Abstract
Background:
Low-level laser therapy (LLLT) has shown a promising effect in ameliorating symptoms of rheumatoid arthritis (RA). The aim of this investigation was to compare the early and late anti-inflammatory effects of LLLT and betamethasone in RA.
Materials and Methods:
In this animal experimental study, after inducing a model of RA in temporomandibular joint (TMJ) of 37 Wistar rats using adjuvant injection, they were randomly distributed into three experimental groups of 12 animals each: (1) LLLT group; (2) steroid group which received a single dose of betamethasone systemically; and (3) positive control group, which did not receive any treatment. One rat served as the negative control. Half of the animals in all the experimental groups were sacrificed on the 21st day after RA induction (early phase), and the other half were sacrificed 2 weeks later (late phase). Then, the severity of TMJ inflammation was assessed histologically in each group on a semi-quantitative scale. Kruskal-Wallis and Mann-Whitney tests were used to compare differences (α = 0.05).
Results:
The LLLT and steroid groups showed significantly (P < 0.05) lower inflammation mean scores in both early (5.66 [±1.86] and 1.66 [±1.21], respectively) and late phases of evaluation (1.16 [±1.47] and 6.50 [±1.04], respectively) compared to positive control group in early and late stages of assessment (11.66 [±3.50] and 8.66 [±1.36], respectively). However, the best results (P < 0.005) were achieved in early phase of the steroid group as well as late phase of the LLLT group.
Conclusion:
Within limitations of this study, it may be concluded that LLLT method has a long-term promising effect on reducing inflammation severity of TMJ similar to betamethasone in earlier stages.
Keywords: Arthritis, betamethasone, low-level laser therapy, temporomandibular joint
INTRODUCTION
Rheumatoid arthritis (RA), the most common form of inflammatory arthritis, is a progressive inflammatory disease that affects the joints. A significant percentage of patients with RA have symptoms and signs of temporomandibular joint (TMJ) involvement.[1] This is a far greater proportion than that in earlier studies,[2] as recently it is believed that TMJ involvement occurs even before the onset of clinical symptoms and autoantibody formation as well as synovial changes precede the clinical onset of the disease. In fact, all the prerequisites for osteoclast differentiation and bone erosion are found in this subclinical preliminary phase.[3] On the other hand, synovitis is not just a pure result of infiltration of the joint tissue by immune cells; rather it is a much more complex process, which consists of mononuclear immune cell infiltration and profound remodeling of the tissue architecture, such as the lining layer hyperplasia, fibrosis and vasculogenesis.[4] These changes provide a suitable setting for the generation of bone-resorbing cells, and the progress of the disease to a more debilitating and active phase.[3]
Rat adjuvant arthritis is an experimental model of RA, which has been widely used in previous studies.[5] Freund complete adjuvant (FCA) is composed of dead and dried mycobacterium tuberculosis in oil, which induces RA that is pathologically identical to that occurring in humans.[5]
Most of the time, RA exacerbates over time unless the inflammation is stopped or slowed by treatment.[2] There is no cure for RA, and the goal of treatment is remission, a state in which inflammation disappears or is very mild.[6] Despite the fact that not much time has elapsed since the initial report on the dramatic effects of cortisone in symptomatic treatment of RA,[7] glucocorticoids have become part of the gold standard of RA treatment and are widely used in this respect.[8] Administration of corticosteroids for RA management can be either intra-articular, local extra-articular or systemic.[9] Siró[10] reported that extra-articular management of cases is initially recommended. Intra-articular administration of steroids not only participates in erosive reactions in bone and cartilage,[11,12] but it also causes some systemic adverse effects.[13]
Of many anti-inflammatory steroid drugs used in the treatment of RA, dexamethasone, prednisone[14] and betamethasone[15] can be mentioned. Betamethasone is a synthetic, long-acting glucocorticoid that depresses formation, release, and activity of endogenous mediators of inflammation, including prostaglandins, kinins, histamine, liposomal enzymes and the complement system. It also modifies body's immune response.[16]
Recently, increasing concerns about the side effects of glucocorticoids have led to a decrease in their indiscriminate use in RA,[17] necessitating administration of new modalities in this field. Low-level laser therapy (LLLT) is one such promising therapy, introduced as an alternative non-invasive treatment for RA about 30 years ago.[18]
Several animal and human trials have demonstrated the modulatory effect of laser radiation on inflammatory markers[19] and cells.[20] The effectiveness of LLLT for RA is still controversial.[21] Many previous investigations have demonstrated dramatic effects of LLLT on relieving clinical symptoms of RA, including reduction in joint swelling,[19] pain[22] and morning stiffness[23] as well as improvement in health status.[22] Nonetheless, Schett[3] reported that even after remission of clinical symptoms, there might be synovitis and inflammation in the affected joint. Studies investigating the histopathological changes in resolution of RA with LLLT are scarce.
In a study by Alves et al.[24] on the histological effects of LLLT in early and late phases of RA, the ability of LLLT on modulating inflammatory responses, both in early as well as late progression stages of RA, was demonstrated. In another investigation, LLLT showed a dramatic effect in modulating inflammatory mediators (interleukin [IL-1β], IL-6) and cells (macrophages and neutrophils), which was in correlation with a reduction in histological inflammatory process.[25]
Although there are many studies assessing LLLT effectiveness in quenching the flame of inflammation, few studies have addressed the effects of LLLT in comparison with conventional treatments.[19,20]
With this perspective in mind, the present study was designed to investigate histological aspects of LLLT in comparison with a systemic corticosteroid in amelioration of TMJ inflammation.
MATERIALS AND METHODS
Animals
In this animal experimental study, 37 male Wistar rats, approximately 13‒15 weeks old and weighing 250-300 g, were employed. The animals were kept under controlled conditions of light and temperature, with water and food provided. The study design and animal experimental procedures were approved by the Institutional Research Ethics Committee for Animal Investigations (Torabinejad Dental Research Center, Isfahan University of Medical Sciences).
For induction of arthritis in TMJ of rats, 50 mL[11] of an emulsion of FCA (Biojen, Mashhad, Iran) was injected into the left TMJ.[12] 7 days later, the animals were randomly distributed into three groups of 12 animals each, as follows:
LLLT group: Animals in this group underwent seven sessions of LLLT.
Steroid group: This group of animals received intra-peritoneal corticosteroid injection in a single dose.
Positive control group: The rats in this group received FCA injection but did not receive any treatment.
In addition, a rat served as the negative control, without receiving any intervention (neither arthritis induction nor treatment) to assess normal TMJ tissues in this rat model.
Low-level laser therapy procedure
For laser irradiation, the animals received a mild sedation of chloroform and were irradiated at an angle of 90° to the surface of the tissue over TMJ area. This region was scrupulously detected in each animal with palpation of a presumptive area, 5-10 mm posterior to the lateral eye canthus, while manipulating the mandible to provide movement of the condyle for further accurate identification of the joint position. Seven sessions of 60s LLLT was performed every other day during a 2-week period (physical parameters were selected in accordance to those applied in previous studies[24,26]).
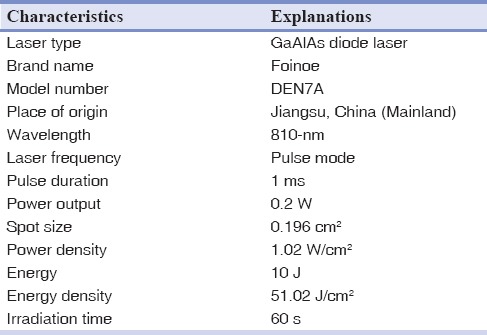
Table 1 shows parameters and specifications of the laser device used in this experiment.
Table 1.
Laser parameters and specifications
Systemic corticosteroid therapy
7 days after induction of arthritis, an intra-peritoneal injection of 1.2 mg/kg of betamethasone[11] was performed according to the technique employed by El-Hakim et al.[12]
Euthanasia and histological procedures
Half of the animals in each group and the rat kept as negative control were sacrificed for the histological procedure 3 weeks after FCA administration (immediately after completion of LLLT sessions: Early phase of evaluation, day 21). To do so, they were identified, weighed, and afterwards, subjected to euthanasia with a lethal dose of ether via inhalation. Thereafter, the left TMJ of each sacrificed rat was dissected by a sharp saw and then fixed in 10% formaldehyde for 24 h. After this period, they were decalcified using formic acid, embedded in paraffin blocks and 5-μm transverse sections were prepared. Slides containing two sections each were prepared and stained using hematoxylin-eosin.
Subsequently, to evaluate the late effects of each treatment modality, 2 weeks after sacrificing half of the animals in each group, the other half were subjected to the same procedure of euthanasia and slide preparation for histological assessment (late phase of evaluation, day 35).
Histopathological assessment
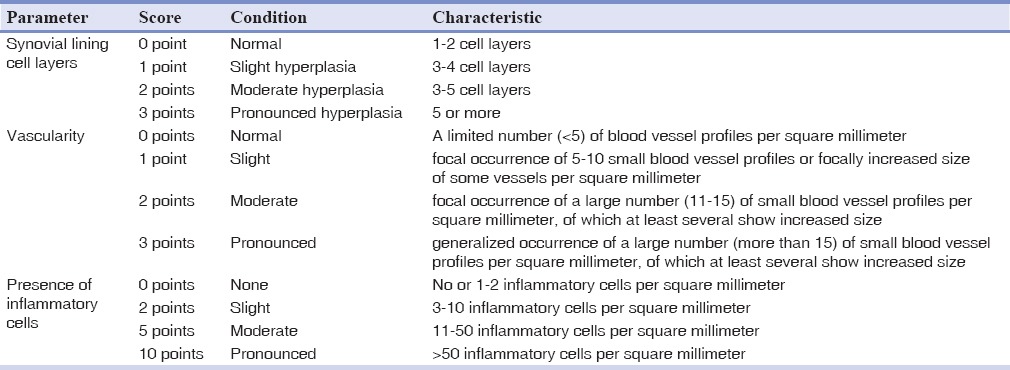
Histopathological evaluation was carried out under a light microscope in a ×400 field (Carl Zeiss Microscopy GmbH, Jena, Germany) by two expert independent pathologists (the intraclass correlation coefficient for grading inflammation features was 0.78). In order to quantify the outcomes analyzed in histological evaluation of the inflammatory events (synovial lining cell layers, vascularity and infiltration of immune cells), blinded operators used a standard scoring method according to Gynther et al. system[27] [Table 2]. In cases in which the synovial cell layers were to be counted, the pathologists chose an area whose status had been repeated in more than one-third of the entire specimen. Estimation of vascularity and inflammatory cells per square millimeter was carried out using grid counting slides (Ted Pella, Inc., California, USA). Both the number of vascular cross-sections per square millimeter and also the size of vessels were used in defining vascularity score of a specimen. Increased size of vessel cross-sections was determined in comparison with those in normal tissue (negative control).
Table 2.
Gynther's scoring system
Moreover, the pathologists investigated the presence of other signs of inflammation qualitatively, including fibrin deposition and synovial cell adhesion (the presence of closely opposed synovial cells intermingled with each other) for all the specimens.
Data analysis and statistics
Synovitis severity was graded from 0 to 16 by adding the scores of the three histological indices. Then, nonparametric Kruskal-Wallis and Mann-Whitney U-tests were used to compare the groups. Data were expressed as means and standard deviations. All the differences were considered significant at P < 0.05.
RESULTS
The histopathological changes, observed in rats injected with the FCA, confirmed the presence of acute inflammation in the synovium.
Positive control group, early and late phases
The synovial tissue appeared hyperplastic and showed a considerable number of inflammatory cells as well as dilated blood vessels in comparison to the negative control [Figure 1]. In addition to these features, an increase in the density of resident cells (synovial cell adhesion) and deposition of fibrous tissue [Figure 1] were observed in the majority of samples. In spite of a lower degree of inflammation scores in the late phase of assessment of the control group, compared to early evaluation of this group, the difference between these two stages was not significant (P = 0.132).
Figure 1.
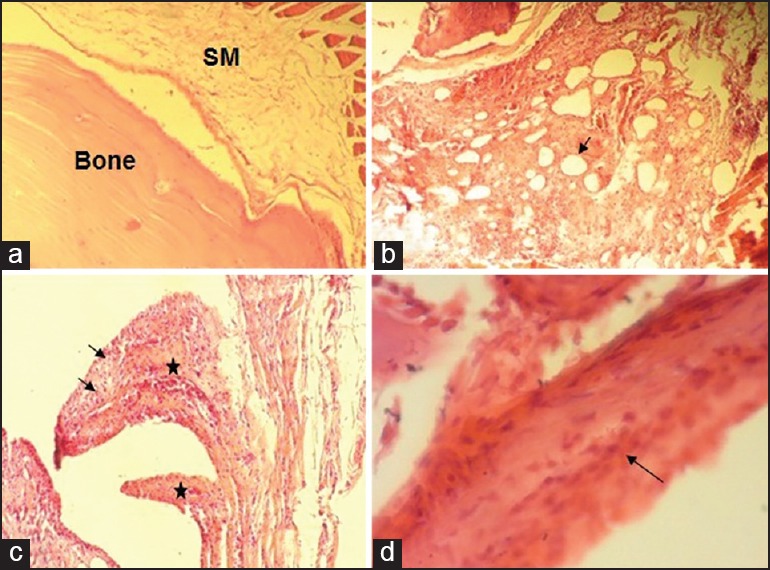
(a) Normal rat temporomandibular joint (TMJ) tissue (×100) showing a thin synovial membrane (synovium) without any evidence of inflammation. (b-d) Adjuvant-induced arthritis in rat TMJ showing: (b) Hypertrophic and hyperplastic synovial membrane with cumulous infiltration of mononuclear inflammatory cells and pronounced dilated vessels (arrow) (×100); (c) redundant folds of hyperplastic synovial lining (asterisks) and infiltration with inflammatory cells (arrows) (×100); (d) increased density and adhesion of synovial resident cells (arrow) (×400). SM: synovial memberane.
Laser group, early phase
Immediately after LLLT sessions, mild to moderate features of inflammation were still observed in the samples [Figure 2]. Moreover, the synovial cell lining in the majority of samples was >2–3 layers [Figure 2].
Figure 2.
Laser group, early phase (×100), showing (a) moderate infiltration of inflammatory cells and slight hypervascularity. Inset: higher magnification (×400) of mononuclear inflammatory cells; (b) moderate hyperplasia of synovial lining layers (outlined by arrows) and a mild inflammatory infiltrate deep in the lining layer (×100).
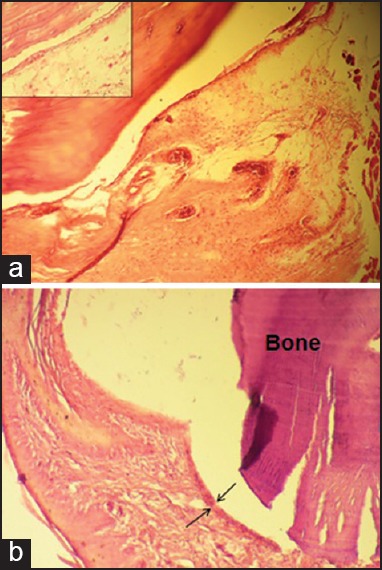
Steroid group, early phase
Two weeks after betamethasone administration, the synovial cell layers appeared normal without a significant inflammation or vasodilation [Figure 3]. However, there was still some evidence of fibrin deposition and fibrinous adhesion between closely opposed synovial membrane and articular surfaces in 50% of cases [Figure 3].
Figure 3.
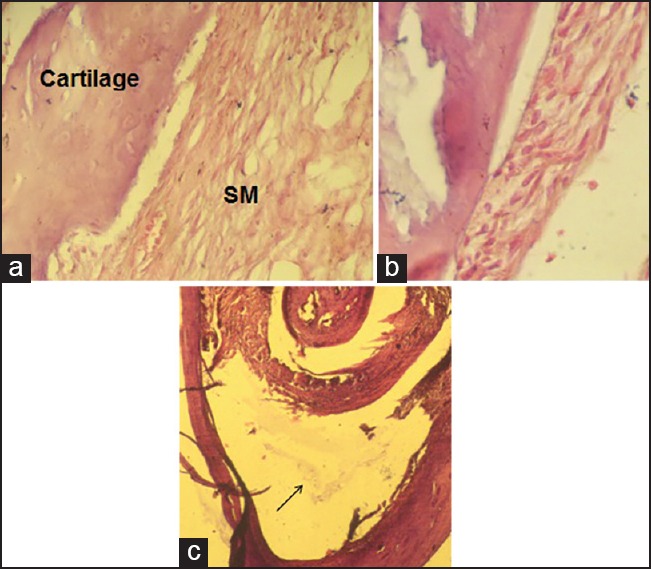
Steroid group, early phase, showing (a) normal synovial memberane (SM) and cartilage (×400); (b) increased synovial cell adhesion (×400); (c) floating fibrin in joint space (arrow) (×100).
Low-level laser therapy group, late phase
Evaluation of late effects of laser showed that LLLT succeeded in modifying the majority of changes due to arthritis [Figure 4].
Figure 4.
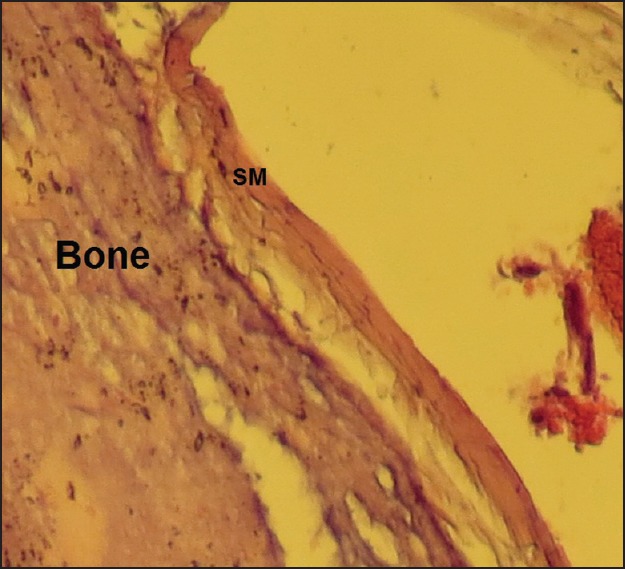
Laser group, late phase, showing a thin layer of synovial memberane (SM) without any inflammation or fibrous tissue (×400).
Steroid group, late phase
In most cases, a marked inflammation was seen although it was milder than that in control samples at this stage. All the three inflammation indices under study were moderately higher in most samples [Figure 5].
Figure 5.
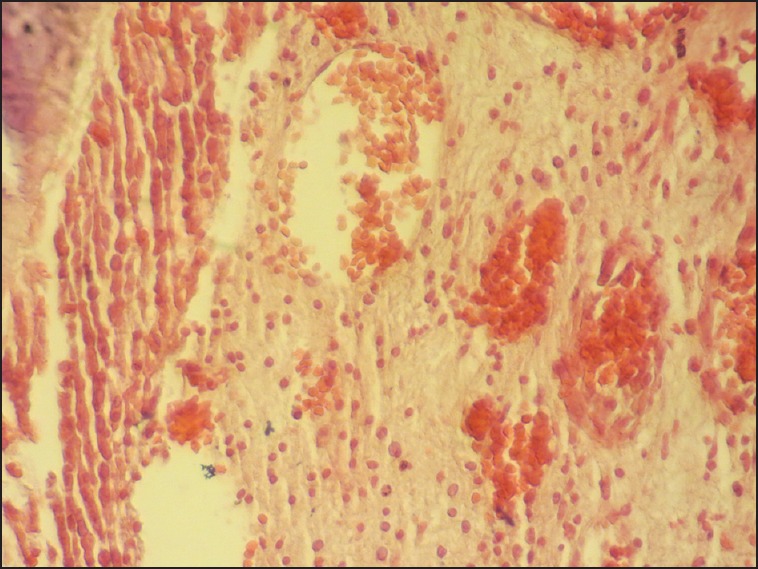
Steroid group, late phase, showing a moderate increase in inflammatory infiltration and vascularity of synovial tissue (×400).
Table 3 shows the mean quantitative severity scores (±standard deviation) of synovitis in experimental and control groups.
Table 3.
Mean values and standard deviations in studied groups*
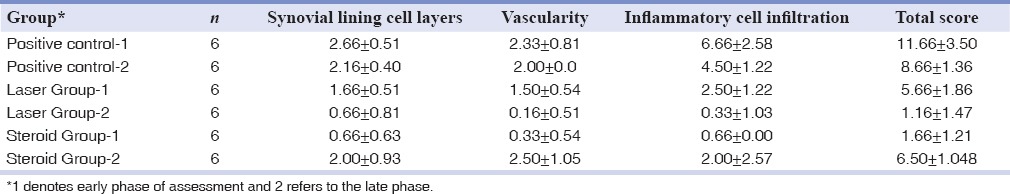
The steroid- and laser-treated groups (early and late phases of treatment) presented a significant decrease in synovial inflammation severity, compared to the control group (P < 0.05). There was also a significant difference between the early and late effects of laser in a way that the late results were markedly better (P = 0.004). This result was contrary to betamethasone which showed a significantly better anti-inflammatory effect in the early phase of evaluation (14 days after administration) compared to the late phase (1-month after administration) (P = 0.002). There was no significant difference between LLLT in the late phase of evaluation and the early results of betamethasone (P = 0.485).
DISCUSSION
Great advances have been made since last decade in exploring the efficacy of LLLT in RA. Molecular[25] and clinical evidence[19,22,23] support the anti-inflammatory effects of this modality. In the present study, the early and late anti-inflammatory efficacy of LLLT and systemic corticosteroid therapy was compared in experimentally induced arthritis in rat TMJ.
The arthritis induced by FCA, in the present study, caused pathologic features similar to that of human disease.[4] The pathogenesis for the development of adjuvant arthritis is not fully understood. However, some have attributed this finding to the heat shock proteins and interactions with intestinal flora.[5]
Studies on RA have indicated that the synovial membrane has a dominant role in joint inflammation and destruction, suggested by the changes in synovial histology:
-
(1)
Thickening of the synovial lining layer as a result of infiltration by CD68+ cells, and both proliferation and reduced apoptosis of type B synoviocytes;
-
(2)
Neo-vascularization of the sub-surface layer;
-
(3)
Infiltration of the sub-surface layer with T and B lymphocytes, plasma cells and macrophages; and
-
(4)
Alteration of the adhesion molecule expression.[4] There are several histologic systems for grading synovial inflammation in TMJs.[27,28,29]
The system used in the present study was the one proposed by Gynther et al.,[27] the accuracy of which was tested by Suzuki et al. in 2001.[30] This system is based on a semi-quantitative evaluation of the following parameters:
-
(1)
Synovial lining cell layers;
-
(2)
Vascularity; and
-
(3)
Inflammatory cell infiltration.
One of the differences between Gynther et al. system and other scoring scales is that all the parameters in this gradation will not necessarily have the same impact. The presence of inflammatory cells is considered more important for grading overall synovial inflammation and is therefore given higher scores, compared with other parameters. The other difference is that it considers even few inflammatory cells as an impressive factor in grading synovitis. It is in line with the results of previous studies which have demonstrated that the presence of several inflammatory cells is always indicative of synovial inflammation in the TMJ.[31] It is believed that human TMJ synovial inflammation differs from that in other synovial joints such as that in the knee, in a way that pronounced synovial inflammation is uncommon in the TMJ synovial lining.[27] Nevertheless, >60% of the rats showed pronounced inflammation in the positive control group of the present study. This discrepancy might be attributed to differences in histopathological features of human and rat models of RA.[5]
The delicacy of the synovial tissue and its partial adherence to articular bone made slide preparation difficult. However, this problem was resolved to a great extent in the present study by using Gynther et al. system for evaluation of the inflammation because in this system not an essentially complete intact synovial tissue is needed.
Previous studies have shown that only a small percentage of patients attain a sustained drug-free remission upon antirheumatic drug withdrawal.[32] This result was also confirmed in the present study, which showed disease exacerbation after betamethasone duration of action in the 4th week of investigation.
There are a large number of studies ranging from clinical controlled trials to molecular surveys to elucidate the efficacy of LLLT to manage RA and other rheumatic conditions. In a review study which explored the effect of nonpharmacological and nonsurgical interventions for patients with RA, it was concluded that there is some evidence that LLLT reduces pain and improves function.[33] In the present study, the GaAlAs diode laser, with a wavelength of 810-nm, energy density of 51 J/cm2 and output power of 200 mW, significantly reduced the inflammation of arthritic joints both in early and late phases of assessment. This result is in contrast with the results of a study by Kucuk et al.,[26] showing no significant difference between laser-treated and control groups in spite of relatively similar laser parameters applied in these two studies. The authors relate this diversity to differences in the methods used to assess inflammation. In Kucuk et al. study, the inflammation in TMJ was recorded through scintigraphic imaging, but in the present study, microscopic examination was used.
The results of the present study are in accordance with histological investigations carried out by Alves et al.[24] who reported a considerable anti-inflammatory effect of LLLT in collagen-induced RA model.
In the present study, the anti-inflammatory efficacy of LLLT in the early phase was significantly lower than that in the betamethasone-treated group, but in the late phase of the investigation, this result was vice versa. Studies comparing the effect of LLLT and conventional therapies in RA are scarce. Castano et al.[19] examined the anti-inflammatory effect of LLLT in comparison with dexamethasone for zymosan-induced arthritis in rats and demonstrated an almost equal effectiveness in these two modalities. Pallotta et al.[20] also compared the anti-inflammatory effect of infrared (810-nm) LLLT with diclofenac on rat experimental knee inflammation and documented a significant decrease in inflammation signs with both therapies.
With regard to the better outcomes of betamethasone in the early phase and LLLT in the later steps, it is suggested that perhaps a combination of the two approaches would bring great benefit in RA management.
It has been concluded from molecular studies that LLLT will start a complex of reactions,[34] which may persist for a long time afterwards. In the present study, the laser effect was evaluated in two phases:
-
(1)
Immediately after completion of treatment course, and
-
(2)
2 weeks after treatment to evaluate early and late effects of laser on joint inflammation.
It was shown that the laser effect in late phase was significantly better than the early stage.
In present study, in addition to a semi-quantitative evaluation of microscopic features of inflammation, the samples were also detected for any evidence of fibrin deposition and fibrinous adhesion between closely opposed synovial membranes; in this respect, these features were only observed in the positive control group and up to 50% of samples in early stage of steroid group. The inhibition of joint fibrinous tissue formation is not surprising in light of general catabolic actions of glucocorticoids on fibroblastic tissues.[35] However, the better late effect of these agents is noteworthy. This result is consistent with El-Hakim et al. findings,[12] which showed a less fibrinous state 6 weeks after intra-peritoneal injection of dexamethasone compared with earlier stages (1-week after injection). On the other hand, LLLT group (early and late phases) exhibited a lower rate of fibrin deposition and fibrinous adhesion compared with the positive control groups, indicating that LLLT was able to reduce synovial cell adhesions and secretion of fibrinous materials to joint space. Perhaps the efficacy of LLLT in eliminating morning stiffness symptoms and improving joint flexibility[23] is attributable to this issue.
The exact mechanism of action of LLLT in the treatment of RA is not yet well understood. It is however believed that it reduces the joint inflammation by suppressing the expression of auto-antigens[34] or inhibiting the expression of cytokines[25] involved in the inflammatory process. The authors of the present study believe that perhaps the better late effects of LLLT in the histological aspect is due to its bio-modulation effects (modulating effects on various biological events),[36] which was previously demonstrated in delayed phases postlaser therapy of wounds[37] and implants.[38] Nevertheless, the symptomatic effects of LLLT on joint inflammatory disorders, such as relief of pain, swelling and improvements in jaw movement, begin immediately after illumination.[23,39]
Despite many advantages in LLLT application for the treatment of RA, there are also some disadvantages, including an increased chair time and the number of treatment sessions in addition to its local effect, compared to systemic corticosteroid therapy.
A limitation of the present study was the lack of data on bone and cartilage changes in the joint.
Further studies are needed to investigate these results in chronic states of joint inflammation.
CONCLUSION
On the basis of the results of the present investigation, it can be concluded that 810-nm LLLT method has a long-term promising effect on reducing the inflammation of the TMJ, similar to betamethasone in earlier stages.
ACKNOWLEDGMENTS
This work was supported by a grant (393529) from the Vice Chancellor of Research of Isfahan University of Medical Sciences.
Footnotes
Source of Support: This work was supported by a grant from the Vice Chancellor of Research of Isfahan University of Medical Sciences.
Conflict of Interest: The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
REFERENCES
- 1.Bessa-Nogueira RV, Vasconcelos BC, Duarte AP, Góes PS, Bezerra TP. Targeted assessment of the temporomandibular joint in patients with rheumatoid arthritis. J Oral Maxillofac Surg. 2008;66:1804–11. doi: 10.1016/j.joms.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 2.Ogus H. Rheumatoid arthritis of the temporomandibular joint. Br J Oral Surg. 1975;12:275–84. doi: 10.1016/0007-117x(75)90058-x. [DOI] [PubMed] [Google Scholar]
- 3.Schett G. Synovitis — An inflammation of joints destroying the bone. Swiss Med Wkly. 2012;142:w13692. doi: 10.4414/smw.2012.13692. [DOI] [PubMed] [Google Scholar]
- 4.Glynn LE. Pathology, pathogenesis, and aetiology of rheumatoid arthritis. Ann Rheum Dis. 1972;31:412–20. doi: 10.1136/ard.31.5.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bendele A. Animal models of rheumatoid arthritis. J Musculoskelet Neuronal Interact. 2001;1:377–85. [PubMed] [Google Scholar]
- 6.Flurey CA, Morris M, Richards P, Hughes R, Hewlett S. It's like a juggling act: Rheumatoid arthritis patient perspectives on daily life and flare while on current treatment regimes. Rheumatology (Oxford) 2014;53:696–703. doi: 10.1093/rheumatology/ket416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hench PS, Kendall EC. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone; compound E) and of pituitary adrenocorticotropic hormone on rheumatoid arthritis. Proc Staff Meet Mayo Clin. 1949;24:181–97. [PubMed] [Google Scholar]
- 8.Kirwan JR. Combination therapy including glucocorticoids: The new gold standard for early treatment in rheumatoid arthritis? Ann Intern Med. 2012;6;156:390–1. doi: 10.7326/0003-4819-156-5-201203060-00014. [DOI] [PubMed] [Google Scholar]
- 9.Caporali R, Todoerti M, Sakellariou G, Montecucco C. Glucocorticoids in rheumatoid arthritis. Drugs. 2013;73:31–43. doi: 10.1007/s40265-013-0008-4. [DOI] [PubMed] [Google Scholar]
- 10.Siró B. The intraarticular and local administration of glucocorticosteroid preparations. Orv Hetil. 2009;150:251–60. doi: 10.1556/OH.2009.28494. [DOI] [PubMed] [Google Scholar]
- 11.Ghalayani P, Razavi SM, Babadi F, Sardari F. Histological assessment of intra-articular versus intra-peritoneal betamethasone L.A on tempromandibular joint arthritis in rat. Dent Res J (Isfahan) 2013;10:518–22. [PMC free article] [PubMed] [Google Scholar]
- 12.El-Hakim IE, Abdel-Hamid IS, Bader A. Tempromandibular joint (TMJ) response to intra-articular dexamethasone injection following mechanical arthropathy: A histological study in rats. Int J Oral Maxillofac Surg. 2005;34:305–10. doi: 10.1016/j.ijom.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Habib GS. Systemic effects of intra-articular corticosteroids. Clin Rheumatol. 2009;28:749–56. doi: 10.1007/s10067-009-1135-x. [DOI] [PubMed] [Google Scholar]
- 14.Sadra V, Khabbazi A, Kolahi S, Hajialiloo M, Ghojazadeh M. Randomized double-blind study of the effect of dexamethasone and methylprednisolone pulse in the control of rheumatoid arthritis flare-up: A preliminary study. Int J Rheum Dis. 2014;17:389–93. doi: 10.1111/1756-185X.12278. [DOI] [PubMed] [Google Scholar]
- 15.Hetland ML. Modern treatment strategies in rheumatoid arthritis. Dan Med Bull. 2011;58:B4320. [PubMed] [Google Scholar]
- 16.Spratto G, Woods AL. 1st ed. Delmar: Cengage Learning; 2011. Delmar Nurse's Drug Handbook. 2012 ed. [Google Scholar]
- 17.Mota LM, Cruz BA, Brenol CV, Pereira IA, Rezende-Fronza LS, Bertolo MB, et al. Guidelines for the drug treatment of rheumatoid arthritis. Rev Bras Reumatol. 2013;53:158–83. [PubMed] [Google Scholar]
- 18.Goldman JA, Chiapella J, Casey H, Bass N, Graham J, McClatchey W, et al. Laser therapy of rheumatoid arthritis. Lasers Surg Med. 1980;1:93–101. doi: 10.1002/lsm.1900010110. [DOI] [PubMed] [Google Scholar]
- 19.Castano AP, Dai T, Yaroslavsky I, Cohen R, Apruzzese WA, Smotrich MH, et al. Low-level laser therapy for zymosan-induced arthritis in rats: Importance of illumination time. Lasers Surg Med. 2007;39:543–50. doi: 10.1002/lsm.20516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pallotta RC, Bjordal JM, Frigo L, Leal Junior EC, Teixeira S, Marcos RL, et al. Infrared (810-nm) low-level laser therapy on rat experimental knee inflammation. Lasers Med Sci. 2012;27:71–8. doi: 10.1007/s10103-011-0906-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall J, Clarke AK, Elvins DM, Ring EF. Low level laser therapy is ineffective in the management of rheumatoid arthritic finger joints. Br J Rheumatol. 1994;33:142–7. doi: 10.1093/rheumatology/33.2.142. [DOI] [PubMed] [Google Scholar]
- 22.Bjordal JM, Couppé C, Chow RT, Tunér J, Ljunggren EA. A systematic review of low level laser therapy with location-specific doses for pain from chronic joint disorders. Aust J Physiother. 2003;49:107–16. doi: 10.1016/s0004-9514(14)60127-6. [DOI] [PubMed] [Google Scholar]
- 23.Brosseau L, Robinson V, Wells G, Debie R, Gam A, Harman K, et al. Low level laser therapy (Classes I, II and III) for treating rheumatoid arthritis. Cochrane Database Syst Rev. 2005;4:CD002049. doi: 10.1002/14651858.CD002049.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alves AC, de Carvalho PT, Parente M, Xavier M, Frigo L, Aimbire F, et al. Low-level laser therapy in different stages of rheumatoid arthritis: A histological study. Lasers Med Sci. 2013;28:529–36. doi: 10.1007/s10103-012-1102-7. [DOI] [PubMed] [Google Scholar]
- 25.Alves AC, Vieira R, Leal-Junior E, dos Santos S, Ligeiro AP, Albertini R, et al. Effect of low-level laser therapy on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation. Arthritis Res Ther. 2013;15:R116. doi: 10.1186/ar4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kucuk BB, Oral K, Selcuk NA, Toklu T, Civi OG. The anti-inflammatory effect of low-level laser therapy on experimentally induced inflammation of rabbit temporomandibular joint retrodiscal tissues. J Orofac Pain. 2010;24:293–7. [PubMed] [Google Scholar]
- 27.Gynther GW, Dijkgraaf LC, Reinholt FP, Holmlund AB, Liem RS, de Bont LG. Synovial inflammation in arthroscopically obtained biopsy specimens from the temporomandibular joint: A review of the literature and a proposed histologic grading system. J Oral Maxillofac Surg. 1998;56:1281–6. doi: 10.1016/s0278-2391(98)90609-7. [DOI] [PubMed] [Google Scholar]
- 28.Krenn V, Morawietz L, Burmester GR, Kinne RW, Mueller-Ladner U, Muller B, et al. Synovitis score: Discrimination between chronic low-grade and high-grade synovitis. Histopathology. 2006;49:358–64. doi: 10.1111/j.1365-2559.2006.02508.x. [DOI] [PubMed] [Google Scholar]
- 29.Muto T, Kawakami J, Kanazawa M, Kaku T, Yajima T. Development and histologic characteristics of synovitis induced by trauma in the rat temporomandibular joint. Int J Oral Maxillofac Surg. 1998;27:470–5. doi: 10.1016/s0901-5027(98)80041-6. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki T, Segami N, Sato J, Nojima T. Accuracy of histologic grading of synovial inflammation in temporomandibular joints with internal derangement using Gynther's system. J Oral Maxillofac Surg. 2001;59:498–501. doi: 10.1053/joms.2001.22676. [DOI] [PubMed] [Google Scholar]
- 31.Gynther GW, Holmlund AB, Reinholt FP, Lindblad S. Temporomandibular joint involvement in generalized osteoarthritis and rheumatoid arthritis: A clinical, arthroscopic, histologic, and immunohistochemical study. Int J Oral Maxillofac Surg. 1997;26:10–6. doi: 10.1016/s0901-5027(97)80838-7. [DOI] [PubMed] [Google Scholar]
- 32.Scott IC, Kingsley GH, Scott DL. Can we discontinue synthetic disease-modifying anti-rheumatic drugs in rheumatoid arthritis? Clin Exp Rheumatol. 2013;31:S4–8. [PubMed] [Google Scholar]
- 33.Christie A, Jamtvedt G, Dahm KT, Moe RH, Haavardsholm EA, Hagen KB. Effectiveness of nonpharmacological and nonsurgical interventions for patients with rheumatoid arthritis: An overview of systematic reviews. Phys Ther. 2007;87:1697–715. doi: 10.2522/ptj.20070039. [DOI] [PubMed] [Google Scholar]
- 34.Bálint G, Barabás K, Zeitler Z, Bakos J, Kékesi KA, Pethes A, et al. Ex vivo soft-laser treatment inhibits the synovial expression of vimentin and a-enolase, potential autoantigens in rheumatoid arthritis. Phys Ther. 2011;91:665–74. doi: 10.2522/ptj.20100065. [DOI] [PubMed] [Google Scholar]
- 35.Baxter JD, Forsham PH. Tissue effects of glucocorticoids. Am J Med. 1972;53:573–89. doi: 10.1016/0002-9343(72)90154-4. [DOI] [PubMed] [Google Scholar]
- 36.Santana-Blank LA, Rodríguez-Santana E, Santana-Rodríguez KE. Photo-infrared pulsed bio-modulation (PIPBM): A novel mechanism for the enhancement of physiologically reparative responses. Photomed Laser Surg. 2005;23:416–24. doi: 10.1089/pho.2005.23.416. [DOI] [PubMed] [Google Scholar]
- 37.Pugliese LS, Medrado AP, Reis SR, Andrade Zde A. The influence of low-level laser therapy on biomodulation of collagen and elastic fibers. Pesqui Odontol Bras. 2003;17:307–13. doi: 10.1590/s1517-74912003000400003. [DOI] [PubMed] [Google Scholar]
- 38.Gokmenoglu C, Ozmeric N, Erguder I, Elgun S. The effect of light-emitting diode photobiomodulation on implant stability and biochemical markers in peri-implant crevicular fluid. Photomed Laser Surg. 2014;32:138–45. doi: 10.1089/pho.2012.3473. [DOI] [PubMed] [Google Scholar]
- 39.Juhl C. Short term beneficial effects of low level laser therapy for patients with rheumatoid arthritis. Aust J Physiother. 2006;52:224. doi: 10.1016/s0004-9514(06)70032-0. [DOI] [PubMed] [Google Scholar]


