pdf/projekter/p06_pervasive-healthcare.pdf (last accessed February24, 2014)
- 3.Smith SM, Elkin SL, Partridge MR. Technology and its role in respiratory care. Prim Care Respir J 2009;18:159–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolton CE, Waters CS, Peirce S, Elwyn G; EPSRC and MRC Grand ChallengeTeam. Insufficient evidence of benefit: A systematic review of home telemonitoring for COPD. J Eval Clin Pract 2011;17:1216–1222 [DOI] [PubMed] [Google Scholar]
- 5.Franek J. Home telehealth for patients with chronic obstructive pulmonary disease (COPD): An evidence-based analysis. Ont Health Technol Assess Ser 2012;12:1–58 [PMC free article] [PubMed] [Google Scholar]
- 6.Jakobsen AS, Laursen LC, Schou L, Emme C, Phanareth KV. Varying effect of telemedicine in the treatment of chronic obstructive pulmonary disease—A systematic review [in Danish]. Ugeskr Laeger 2012;174:936–942 [PubMed] [Google Scholar]
- 7.McLean S, Nurmatov U, Liu JL, Pagliari C, Car J, Sheikh A. Telehealthcare for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2011;7:CD007718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polisena J, Tran K, Cimon K, Hutton B, McGill S, Palmer K, et al. Hometelehealth for chronic obstructive pulmonary disease: A systematic review and meta-analysis. J Telemed Telecare 2010;16:120–127 [DOI] [PubMed] [Google Scholar]
- 9.Kitsiou S, Paré G, Jaana M. Systematic reviews and meta-analyses of home telemonitoring interventions for patients with chronic diseases: A critical assessment of their methodological quality. J Med Internet Res 2013;15:e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steventon A, Bardsley M, Billings J, Dixon J, Doll H, Hirani S, et al. Effect of telehealth on use of secondary care and mortality: Findings from the Whole System Demonstrator cluster randomized trial. BMJ 2012;344:e3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinnock H, Hanley J, McCloughan L, Todd A, Krishan A, Lewis S, et al. Effectiveness of telemonitoring integrated into existing clinical services on hospital admission for exacerbation of chronic obstructive pulmonary disease: Researcher blind, multicentre, randomised controlled trial. BMJ 2013;347:f6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jakobsen AS, Laursen LC, Østergaard B, Rydahl-Hansen S, Phanareth KV. Hospital-admitted COPD patients treated at home using telemedicine technology in The Virtual Hospital Trial: Methods of a randomized effectiveness trial. Trials 2013;14:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suter P, Suter WN, Johnston D. Theory-based telehealth and patient empowerment. Popul Health Manag 2011;14:87–92 [DOI] [PubMed] [Google Scholar]
- 14.Haughney J, Partridge MR, Vogelmeier C, Larsson T, Kessler R, Ståhl E, et al. Exacerbations of COPD: Quantifying the patient's perspective using discrete choice modeling. Eur Respir J 2005;26:623–629 [DOI] [PubMed] [Google Scholar]
- 15.Gravil JH, Al-Rawas OA, Cotton MM, Flanigan U, Irwin A, Stevenson RD. Home treatment of exacerbations of chronic obstructive pulmonary disease by an acute respiratory assessment service. Lancet 1998;351:1853–1855 [DOI] [PubMed] [Google Scholar]
- 16.Ram FS, Wedzicha JA, Wright J, Greenstone M. Hospital at home for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2003;4:CD003573. [DOI] [PubMed] [Google Scholar]
- 17.Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG; CONSORT Group. Reporting of noninferiority and equivalence randomized trials: Extension of the CONSORT 2010 statement. JAMA 2012;308:2594–2604 [DOI] [PubMed] [Google Scholar]
- 18.Phanareth K, Hansen LS, Christensen LK, Laursen LC. A proposal for a practical treatment guideline designed for the initial two-hours of the management of patients with acute severe asthma and COPD using the principles of evidence-based medicine. Respir Med 2002;96:659–671 [DOI] [PubMed] [Google Scholar]
- 19.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD. 2011. Available at www.goldcopd.org/ (last accessed February24, 2014)
- 20.Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987;106:196–204 [DOI] [PubMed] [Google Scholar]
- 21.Emme C, Rydahl-Hansen S, Ostergaard B, Schou L, Svarre Jakobsen A, Phanareth K. How virtual admission affects coping—Telemedicine for patients with chronic obstructive pulmonary disease. J Clin Nurs 2014;23:1445–1458 [DOI] [PubMed] [Google Scholar]
- 22.Emme C, Mortensen EL, Rydahl-Hansen S, Ostergaard B, Svarre Jakobsen A, Schou L, Phanareth K. The impact of virtual admission on self-efficacy in patients with chronic obstructive pulmonary disease—A randomised clinical trial. J Clin Nurs 2014;23:3124–3137 [DOI] [PubMed] [Google Scholar]
- 23.Schou L, Ostergaard B, Rydahl-Hansen S, Rasmussen LS, Emme C, Jakobsen AS, et al. A randomised trial of telemedicine-based treatment versus conventional hospitalisation in patients with severe COPD and exacerbation—Effect on self-reported outcome. J Telemed Telecare 2013April23 [Epub ahead of print]. doi: 10.1177/1357633X13483255 [DOI] [PubMed] [Google Scholar]
- 24.Niewoehner DE, Erbland ML, Deupree RH, Collins D, Gross NJ, Light RW, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. N Engl J Med 1999;340:1941–1947 [DOI] [PubMed] [Google Scholar]
- 25.Erbland ML, Deupree RH, Niewoehner DE. Systemic corticosteroids in chronic obstructive pulmonary disease exacerbations (SCCOPE): Rationale and design of an equivalence trial. Veterans Administration Cooperative Trials SCCOPE Study Group. Control Clin Trials 1998;19:404–417 [DOI] [PubMed] [Google Scholar]
- 26.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at www.R-project.org/ (last accessed February24, 2014)
- 27.Andersen PK, Abildstrøm SZ, Rosthøj S. Competing risks as a multi-state model. Stat Methods Med Res 2002;11:203–215 [DOI] [PubMed] [Google Scholar]
- 28.Marubini E, Valsecchi MG. Analysing survival data from clinical trials and observational studies. Chichester, United Kingdom: Wiley, 1995 [Google Scholar]
- 29.Tunes da Silva G, Logan BR, Klein JP. Methods for equivalence and noninferiority testing. Biol Blood Marrow Transplant 2009;15:120–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cook RJ, Lawless J. The statistical analysis of recurrent cvents. New York: Springer, 2007 [Google Scholar]
- 31.Kurland BF, Johnson LL, Egleston BL, Diehr PH. Longitudinal data with follow-up truncated by death: Match the analysis method to research aims. Stat Sci 2009;24:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eriksen N, Hansen EF, Munch EP, Rasmussen FV, Vestbo J. Chronic obstructive pulmonary disease. Admission, course and prognosis [in Danish]. Ugeskr Laeger 2003;165:3499–3502 [PubMed] [Google Scholar]
- 33.McCurdy BR. Hospital-at-home programs for patients with acute exacerbations of chronic obstructive pulmonary disease (COPD): An evidence-based analysis. Ont Health Technol Assess Ser 2012;12:1–65 [PMC free article] [PubMed] [Google Scholar]
- 34.Report from the Swedish Council on Technology Assessment in Health Care (SBU). Literature searching and evidence interpretation for assessing health care practices. Int J Technol Assess Health Care 1994;10:714–715 [DOI] [PubMed] [Google Scholar]
- 35.Sanders C, Rogers A, Bowen R, Bower P, Hirani S, Cartwright M, et al. Exploring barriers to participation and adoption of telehealth and telecare within the Whole System Demonstrator trial: A qualitative study. BMC Health Serv Res 2012;12:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cartwright M, Hirani SP, Rixon L, Beynon M, Doll H, Bower P, et al. Effect of telehealth on quality of life and psychological outcomes over 12 months (Whole Systems Demonstrator telehealth questionnaire study): Nested study of patient reported outcomes in a pragmatic, cluster randomised controlled trial. BMJ 2013;346:f653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finkelstein SM, Speedie SM, Demiris G, Veen M, Lundgren JM, Potthoff S. Telehomecare: Quality, perception, satisfaction. Telemed J E Health 2004;10:122–128 [DOI] [PubMed] [Google Scholar]