Abstract
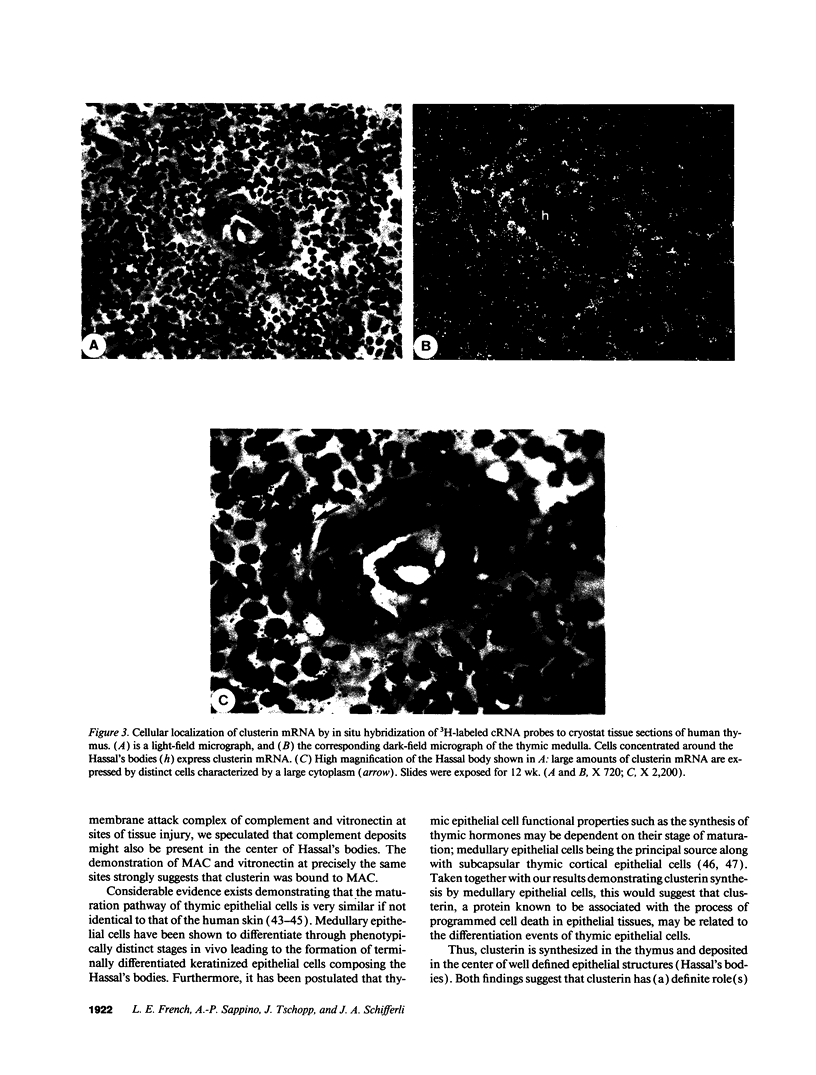
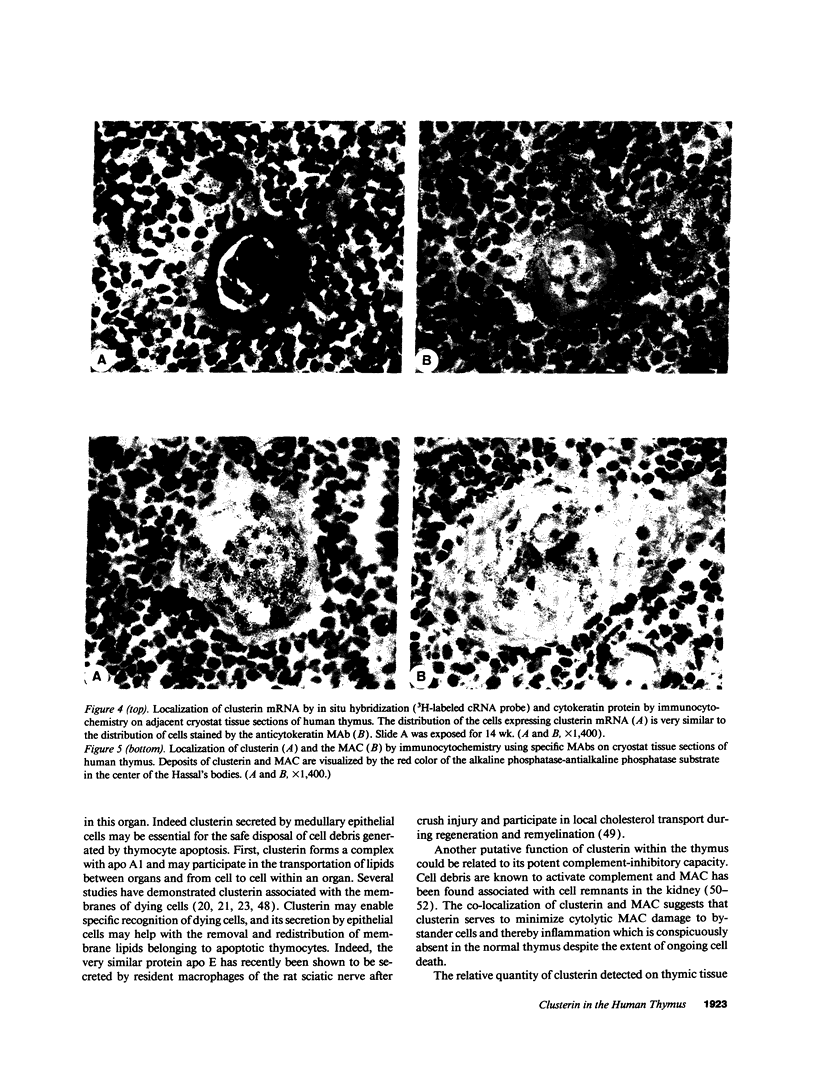
Clusterin is a multifunctional protein endowed with cell-aggregating, complement-inhibitory, and lipid-binding properties. Since several studies have demonstrated highly increased clusterin gene expression in epithelial and nervous tissues regressing as a consequence of tissue involution and apoptotic cell death, clusterin is also considered as a specific marker of dying cells. To determine whether clusterin expression is also upregulated during thymocyte death occurring during the negative selection process we analyzed the cellular distribution of clusterin mRNA and protein by in situ hybridization and immunocytochemistry in the human thymus. We observed that the expression of clusterin mRNA was confined to cells present in the thymic medulla, concentrated mainly around Hassal's bodies. Immunostaining of adjacent sections with antikeratin Ab revealed that cells containing clusterin mRNA were predominantly epithelial. By contrast no clusterin mRNA was found in thymocytes by in situ hybridization and Northern blot analysis of total RNA from purified thymocyte populations. Clusterin protein colocalized with the membrane attack complex of complement and vitronectin in the center of the largest Hassal's bodies, but was not detectable by immunocytochemistry in or at the surface of epithelial cells. Our results demonstrate that clusterin gene expression does not take place in apoptotic thymocytes, and therefore that clusterin synthesis by the dying cell is probably not a prerequisite to its death. However, synthesis of clusterin by medullary epithelial cells may be related to their terminal differentiation, and, furthermore, its presence in Hassal's bodies raises the possibility that the secreted protein is involved in the disposal of cell debris resulting from thymocyte apoptosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandyk M. G., Sawczuk I. S., Olsson C. A., Katz A. E., Buttyan R. Characterization of the products of a gene expressed during androgen-programmed cell death and their potential use as a marker of urogenital injury. J Urol. 1990 Feb;143(2):407–413. doi: 10.1016/s0022-5347(17)39975-5. [DOI] [PubMed] [Google Scholar]
- Blaschuk O., Burdzy K., Fritz I. B. Purification and characterization of a cell-aggregating factor (clusterin), the major glycoprotein in ram rete testis fluid. J Biol Chem. 1983 Jun 25;258(12):7714–7720. [PubMed] [Google Scholar]
- Boyles J. K., Zoellner C. D., Anderson L. J., Kosik L. M., Pitas R. E., Weisgraber K. H., Hui D. Y., Mahley R. W., Gebicke-Haerter P. J., Ignatius M. J. A role for apolipoprotein E, apolipoprotein A-I, and low density lipoprotein receptors in cholesterol transport during regeneration and remyelination of the rat sciatic nerve. J Clin Invest. 1989 Mar;83(3):1015–1031. doi: 10.1172/JCI113943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttyan R., Olsson C. A., Pintar J., Chang C., Bandyk M., Ng P. Y., Sawczuk I. S. Induction of the TRPM-2 gene in cells undergoing programmed death. Mol Cell Biol. 1989 Aug;9(8):3473–3481. doi: 10.1128/mcb.9.8.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi N. H., Mazda T., Tomita M. A serum protein SP40,40 modulates the formation of membrane attack complex of complement on erythrocytes. Mol Immunol. 1989 Sep;26(9):835–840. doi: 10.1016/0161-5890(89)90139-9. [DOI] [PubMed] [Google Scholar]
- Choi N. H., Nakano Y., Tobe T., Mazda T., Tomita M. Incorporation of SP-40,40 into the soluble membrane attack complex (SMAC, SC5b-9) of complement. Int Immunol. 1990;2(5):413–417. doi: 10.1093/intimm/2.5.413. [DOI] [PubMed] [Google Scholar]
- Cohen J. J. Programmed cell death in the immune system. Adv Immunol. 1991;50:55–85. doi: 10.1016/s0065-2776(08)60822-6. [DOI] [PubMed] [Google Scholar]
- Collard M. W., Griswold M. D. Biosynthesis and molecular cloning of sulfated glycoprotein 2 secreted by rat Sertoli cells. Biochemistry. 1987 Jun 16;26(12):3297–3303. doi: 10.1021/bi00386a008. [DOI] [PubMed] [Google Scholar]
- Compton M. M., Cidlowski J. A. Identification of a glucocorticoid-induced nuclease in thymocytes. A potential "lysis gene" product. J Biol Chem. 1987 Jun 15;262(17):8288–8292. [PubMed] [Google Scholar]
- Connor J., Buttyan R., Olsson C. A., D'Agati V., O'Toole K., Sawczuk I. S. SGP-2 expression as a genetic marker of progressive cellular pathology in experimental hydronephrosis. Kidney Int. 1991 Jun;39(6):1098–1103. doi: 10.1038/ki.1991.139. [DOI] [PubMed] [Google Scholar]
- Dent A. L., Matis L. A., Hooshmand F., Widacki S. M., Bluestone J. A., Hedrick S. M. Self-reactive gamma delta T cells are eliminated in the thymus. Nature. 1990 Feb 22;343(6260):714–719. doi: 10.1038/343714a0. [DOI] [PubMed] [Google Scholar]
- Falk R. J., Dalmasso A. P., Kim Y., Tsai C. H., Scheinman J. I., Gewurz H., Michael A. F. Neoantigen of the polymerized ninth component of complement. Characterization of a monoclonal antibody and immunohistochemical localization in renal disease. J Clin Invest. 1983 Aug;72(2):560–573. doi: 10.1172/JCI111004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk R. J., Sisson S. P., Dalmasso A. P., Kim Y., Michael A. F., Vernier R. L. Ultrastructural localization of the membrane attack complex of complement in human renal tissues. Am J Kidney Dis. 1987 Feb;9(2):121–128. doi: 10.1016/s0272-6386(87)80089-6. [DOI] [PubMed] [Google Scholar]
- French L. E., Polla L. L., Tschopp J., Schifferli J. A. Membrane attack complex (MAC) deposits in skin are not always accompanied by S-protein and clusterin. J Invest Dermatol. 1992 May;98(5):758–763. doi: 10.1111/1523-1747.ep12499946. [DOI] [PubMed] [Google Scholar]
- French L. E., Tschopp J., Schifferli J. A. Clusterin in renal tissue: preferential localization with the terminal complement complex and immunoglobulin deposits in glomeruli. Clin Exp Immunol. 1992 Jun;88(3):389–393. doi: 10.1111/j.1365-2249.1992.tb06459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz I. B., Burdzy K., Sétchell B., Blaschuk O. Ram rete testis fluid contains a protein (clusterin) which influences cell-cell interactions in vitro. Biol Reprod. 1983 Jun;28(5):1173–1188. doi: 10.1095/biolreprod28.5.1173. [DOI] [PubMed] [Google Scholar]
- Golstein P., Ojcius D. M., Young J. D. Cell death mechanisms and the immune system. Immunol Rev. 1991 Jun;121:29–65. doi: 10.1111/j.1600-065x.1991.tb00822.x. [DOI] [PubMed] [Google Scholar]
- Grima J., Zwain I., Lockshin R. A., Bardin C. W., Cheng C. Y. Diverse secretory patterns of clusterin by epididymis and prostate/seminal vesicles undergoing cell regression after orchiectomy. Endocrinology. 1990 Jun;126(6):2989–2997. doi: 10.1210/endo-126-6-2989. [DOI] [PubMed] [Google Scholar]
- Griswold M. D., Roberts K., Bishop P. Purification and characterization of a sulfated glycoprotein secreted by Sertoli cells. Biochemistry. 1986 Nov 18;25(23):7265–7270. doi: 10.1021/bi00371a003. [DOI] [PubMed] [Google Scholar]
- Hartmann K., Rauch J., Urban J., Parczyk K., Diel P., Pilarsky C., Appel D., Haase W., Mann K., Weller A. Molecular cloning of gp 80, a glycoprotein complex secreted by kidney cells in vitro and in vivo. A link to the reproductive system and to the complement cascade. J Biol Chem. 1991 May 25;266(15):9924–9931. [PubMed] [Google Scholar]
- Haynes B. F. Phenotypic characterization and ontogeny of components of the human thymic microenvironment. Clin Res. 1984 Dec;32(5):500–507. [PubMed] [Google Scholar]
- Hinglais N., Kazatchkine M. D., Bhakdi S., Appay M. D., Mandet C., Grossetete J., Bariety J. Immunohistochemical study of the C5b-9 complex of complement in human kidneys. Kidney Int. 1986 Sep;30(3):399–410. doi: 10.1038/ki.1986.198. [DOI] [PubMed] [Google Scholar]
- Huarte J., Belin D., Bosco D., Sappino A. P., Vassalli J. D. Plasminogen activator and mouse spermatozoa: urokinase synthesis in the male genital tract and binding of the enzyme to the sperm cell surface. J Cell Biol. 1987 May;104(5):1281–1289. doi: 10.1083/jcb.104.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R. W., Hochstrasser A. C., Borghini I., Martin B., Pometta D., Hochstrasser D. Characterization of a human high density lipoprotein-associated protein, NA1/NA2. Identity with SP-40,40, an inhibitor of complement-mediated cytolysis. Arterioscler Thromb. 1991 May-Jun;11(3):645–652. doi: 10.1161/01.atv.11.3.645. [DOI] [PubMed] [Google Scholar]
- Jenne D. E., Lowin B., Peitsch M. C., Böttcher A., Schmitz G., Tschopp J. Clusterin (complement lysis inhibitor) forms a high density lipoprotein complex with apolipoprotein A-I in human plasma. J Biol Chem. 1991 Jun 15;266(17):11030–11036. [PubMed] [Google Scholar]
- Jenne D. E., Tschopp J. Molecular structure and functional characterization of a human complement cytolysis inhibitor found in blood and seminal plasma: identity to sulfated glycoprotein 2, a constituent of rat testis fluid. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7123–7127. doi: 10.1073/pnas.86.18.7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Kirszbaum L., Sharpe J. A., Murphy B., d'Apice A. J., Classon B., Hudson P., Walker I. D. Molecular cloning and characterization of the novel, human complement-associated protein, SP-40,40: a link between the complement and reproductive systems. EMBO J. 1989 Mar;8(3):711–718. doi: 10.1002/j.1460-2075.1989.tb03430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laster A. J., Itoh T., Palker T. J., Haynes B. F. The human thymic microenvironment: thymic epithelium contains specific keratins associated with early and late stages of epidermal keratinocyte maturation. Differentiation. 1986;31(1):67–77. doi: 10.1111/j.1432-0436.1986.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Lobach D. F., Haynes B. F. Ontogeny of the human thymus during fetal development. J Clin Immunol. 1987 Mar;7(2):81–97. doi: 10.1007/BF00916002. [DOI] [PubMed] [Google Scholar]
- Lobach D. F., Itoh T., Singer K. H., Haynes B. F. The thymic microenvironment. Characterization of in vitro differentiation of the IT26R21 rat thymic epithelial cell line. Differentiation. 1987;34(1):50–59. doi: 10.1111/j.1432-0436.1987.tb00050.x. [DOI] [PubMed] [Google Scholar]
- Lobach D. F., Scearce R. M., Haynes B. F. The human thymic microenvironment. Phenotypic characterization of Hassall's bodies with the use of monoclonal antibodies. J Immunol. 1985 Jan;134(1):250–257. [PubMed] [Google Scholar]
- MacDonald H. R., Howe R. C., Pedrazzini T., Lees R. K., Budd R. C., Schneider R., Liao N. S., Zinkernagel R. M., Louis J. A., Raulet D. H. T-cell lineages, repertoire selection and tolerance induction. Immunol Rev. 1988 Aug;104:157–182. doi: 10.1111/j.1600-065x.1988.tb00762.x. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Lees R. K. Programmed death of autoreactive thymocytes. Nature. 1990 Feb 15;343(6259):642–644. doi: 10.1038/343642a0. [DOI] [PubMed] [Google Scholar]
- May P. C., Lampert-Etchells M., Johnson S. A., Poirier J., Masters J. N., Finch C. E. Dynamics of gene expression for a hippocampal glycoprotein elevated in Alzheimer's disease and in response to experimental lesions in rat. Neuron. 1990 Dec;5(6):831–839. doi: 10.1016/0896-6273(90)90342-d. [DOI] [PubMed] [Google Scholar]
- Mollnes T. E., Lea T., Harboe M., Tschopp J. Monoclonal antibodies recognizing a neoantigen of poly(C9) detect the human terminal complement complex in tissue and plasma. Scand J Immunol. 1985 Aug;22(2):183–195. doi: 10.1111/j.1365-3083.1985.tb01870.x. [DOI] [PubMed] [Google Scholar]
- Murphy B. F., Davies D. J., Morrow W., d'Apice A. J. Localization of terminal complement components S-protein and SP-40,40 in renal biopsies. Pathology. 1989 Oct;21(4):275–278. doi: 10.3109/00313028909061073. [DOI] [PubMed] [Google Scholar]
- Murphy B. F., Kirszbaum L., Walker I. D., d'Apice A. J. SP-40,40, a newly identified normal human serum protein found in the SC5b-9 complex of complement and in the immune deposits in glomerulonephritis. J Clin Invest. 1988 Jun;81(6):1858–1864. doi: 10.1172/JCI113531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. F., Saunders J. R., O'Bryan M. K., Kirszbaum L., Walker I. D., d'Apice A. J. SP-40,40 is an inhibitor of C5b-6-initiated haemolysis. Int Immunol. 1989;1(5):551–554. doi: 10.1093/intimm/1.5.551. [DOI] [PubMed] [Google Scholar]
- O'Bryan M. K., Baker H. W., Saunders J. R., Kirszbaum L., Walker I. D., Hudson P., Liu D. Y., Glew M. D., d'Apice A. J., Murphy B. F. Human seminal clusterin (SP-40,40). Isolation and characterization. J Clin Invest. 1990 May;85(5):1477–1486. doi: 10.1172/JCI114594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens G. P., Hahn W. E., Cohen J. J. Identification of mRNAs associated with programmed cell death in immature thymocytes. Mol Cell Biol. 1991 Aug;11(8):4177–4188. doi: 10.1128/mcb.11.8.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappino A. P., Busso N., Belin D., Vassalli J. D. Increase of urokinase-type plasminogen activator gene expression in human lung and breast carcinomas. Cancer Res. 1987 Aug 1;47(15):4043–4046. [PubMed] [Google Scholar]
- Sappino A. P., Huarte J., Vassalli J. D., Belin D. Sites of synthesis of urokinase and tissue-type plasminogen activators in the murine kidney. J Clin Invest. 1991 Mar;87(3):962–970. doi: 10.1172/JCI115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawczuk I. S., Hoke G., Olsson C. A., Connor J., Buttyan R. Gene expression in response to acute unilateral ureteral obstruction. Kidney Int. 1989 Jun;35(6):1315–1319. doi: 10.1038/ki.1989.128. [DOI] [PubMed] [Google Scholar]
- Sensibar J. A., Griswold M. D., Sylvester S. R., Buttyan R., Bardin C. W., Cheng C. Y., Dudek S., Lee C. Prostatic ductal system in rats: regional variation in localization of an androgen-repressed gene product, sulfated glycoprotein-2. Endocrinology. 1991 Apr;128(4):2091–2102. doi: 10.1210/endo-128-4-2091. [DOI] [PubMed] [Google Scholar]
- Steinmann G. G., Klaus B., Müller-Hermelink H. K. The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand J Immunol. 1985 Nov;22(5):563–575. doi: 10.1111/j.1365-3083.1985.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G., Smith A. L., Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984 Jan;142(1):67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]
- de Silva H. V., Harmony J. A., Stuart W. D., Gil C. M., Robbins J. Apolipoprotein J: structure and tissue distribution. Biochemistry. 1990 Jun 5;29(22):5380–5389. doi: 10.1021/bi00474a025. [DOI] [PubMed] [Google Scholar]
- de Silva H. V., Stuart W. D., Duvic C. R., Wetterau J. R., Ray M. J., Ferguson D. G., Albers H. W., Smith W. R., Harmony J. A. A 70-kDa apolipoprotein designated ApoJ is a marker for subclasses of human plasma high density lipoproteins. J Biol Chem. 1990 Aug 5;265(22):13240–13247. [PubMed] [Google Scholar]
- von Boehmer H., Teh H. S., Kisielow P. The thymus selects the useful, neglects the useless and destroys the harmful. Immunol Today. 1989 Feb;10(2):57–61. doi: 10.1016/0167-5699(89)90307-1. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. The developmental biology of T lymphocytes. Annu Rev Immunol. 1988;6:309–326. doi: 10.1146/annurev.iy.06.040188.001521. [DOI] [PubMed] [Google Scholar]