Abstract
Background:
The aim of this study was to compare the shear bond strength of four one-step self-etch adhesives with different pH values to enamel and dentin.
Materials and Methods:
In this in vitro study, 200 bovine permanent mandibular incisors were used. Four one-step self-etch adhesives with different pH values were tested both on enamel and on dentin: Adper™ Easy Bond Self-Etch Adhesive (pH = 0.8-1), Futurabond NR (pH=2), G-aenial Bond (pH = 1.5), Clearfil S3 Bond (pH = 2.7). After adhesive systems application, a nanohybrid composite resin was inserted into the bonded surface. The specimens were placed in a universal testing machine. The shear bond strength was performed at a cross-head speed of 1 mm/min until the sample rupture. The shear bond strength values (MPa) of the different groups were compared with analysis of variance after that Kolmogorov and Smirnov tests were applied to assess normality of distributions. P < 0.05 was considered as significant.
Results:
In enamel shear bond strength, the highest shear bond strength values were reported with Futurabond NR (P < 0.01); however, no significant differences were found with Clearfil S3 Bond. The others adhesive systems showed lower shear bond strength values with significant differences between them (P < 0.05). When comparing the dentin shear bond strength, the lowest shear bond strength values were reported with Clearfil S3 Bond (P < 0.05), while there were no significant differences among the other three products (P > 0.05).
Conclusion:
The pH values of adhesive systems did not influence significantly their shear bond strength to enamel or dentin.
Keywords: Dentin, enamel, one-step self-etch adhesives, bond strength
INTRODUCTION
The contemporary adhesive systems can be classified into etch-and-rinse and self-etch adhesives on the basis of the adhesive strategy with enamel and dentin substrates.[1] The etch-and-rinse approach with phosphoric acid has become a standard procedure for the surface conditioning of enamel and dentin prior to adhesive resin application: The infiltration of adhesive resin into the porous zone results in the formation of resin tags, establishing micromechanical retention to etched enamel and dentin. In the self-etch approach demineralization and infiltration occur simultaneously,[2] although with no perfect synchronism.[3] These simplified systems are suggested to reduce technique sensitivity and shorten clinical procedures. Depending on the number of procedures required for bonding, the self-etch adhesives can be subdivided into two-step systems (that require a separate bonding step) or one-step systems (that combine all bonding procedures in a single application).[4] The self-etch adhesives are also classified into three categories based upon their initial pH-value: Mild (pH of 2.5 or more), moderate (pH of approximately 1) and strong (pH < 1), depending on their composition and concentration of polymerizable acids and/or acid resin monomers.[5] Their interaction with enamel is characterized as nanoretentive interlocking with the dissolution of peripheral and central parts of the crystallites and an additional inter- and intra-crystallite monomer infiltration. In literature, some authors[6] controversially discussed the bond strength of adhesive systems with different pH values to enamel and dentin. A morphological study conducted by Moura et al.[6] demonstrated that the application of a self-etch primer did not create a deep enamel etching pattern, unlike the application of phosphoric acid. The performance of self-etch systems in enamel and dentin are controversial because several factors such as the type of the acid, the acid concentration, the duration of etching, the method of application and the bond strength test vary among studies. Due to this fact, while some authors[7,8] have reported superior performance of more acidic systems in particular conditions, others authors[9] have shown that only mild self-etch systems can provide bond strength values as high as those of etch-and-rinse adhesives.
Therefore, the aim of this study was to compare the shear bond strength of four one-step self-etch adhesives with different pH values to enamel and dentin. The null hypothesis to be tested was that there was no difference in bond strengths among one-step self-etch adhesives with different pH values when bonding to dentin and to enamel.
MATERIALS AND METHODS
In this in vitro study, two hundred bovine permanent mandibular incisors freshly extracted and stored in a solution of 0.1% (weight/volume) thymol were used as a substitute for human teeth.[10,11] The criteria for tooth selection included intact buccal enamel with no crack caused by extraction. The teeth were cleansed of soft tissue and embedded in self-curing, fast-setting acrylic resin (Rapid Repair, DeguDent GmbH, Hanau, Germany). Specially fabricated cubical Teflon mould (SSD- Rubber) were filled with the acrylic resin and allowed to cure, thus encasing each specimen while allowing the buccal surface of enamel to be exposed. Each tooth was oriented so that its labial surface was parallel to the shearing force.
Preparation of specimens (enamel)
Half of the incisors collected were used to investigate the pH influence when the adhesion was conducted on enamel surface. The buccal enamel surface of specimens was flattened with aluminum oxide discs of sequentially decreasing granulation (400, 600, 1200 grit) with copious water coolant to obtain flat enamel surfaces.[12] This process standardizes the orientation of enamel prisms, removes the outer hypermineralized and acid-resistant enamel and it is also consistent with clinical practice when the outer 0.5 mm of labial enamel is removed during preparation for veneering.[13]
Preparation of specimens (dentin)
The remaining half of the incisors collected were used to investigate the pH influence when the adhesion was conducted on dentinal surface. The teeth were sectioned parallel to the occlusal surface to expose midcoronal dentin. The exposed dentin surfaces were polished using an automated machine (APL-4; Arotec S.A. Ind Com, Cotia, SP, Brazil) with 600-grit abrasive silicon carbide paper (SiC) disks under water irrigation to obtain a flat and uniform dentin surface.
Materials tested
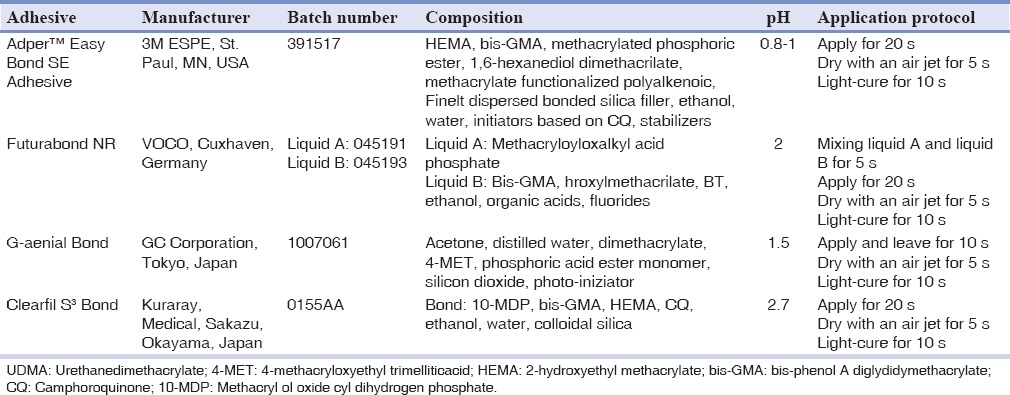
The materials tested in this study included four one-step self-etch adhesives with different pH values: Adper™ Easy Bond Self-Etch Adhesive (pH = 0.8-1), Futurabond NR (pH = 2), G-aenial Bond (pH = 1.5), Clearfil S3 Bond (pH = 2.7). The specifications of all adhesive systems are listed in Table 1.
Table 1.
Adhesive systems tested
Application of adhesive systems (enamel)
The teeth were randomly assigned to four groups of 25 teeth in each and treated as follow:
Group 1e: Adper™ Easy Bond Self-Etch Adhesive (pH = 0.8-1),
Group 2e: Futurabond NR (pH = 2),
Group 3e: G-aenial Bond (pH = 1.5),
Group 4e: Clearfil S3 Bond (pH = 2.7).
The adhesive systems were applied to the demarcated bonding area following the manufacturer's instructions. Before application of the adhesive systems, the labial surface of each incisor was cleaned for 10 s with a mixture of water and fluoride-free pumice in a rubber-polishing cup with a low-speed handpiece. The enamel surface was rinsed with water to remove pumice or debris and then dried with an oil-free air stream.
All adhesive systems were applied to the demarcated bonding area and then cured using a light emitting diode (LED) curing light in one light polymerization mode (Celalux 2 High-Power LED curing-light, Voco GmbH, Cuxhaven, Germany) for 20 s at a light intensity of 1000 mW/cm2.
Application of adhesive systems (dentin)
The teeth were randomly assigned to four groups of 25 teeth in each and treated as follow:
Group 1d: Adper™ Easy Bond Self-Etch Adhesive (pH = 0.8-1),
Group 2d: Futurabond NR (pH = 2),
Group 3d: G-aenial Bond (pH = 1.5),
Group 4d: Clearfil S3 Bond (pH = 2.7).
The adhesive systems were applied to the demarcated bonding area following each manufacturer's instructions. Manufacturers do not specify if the substrate of adhesion should be wet or dry. Due to this fact, in this study, the substrate was gently dried with a cotton pellet in order to avoid dentin dehydration. All adhesives were cured using a LED curing light in one light polymerization mode (Celalux 2 High-Power LED curing-light, Voco GmbH, Cuxhaven, Germany) for 20 s at a light intensity of 1000 mW/cm2.
Application of composite resin
After adhesive systems application, a nanohybrid composite resin (Grandio, Voco GmbH, Cuxhaven, Germany) was carefully inserted into the enamel or dentin surface by packing the material into cylindrical-shaped plastic matrices with an internal diameter of 2 mm and a height of 2 mm. Excess composite was carefully removed from the periphery of the matrix with an explorer. The composite was cured with a LED curing light in one light polymerization mode (Celalux 2 High-Power LED curing-light, Voco GmbH, Cuxhaven, Germany) for 20 s at a light intensity of 1000 mW/cm2 according to manufacturer's instructions. The composite buildups were created. Following polymerization, specimens were stored in distilled water for 24 h at 37°C.
Shear bond strength testing
After storing, all specimens were placed in a universal testing machine (Instron Single Column Testing Systems for Low-Force Testing-Model 3343, Instron Corporation, Norwood, MA, USA). Specimens were secured in the lower jaw of the machine so that the bonded cylinder base was parallel to the shear force direction. The bond strength was performed at a cross-head speed of 1 mm/min for both enamel specimens and dentinal specimens until the sample rupture. The maximum load necessary to debond was recorded in Newton (N) and calculated in MPa as a ratio of Newton to surface area of the cylinder.[14,15] After the testing procedure, the fractured surfaces were examined with an optical microscope (Stereomicroscope SR, Zeiss, Oberkochen, Germany) at a magnification of ×10 to determine failure modes and classified as adhesive failures, cohesive failures within the composite, or cohesive failures within the tooth.
Statistical analysis
Statistical analysis was performed with Stata 9.0 software (Stata, College Station, TX, USA). Descriptive statistics, including the mean, standard deviation, median, and minimum and maximum values were calculated for all groups. Kolmogorov and Smirnov test was applied to assess normality of distributions. An analysis of variance was applied to determine whether significant differences in debond values existed among the groups. The Tukey test was used as post-hoc. P < 0.05 was considered as significant.
RESULTS
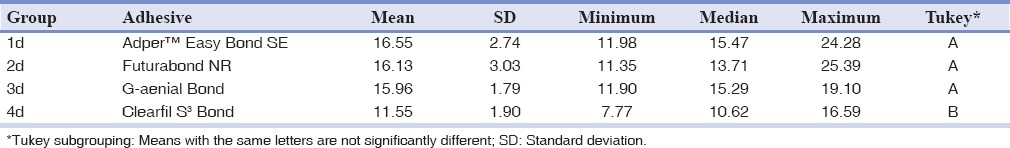
The shear bond strength values (MPa) of the different groups were initially analyzed with descriptive statistics and illustrated in Tables 2 and 3. In the evaluation of the adhesive systems on enamel surface, the highest shear bond strength values were reported with Futurabond NR (P < 0.01); however, no significant differences were found with Clearfil S3 Bond (P > 0.05). The others adhesive systems showed lower shear bond strength values with significant differences between them (P < 0.05). When comparing the adhesive systems values on dentin surface, the lowest shear bond strength values were reported with Clearfil S3 Bond (P < 0.05), while there were no significant differences among the other three products (P > 0.05).
Table 2.
Descriptive statistics (in MPa) of shear bond strengths values when bonding to enamel of the four groups tested
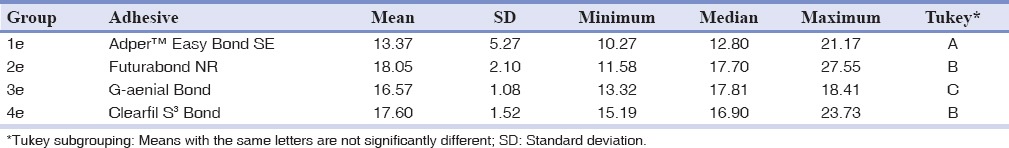
Table 3.
Descriptive statistics (in MPa) of shear bond strengths values when bonding to dentin of the four groups tested
DISCUSSION
Although it is preferable to use extracted human teeth for bonding research,[16,17] it has become increasingly difficult to obtain such samples for laboratory studies in Italy. In order to compare data from the current study with that reported in previous bovine enamel and dentin bond strength tests,[18] bovine teeth were used as a substitute for human teeth in the current study. Bovine teeth have some advantages, as they are easy to obtain in large quantities, are in good condition and have less composition variables than human enamel and dentin.[19] Bovine teeth also have large, flat surfaces and are unlikely to have undergone prior caries challenges that could affect the test results. The mineral distribution within the carious lesions in bovine teeth is reportedly similar to that found in human teeth, and the structural changes that occur in human and bovine teeth are also similar.[20]
As reported in previous studies,[6] it is clear from micro-morphological findings that the use of a stronger acid resulted in a considerable dissolution and a more defined etching pattern; however, this fact did not translate into higher bond strengths, as confirmed in this study. In fact, Adper™ Easy Bond Self-Etch Adhesive (pH = 0.8-1) provides lower bond strength values, when tested on enamel surfaces, when compared under shear bond strength approach. The reason of these results is amenable to other factors, apart from the etching pattern, such as the viscosity of the adhesive, the surface tension of the adhesive itself, the chemical interaction of acidic monomers with enamel and the water concentration.[21,22,23] In particular, as reported in other studies and reviews,[24,25] the methacrylates monomers show low hydrolytic stability in acidic solutions because of the ester portion of the molecule, which could be hydrolyzed in aqueous solutions when pH values are below 1. Adper™ Easy Bond Self-Etch Adhesive contains different functional monomers, such as 2-hydroxyethyl methacrylate (HEMA) and bis-phenol A glycidyl-methacrylate, dissolved in water and ethanol to result in a pH of around 0.9. This composition provides an optimal ability to infilitrate demineralized dentin, which justify the high bond strength values of this adhesive system when tested on dentin. Futurabond NR, which is classified as a moderate acidic adhesive system (pH = 2), maintains an acceptable hydrolytic stability in acidic solutions despite of the ester portion of the molecule. This fact helps to create a balance between the advantages and disadvantages of the influence of pH values, allowing the adhesive system to provide high shear bond strength values on enamel surfaces. The optimal shear bond strength values obtained with G-aenial Bond both in enamel surfaces and in dentin surfaces are due to the composition of the adhesive and to the methodology of use provided. On enamel surfaces, the low pH value allows the dissolution of peripheral and central parts of the crystallites and an additional inter- and intra-crystallite monomer infiltration. The adhesion involves the removal of calcium phosphates and the creation of micro-porosities followed by infiltration and polymerization of resin. On dentin surfaces, the high shear bond strength provided by the adhesive system is due to the composition of the solution, which is based on 4-methacryloxyethyl trimellitic acid (4-META). In fact, the carboxylic group of 4-META renders G-aenial Bond more hydrophilic than the other adhesive systems and more suitable for dentinal surfaces, which are rich in water. However, for the right application of the adhesive, a strong air blowing of the primed surface is requested to accelerate the evaporation of the solvent and of the resultant water droplets formed due to phase separation. The different adhesion strength in enamel and in dentin provided by the Clearfil S3 Bond is due to the monomer and solvent contained in the bonding agent. The presence of 10-methacry lol oxy decyl dihydrogen phosphate provides intense and stable molecular adhesion to the enamel hydroxyapatite-based structure, which justifies high values in shear bond strength when tested on enamel surfaces. However, when the adhesive system is tested on dentinal surfaces, the shear bond values significantly decreased. The presence of HEMA, a monomer with high water dispersion capacity, could allow, in association with alcohol (in particular ethanol), high bond strength values to dentin, but the association with water, used in Clearfil S3 system as solvent together with ethanol, may interfere and provide an incomplete polymerization. Different from thermocycling, which it is demonstrated that should not influence significantly the shear bond strength as reported in previous studies,[26] the formation of a porous hydrogel which allows water to permeate through the adhesive layer, compromising the adhesion, should be investigated with scanning electron microscopy and microleakage studies.
CONCLUSION
Based on the findings of this research, the null hypothesis was supported. The pH values did not influence significantly the shear bond strength of the tested adhesive systems. Enamel was the dental substrate that showed larger adhesive strength and the Futurabond NR (pH = 2) system showed the best performance. On dentinal surfaces the Adper™ Easy Bond SE (pH = 0.8-1) system showed the best performance.
Footnotes
Source of Support: Nil.
Conflict of Interest: The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
REFERENCES
- 1.Van Meerbeek B, De Munck J, Yoshida Y, Inoue S, Vargas M, Vijay P, et al. Buonocore memorial lecture. Adhesion to enamel and dentin: Current status and future challenges. Oper Dent. 2003;28:215–35. [PubMed] [Google Scholar]
- 2.Sano H, Shono T, Takatsu T, Hosoda H. Microporous dentin zone beneath resin-impregnated layer. Oper Dent. 1994;19:59–64. [PubMed] [Google Scholar]
- 3.Carvalho RM, Chersoni S, Frankenberger R, Pashley DH, Prati C, Tay FR. A challenge to the conventional wisdom that simultaneous etching and resin infiltration always occurs in self-etch adhesives. Biomaterials. 2005;26:1035–42. doi: 10.1016/j.biomaterials.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Nikaido T, Nakajima M, Higashi T, Kanemura N, Pereira PN, Tagami J. Shear bond strengths of a single-step bonding system to enamel and dentin. Dent Mater J. 1997;16:40–7. doi: 10.4012/dmj.16.40. [DOI] [PubMed] [Google Scholar]
- 5.Lührs AK, Guhr S, Schilke R, Borchers L, Geurtsen W, Günay H. Shear bond strength of self-etch adhesives to enamel with additional phosphoric acid etching. Oper Dent. 2008;33:155–62. doi: 10.2341/07-63. [DOI] [PubMed] [Google Scholar]
- 6.Moura SK, Reis A, Pelizzaro A, Dal-Bianco K, Loguercio AD, Arana-Chavez VE, et al. Bond strength and morphology of enamel using self-etching adhesive systems with different acidities. J Appl Oral Sci. 2009;17:315–25. doi: 10.1590/S1678-77572009000400009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai YL, Nakajima M, Wang CY, Foxton RM, Lin CP, Tagami J. Influence of etching ability of one-step self-etch adhesives on bonding to sound and non-carious cervical sclerotic dentin. Dent Mater J. 2011;30:941–7. doi: 10.4012/dmj.2011-111. [DOI] [PubMed] [Google Scholar]
- 8.Samimi P, Filsoufi A, Fathpour K. Composite-dentin bond strength of two adhesives in different conditions. Dent Res J. 2007;4:36–9. [Google Scholar]
- 9.Kandaswamy D, Rajan KJ, Venkateshbabu N, Porkodi I. Shear bond strength evaluation of resin composite bonded to glass-ionomer cement using self-etching bonding agents with different pH: In vitro study. J Conserv Dent. 2012;15:27–31. doi: 10.4103/0972-0707.92602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schilke R, Bauss O, Lisson JA, Schuckar M, Geurtsen W. Bovine dentin as a substitute for human dentin in shear bond strength measurements. Am J Dent. 1999;12:92–6. [PubMed] [Google Scholar]
- 11.Tsuchiya H, Tsubota K, Iwasa M, Ando S, Miyazaki M, Platt JA. Influence of adhesive application time on enamel bond strength of single-step self-etch adhesive systems. Oper Dent. 2010;35:77–83. doi: 10.2341/09-064-L. [DOI] [PubMed] [Google Scholar]
- 12.De Munck J, Van Meerbeek B, Satoshi I, Vargas M, Yoshida Y, Armstrong S, et al. Microtensile bond strengths of one- and two-step self-etch adhesives to bur-cut enamel and dentin. Am J Dent. 2003;16:414–20. [PubMed] [Google Scholar]
- 13.Weerasinghe DS, Nikaido T, Wettasinghe KA, Abayakoon JB, Tagami J. Micro-shear bond strength and morphological analysis of a self-etching primer adhesive system to fluorosed enamel. J Dent. 2005;33:419–26. doi: 10.1016/j.jdent.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Jobalia SB, Valente RM, de Rijk WG, BeGole EA, Evans CA. Bond strength of visible light-cured glass ionomer orthodontic cement. Am J Orthod Dentofacial Orthop. 1997;112:205–8. doi: 10.1016/s0889-5406(97)70247-6. [DOI] [PubMed] [Google Scholar]
- 15.Cacciafesta V, Sfondrini MF, De Angelis M, Scribante A, Klersy C. Effect of water and saliva contamination on shear bond strength of brackets bonded with conventional, hydrophilic, and self-etching primers. Am J Orthod Dentofacial Orthop. 2003;123:633–40. doi: 10.1016/s0889-5406(03)00198-7. [DOI] [PubMed] [Google Scholar]
- 16.Mirzakouchaki B, Kimyai S, Hydari M, Shahrbaf S, Mirzakouchaki-Boroujeni P. Effect of self-etching primer/adhesive and conventional bonding on the shear bond strength in metallic and ceramic brackets. Med Oral Patol Oral Cir Bucal. 2012;17:e164–70. doi: 10.4317/medoral.17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Artun J, Bergland S. Clinical trials with crystal growth conditioning as an alternative to acid-etch enamel pretreatment. Am J Orthod. 1984;85:333–40. doi: 10.1016/0002-9416(84)90190-8. [DOI] [PubMed] [Google Scholar]
- 18.Atash R, Van den Abbeele A. Bond strengths of eight contemporary adhesives to enamel and to dentine: An in vitro study on bovine primary teeth. Int J Paediatr Dent. 2005;15:264–73. doi: 10.1111/j.1365-263X.2005.00650.x. [DOI] [PubMed] [Google Scholar]
- 19.Fowler CS, Swartz ML, Moore BK, Rhodes BF. Influence of selected variables on adhesion testing. Dent Mater. 1992;8:265–9. doi: 10.1016/0109-5641(92)90097-v. [DOI] [PubMed] [Google Scholar]
- 20.Nakamichi I, Iwaku M, Fusayama T. Bovine teeth as possible substitutes in the adhesion test. J Dent Res. 1983;62:1076–81. doi: 10.1177/00220345830620101501. [DOI] [PubMed] [Google Scholar]
- 21.De Munck J, Van Landuyt K, Peumans M, Poitevin A, Lambrechts P, Braem M, et al. A critical review of the durability of adhesion to tooth tissue: Methods and results. J Dent Res. 2005;84:118–32. doi: 10.1177/154405910508400204. [DOI] [PubMed] [Google Scholar]
- 22.Inoue S, Vargas MA, Abe Y, Yoshida Y, Lambrechts P, Vanherle G, et al. Microtensile bond strength of eleven contemporary adhesives to enamel. Am J Dent. 2003;16:329–34. [PubMed] [Google Scholar]
- 23.Kanemura N, Sano H, Tagami J. Tensile bond strength to and SEM evaluation of ground and intact enamel surfaces. J Dent. 1999;27:523–30. doi: 10.1016/s0300-5712(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 24.Moszner N, Salz U, Zimmermann J. Chemical aspects of self-etching enamel-dentin adhesives: A systematic review. Dent Mater. 2005;21:895–910. doi: 10.1016/j.dental.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Nishiyama N, Suzuki K, Takahashi K, Nemoto K. The pKa effects of the carboxylic acid in N-methacryloyl-omega-amino acid on the demineralization and bond strengths to the teeth. Biomaterials. 2004;25:5441–7. doi: 10.1016/j.biomaterials.2003.12.044. [DOI] [PubMed] [Google Scholar]
- 26.Dos Santos PA, Garcia PP, Palma-Dibb RG. Shear bond strength of adhesive systems to enamel and dentin. Thermocycling influence. J Mater Sci Mater Med. 2005;16:727–32. doi: 10.1007/s10856-005-2609-2. [DOI] [PubMed] [Google Scholar]


