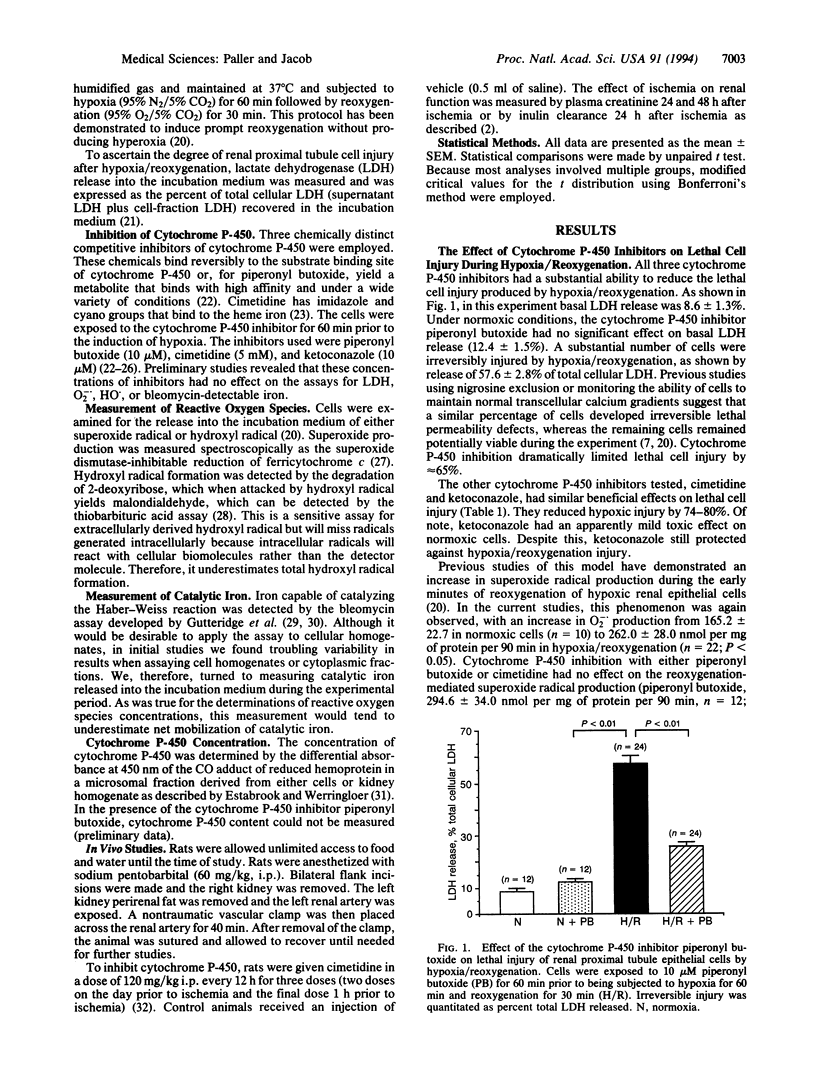
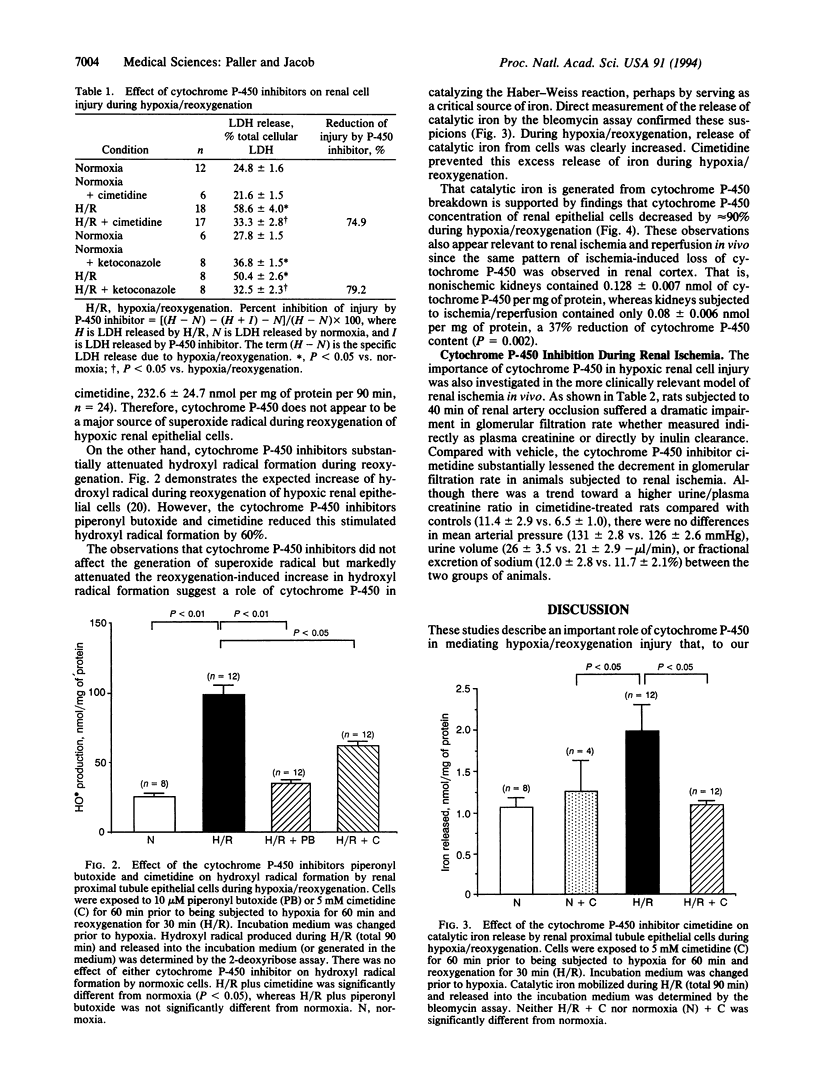
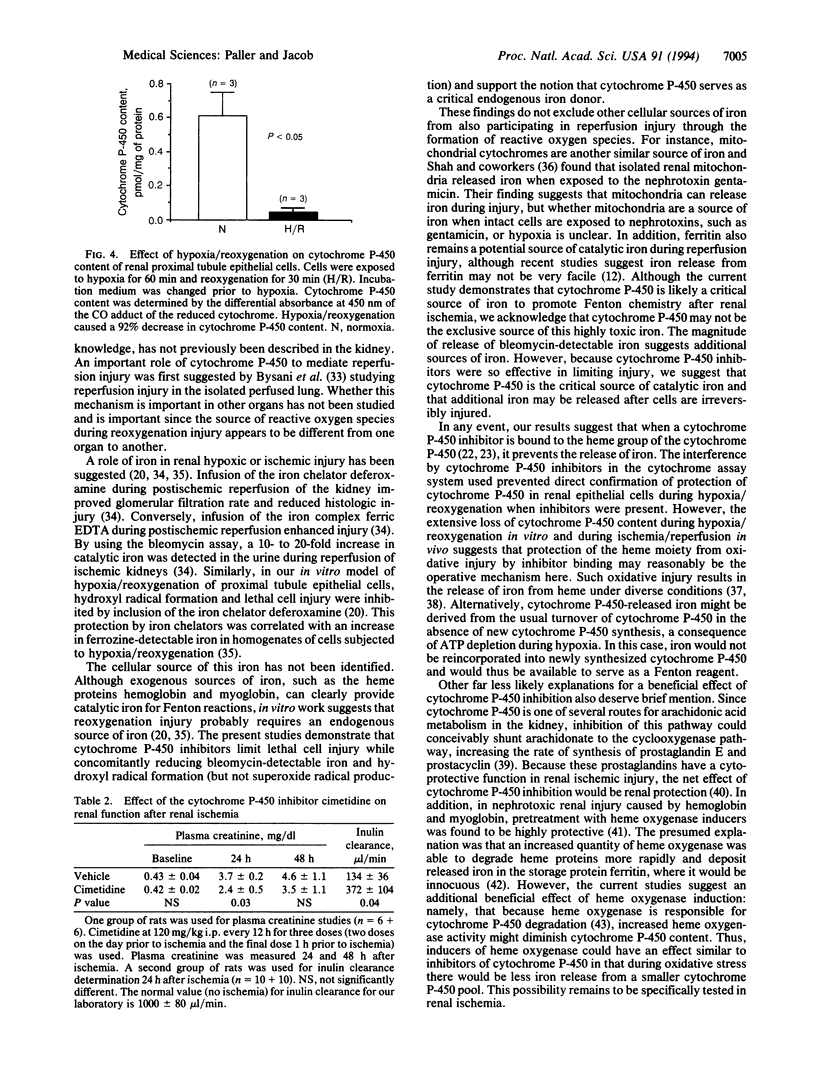
Abstract
Renal reperfusion injury results from oxygen radical generation. During reoxygenation of hypoxic kidney cells, xanthine oxidase produces superoxide radical, which eventuates in hydroxyl radical formation by the Fenton reaction. This reaction, catalyzed by transition metals such as iron, is particularly important because hydroxyl radical is highly reactive with a wide variety of biomolecules. We tested the hypothesis that this catalytic function is fostered by iron released from the heme moiety of cytochrome P-450. Primary cultures of rat proximal tubule epithelial cells studied in a subconfluent stage were subjected to 60 min of hypoxia and 30 min of reoxygenation. When cells were pretreated with one of three cytochrome P-450 inhibitors (piperonyl butoxide, cimetidine, or ketoconazole), lethal cell injury was attenuated. There was the expected increase in O2-. production during hypoxia/reoxygenation that cytochrome P-450 inhibitors did not prevent; on the other hand, inhibitors did prevent reoxygenation-induced hydroxyl radical formation. Analogously, the increase in catalytic iron (bleomycin-detectable iron) that accompanies hypoxia/reoxygenation did not occur in the presence of cytochrome P-450 inhibitors. In vivo studies confirmed a protective effect of cytochrome P-450 inhibition because glomerular filtration rate was better preserved in rats pretreated with cimetidine and then subjected to renal artery occlusion. In summary, several chemically distinct cytochrome P-450 inhibitors reduced iron release, and thereby, hydroxyl radical formation and reoxygenation-induced lethal cell injury, without inhibiting superoxide radical formation. We conclude that highly labile P-450 may act as an Fe-donating catalyst for Fenton reaction production of HO.-mediated reperfusion injury.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham N. G., Lin J. H., Schwartzman M. L., Levere R. D., Shibahara S. The physiological significance of heme oxygenase. Int J Biochem. 1988;20(6):543–558. doi: 10.1016/0020-711x(88)90093-6. [DOI] [PubMed] [Google Scholar]
- Balla G., Jacob H. S., Balla J., Rosenberg M., Nath K., Apple F., Eaton J. W., Vercellotti G. M. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem. 1992 Sep 5;267(25):18148–18153. [PubMed] [Google Scholar]
- Bast A., Savenije-Chapel E. M., Kroes B. H. Inhibition of mono-oxygenase and oxidase activity of rat-hepatic cytochrome P-450 by H2-receptor blockers. Xenobiotica. 1984 May;14(5):399–408. doi: 10.3109/00498258409151428. [DOI] [PubMed] [Google Scholar]
- Burgess E., Blair A., Krichman K., Cutler R. E. Inhibition of renal creatinine secretion by cimetidine in humans. Ren Physiol. 1982;5(1):27–30. doi: 10.1159/000172836. [DOI] [PubMed] [Google Scholar]
- Bysani G. K., Kennedy T. P., Ky N., Rao N. V., Blaze C. A., Hoidal J. R. Role of cytochrome P-450 in reperfusion injury of the rabbit lung. J Clin Invest. 1990 Nov;86(5):1434–1441. doi: 10.1172/JCI114859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumond M. C., Deen W. M. Structural determinants of glomerular hydraulic permeability. Am J Physiol. 1994 Jan;266(1 Pt 2):F1–12. doi: 10.1152/ajprenal.1994.266.1.F1. [DOI] [PubMed] [Google Scholar]
- Estabrook R. W., Werringloer J. The measurement of difference spectra: application to the cytochromes of microsomes. Methods Enzymol. 1978;52:212–220. doi: 10.1016/s0076-6879(78)52024-7. [DOI] [PubMed] [Google Scholar]
- Ferreri N. R., Schwartzman M., Ibraham N. G., Chander P. N., McGiff J. C. Arachidonic acid metabolism in a cell suspension isolated from rabbit renal outer medulla. J Pharmacol Exp Ther. 1984 Nov;231(2):441–448. [PubMed] [Google Scholar]
- Fleming S. Immunocytochemical localization of ferritin in the kidney and renal tumours. Eur Urol. 1987;13(6):407–411. doi: 10.1159/000472835. [DOI] [PubMed] [Google Scholar]
- Gesek F. A., Wolff D. W., Strandhoy J. W. Improved separation method for rat proximal and distal renal tubules. Am J Physiol. 1987 Aug;253(2 Pt 2):F358–F365. doi: 10.1152/ajprenal.1987.253.2.F358. [DOI] [PubMed] [Google Scholar]
- González-Flecha B., Cutrin J. C., Boveris A. Time course and mechanism of oxidative stress and tissue damage in rat liver subjected to in vivo ischemia-reperfusion. J Clin Invest. 1993 Feb;91(2):456–464. doi: 10.1172/JCI116223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. N., Rutili G., McCord J. M. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981 Jul;81(1):22–29. [PubMed] [Google Scholar]
- Greene E. L., Paller M. S. Xanthine oxidase produces O2-. in posthypoxic injury of renal epithelial cells. Am J Physiol. 1992 Aug;263(2 Pt 2):F251–F255. doi: 10.1152/ajprenal.1992.263.2.F251. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Rowley D. A., Halliwell B. Superoxide-dependent formation of hydroxyl radicals and lipid peroxidation in the presence of iron salts. Detection of 'catalytic' iron and anti-oxidant activity in extracellular fluids. Biochem J. 1982 Sep 15;206(3):605–609. doi: 10.1042/bj2060605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Rowley D. A., Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Detection of 'free' iron in biological systems by using bleomycin-dependent degradation of DNA. Biochem J. 1981 Oct 1;199(1):263–265. doi: 10.1042/bj1990263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Grootveld M., Gutteridge J. M. Methods for the measurement of hydroxyl radicals in biomedical systems: deoxyribose degradation and aromatic hydroxylation. Methods Biochem Anal. 1988;33:59–90. doi: 10.1002/9780470110546.ch2. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel S., Salan M. A., Kanner J. Iron release from metmyoglobin, methaemoglobin and cytochrome c by a system generating hydrogen peroxide. Free Radic Res Commun. 1988;5(1):11–19. doi: 10.3109/10715768809068554. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr Measurement of O2- secreted by monocytes and macrophages. Methods Enzymol. 1984;105:365–369. doi: 10.1016/s0076-6879(84)05049-7. [DOI] [PubMed] [Google Scholar]
- Kennedy T. P., Rao N. V., Hopkins C., Pennington L., Tolley E., Hoidal J. R. Role of reactive oxygen species in reperfusion injury of the rabbit lung. J Clin Invest. 1989 Apr;83(4):1326–1335. doi: 10.1172/JCI114019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuthan H., Tsuji H., Graf H., Ullrich V. Generation of superoxide anion as a source of hydrogen peroxide in a reconstituted monooxygenase system. FEBS Lett. 1978 Jul 15;91(2):343–345. doi: 10.1016/0014-5793(78)81206-x. [DOI] [PubMed] [Google Scholar]
- Liochev S. I., Fridovich I. The role of O2.- in the production of HO.: in vitro and in vivo. Free Radic Biol Med. 1994 Jan;16(1):29–33. doi: 10.1016/0891-5849(94)90239-9. [DOI] [PubMed] [Google Scholar]
- Mason D. Y., Taylor C. R. Distribution of transferrin, ferritin, and lactoferrin in human tissues. J Clin Pathol. 1978 Apr;31(4):316–327. doi: 10.1136/jcp.31.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath K. A., Balla G., Vercellotti G. M., Balla J., Jacob H. S., Levitt M. D., Rosenberg M. E. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992 Jul;90(1):267–270. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netter K. J. Inhibition of oxidative drug metabolism in microsomes. Pharmacol Ther. 1980;10(3):515–535. doi: 10.1016/0163-7258(80)90029-7. [DOI] [PubMed] [Google Scholar]
- Pai K. S., Ravindranath V. Protection and potentiation of MPTP-induced toxicity by cytochrome P-450 inhibitors and inducer: in vitro studies with brain slices. Brain Res. 1991 Aug 2;555(2):239–244. doi: 10.1016/0006-8993(91)90347-x. [DOI] [PubMed] [Google Scholar]
- Paller M. S., Hedlund B. E. Role of iron in postischemic renal injury in the rat. Kidney Int. 1988 Oct;34(4):474–480. doi: 10.1038/ki.1988.205. [DOI] [PubMed] [Google Scholar]
- Paller M. S. Hemoglobin- and myoglobin-induced acute renal failure in rats: role of iron in nephrotoxicity. Am J Physiol. 1988 Sep;255(3 Pt 2):F539–F544. doi: 10.1152/ajprenal.1988.255.3.F539. [DOI] [PubMed] [Google Scholar]
- Paller M. S., Hoidal J. R., Ferris T. F. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984 Oct;74(4):1156–1164. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller M. S., Manivel J. C. Prostaglandins protect kidneys against ischemic and toxic injury by a cellular effect. Kidney Int. 1992 Dec;42(6):1345–1354. doi: 10.1038/ki.1992.426. [DOI] [PubMed] [Google Scholar]
- Paller M. S., Neumann T. V. Reactive oxygen species and rat renal epithelial cells during hypoxia and reoxygenation. Kidney Int. 1991 Dec;40(6):1041–1049. doi: 10.1038/ki.1991.312. [DOI] [PubMed] [Google Scholar]
- Reif D. W. Ferritin as a source of iron for oxidative damage. Free Radic Biol Med. 1992;12(5):417–427. doi: 10.1016/0891-5849(92)90091-t. [DOI] [PubMed] [Google Scholar]
- Rendić S., Kajfez F., Ruf H. H. Characterization of cimetidine, ranitidine, and related structures' interaction with cytochrome P-450. Drug Metab Dispos. 1983 Mar-Apr;11(2):137–142. [PubMed] [Google Scholar]
- Sadrzadeh S. M., Anderson D. K., Panter S. S., Hallaway P. E., Eaton J. W. Hemoglobin potentiates central nervous system damage. J Clin Invest. 1987 Feb;79(2):662–664. doi: 10.1172/JCI112865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S. V., Walker P. D. Evidence suggesting a role for hydroxyl radical in glycerol-induced acute renal failure. Am J Physiol. 1988 Sep;255(3 Pt 2):F438–F443. doi: 10.1152/ajprenal.1988.255.3.F438. [DOI] [PubMed] [Google Scholar]
- Testa B., Jenner P. Inhibitors of Cytochrome P-450s and their mechanism of action. Drug Metab Rev. 1981;12(1):1–117. doi: 10.3109/03602538109011082. [DOI] [PubMed] [Google Scholar]
- Thomas C. E., Morehouse L. A., Aust S. D. Ferritin and superoxide-dependent lipid peroxidation. J Biol Chem. 1985 Mar 25;260(6):3275–3280. [PubMed] [Google Scholar]
- Todd N. W., Hunt C. M., Kennedy T. P., Piantadosi C. A., Whorton A. R. Effects of inhibition and induction of cytochrome P-450 isozymes on hyperoxic lung injury in rats. Am J Respir Cell Mol Biol. 1992 Aug;7(2):222–229. doi: 10.1165/ajrcmb/7.2.222. [DOI] [PubMed] [Google Scholar]
- Ueda N., Guidet B., Shah S. V. Gentamicin-induced mobilization of iron from renal cortical mitochondria. Am J Physiol. 1993 Sep;265(3 Pt 2):F435–F439. doi: 10.1152/ajprenal.1993.265.3.F435. [DOI] [PubMed] [Google Scholar]
- White R. E., Coon M. J. Oxygen activation by cytochrome P-450. Annu Rev Biochem. 1980;49:315–356. doi: 10.1146/annurev.bi.49.070180.001531. [DOI] [PubMed] [Google Scholar]
- Williams J. F. Induction of tolerance in mice and rats to the effect of endotoxin to decrease the hepatic microsomal mixed-function oxidase system. Evidence for a possible macrophage-derived factor in the endotoxin effect. Int J Immunopharmacol. 1985;7(4):501–509. doi: 10.1016/0192-0561(85)90069-4. [DOI] [PubMed] [Google Scholar]
- Zweier J. L., Flaherty J. T., Weisfeldt M. L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]



