Abstract
Background:
The issue of a possible malignant transformation in the lesions like epithelial dysplasia and oral lichen planus (OLP) is a matter of serious controversy. The purpose of this study was to suggest the malignant transformation rate in OLP and oral epithelial dysplasia (OED) by using immunohistochemical expression of the tumor markers Ki-67, p53, BCL-2, and BAX.
Materials and Methods:
This was a cross-sectional study carried out among 70 samples, including 10 samples from normal healthy mucosa categorized into Group 1, Group 2 (30 OLP), and Group 3 (30 OED) samples. Five sections (4 μm thick) were obtained and stained with monoclonal antibodies such as Ki-67, p53, BCL-2, and BAX and analyzed for number of positive cells and also for intensity of staining. Statistical analysis was done using Mann-Whitney U-test (P < 0.05).
Results:
Significant results were found only for expressions of Ki-67, p53, BCL-2 markers in both study groups (P < 0.05). In these groups, the intensity of staining was mostly mild to moderate for all studied tumor markers. In this study, subjects with an average positive IHC expression of Ki-67, p53, BCL-2, and BAX markers in normal mucosa was about 22.5%, which was significantly lower when compared with OLP (54.9%) and OED (64.9%).
Conclusion:
The high propensity for malignant transformation in OED followed by OLP suggests that a wide range of inherent and extrinsic factors contribute to the disease progression and malignant transformation.
Keywords: Epithelial dysplasia, immunohistochemistry, oral lichen planus, proliferative marker, tumor marker
INTRODUCTION
Oral cancer is commonly preceded by premalignant lesions and conditions grouped under common terminology as potentially malignant disorders.[1] The precursor lesions that precede oral cancer are defined as an altered epithelium that shows a variety of cytological and architectural changes that have been traditionally brought under the common denominator dysplasia with an increased likelihood for progression to malignancy, squamous cell carcinoma.[2]
The presence of oral epithelial dysplasia (OED) is generally accepted as one of the most important predictors of malignant development in premalignant disorders. Silverman et al.[3] in their study have reported malignant transformation in 36% of cases with OED. Lichen planus a unique chronic inflammatory mucocutaneous disorder was considered as a precancerous condition by WHO in 1978.[4,5,6,7,8] The etiopathogenesis in oral lichen planus (OLP) appears to be complex, with interactions between and among genetic, immune, environmental, and lifestyle factors.[9] OLP affects 1-2% of the general adult population affecting women more than men (1.4:1) and occurs predominantly in adults over 40 years of age, although occurrence of this disease in younger adults and children is not unusual.[10,11,12] According to location, 72.9% of lesions occurred on posterior buccal mucosa with a 1.03% concurrent occurrence of buccal mucosa and tongue.[12]
The common denominator of the various neoplastic processes is the anarchic and autonomous increase in the number of their constituent cells. This increase is due to either abundant cell proliferation or insufficient cell death. Consequently, mutations and other molecular alterations that impede physiologic apoptosis can play a decisive part in the pathogenesis and progression of neoplasms. This is exemplified by the high frequency of mutations affecting the tumor proliferation gene Ki-67, tumor suppressor gene p53 and its regulators or the oncogene BCL-2 and BAX; a BCL-2 related protein that counteracts BCL-2 and is required for p53 mediated apoptosis in diverse types of cancer.[13,14,15]
The malignant potential of these lesions are still debatable and is a matter of serious controversy.[6,7] Recent techniques, such as immunohistochemistry (IHC) and molecular biology have been contributing to evaluate the expression of proteins related to the regulation of the cellular cycle.[16] To detect early cancer development and prevention of disease progression to malignancy, IHC is preferred as one of the precise diagnostic tools.[8] Loss of BCL-2 in basal cells of potentially malignant and malignant oral epithelial lesions and loss of BAX in poorly differentiated oral squamous cell carcinoma (OSCC) is not associated with mutations in the coding regions of these genes. The aberrant expression of BCL-2 and BAX suggests a role, in cooperation with other molecular changes in the progression of oral dysplasia and OSCC.[17]
As both apoptosis and abnormal proliferation play an important role in tumor formation, this study was conducted to suggest the malignant transformation rate in OLP and OED cases using immunohistochemical expression of the tumor markers Ki-67, p53, BCL-2, and BAX.
MATERIALS AND METHODS
A cross-sectional descriptive analytical study was done to observe the expression of Ki-67, p53, BCL-2, and BAX antigens in basal keratinocytes and to evaluate the malignant transformation by using IHC procedure in normal oral mucosa, OLP and OED. The study was conducted among 70 samples (Group 1- controls [10], Group 2- OLP [30] and Group 3- OED [30]) in the Department of Oral Pathology and Microbiology, Panineeya Mahavidyalaya Institute of Dental Sciences, Hyderabad. Study group comprised a total of 60 formalin fixed archival blocks after histopathological confirmation, which were divided into two groups (30 each of OLP and OED) and a control group of 10 normal healthy controls. Though, it was challenging to obtain the normal individuals, we could motivate them and procure the tissues only after they gave us the consent voluntarily. The site selected was buccal mucosa in all the samples. This study was approved by the Institutional Ethical Committee (PDC/126/2012-13).
Cases of clinically and histopathologically diagnosed OLP and OED, without any previous history of treatment for any type of oral diseases and subjects who were not on any sort of medication were included in the study. Pregnant women with OLP and OED, subjects with OLP along with OED, immune compromised patients and patients with multiple oral and/head and neck lesions were excluded.
Five sections (4 μm thick) were obtained from each of the formalin fixed, paraffin embedded tissue blocks. The first section was stained with hematoxylin and eosin for histological confirmation, while the remaining four sections were taken on 3- aminopropyltriethoxysilane (APES) coated glass slides and were stained with monoclonal antibodies p53, Ki-67, BCL-2, and BAX and analyzed.
The IHC method used was a new direct technique (enhanced polymer one-step staining method) by DAKO (India). Tissue sections were made from paraffin blocks stored in neutral buffered formalin using a microtome. These sections of 4 μm mounted on APES coated slides were then deparaffinized in xylene followed by rehydration through graded alcohol. Microwave oven was used for antigen retrieval, which was performed in citrate based buffer, with pH 6.0 and then allowed to cool until room temperature. Endogenous peroxide was neutralized using peroxide — block for 5 min to block the nonspecific antigen sites. Slides were washed in phosphate buffered saline (PBS) for 5 min in two changes. Later the slides were incubated with protein block for 5 min followed by washing in PBS for 5 min in two changes. The slides were then incubated with liquid mouse monoclonal primary antibody (p53 [Clone DO7], Ki-67 [MIB-1], BCL-2 [Clone 124] and BAX [1:70,000 dilution]) — (Sigma Aldrich companies, USA) for 1 h. Slides were incubated with post primary block for 30 min, washed in three changes of PBS for 5 min each and excess PBS was removed. Slides were incubated with secondary antibody for 30 min. The peroxidase activity was developed with a drop of freshly prepared 3’ diaminobenzidine tetrahydrochloride working solution for 5 min to aid in visualization of the sections. Slides were rinsed in distilled water and then sections were counterstained with Harris hematoxylin for 3 min followed by rinsing in tap water for 5 min. Later dehydrated, cleared and mounted.
Histopathologically confirmed case of OSCC was taken as positive control for the expression of p53, Ki-67, BCL-2, and BAX markers. Omitting the use of primary antibody and carrying out the successive steps of IHC as usual, gave negatively stained slides for p53, Ki-67, BCL-2, and BAX markers.
Quantitative analysis was performed on one section per biopsy. The immunoexpression of Ki-67, p53, BCL-2, and BAX was assessed using light microscope (Olympus CH 20i-Japan) under ×10 and ×20 magnifications by one blinded pathologist. Criteria given by Sousa et al.[13] and Dekker et al.[18] were followed for identification of keratinocytes in the basal and parabasal layers. Cells were considered immunopositive if they presented a brown nuclear coloring, regardless of intensity for p53 and Ki-67 (p53 and Ki-67 are nuclear markers). Cells were considered positive if they presented cytoplasmic staining for BCL-2 and BAX (BCL-2 and BAX are cytoplasmic markers). The basal keratinocytes positive for the antigen expression in the basal and parabasal layers of the epithelium were considered as the positive cells and those negative for the expression were considered as negative cells.
In labeling index positive staining areas for particular IHC marker or percent tumor cells staining positive as measured by IHC staining were counted by analyzing five high power fields (×20) for each slide [Figures 1 and 2 - OLP; Figures 3 and 4 - OED]. Ki-67, p53, BCL-2, and BAX expression were classified according to the number of positively stained cells per 1000 counted cells. The percentage of positive cells was scored according to the method of Nakagawa et al.[19] [Table 1].
Figure 1.
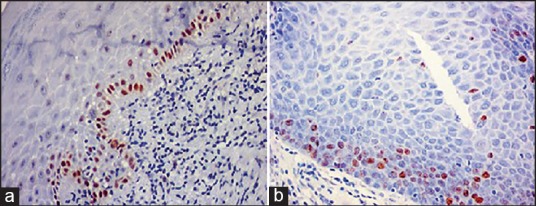
Immonohistochemical view (×20) (a) P53 and (b) Ki-67. Showing nuclear brown staining of tumor markers in basal and parabasal cells of oral lichen planus.
Figure 2.
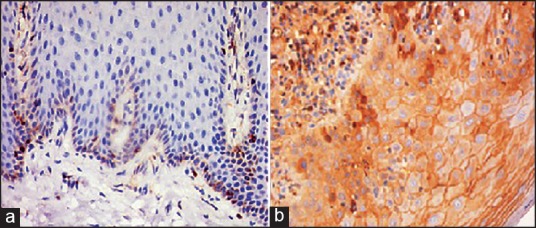
Immonohistochemical view (×20) (a) BCL-2 and (b) BAX. Showing cytoplasmic high intensity brown staining of tumor markers in basal and parabasal cells of oral lichen planus.
Figure 3.
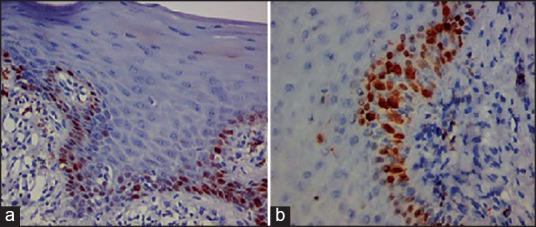
Immonohistochemical view (×20) (a) P53 and (b) Ki-67. Showing nuclear brown staining of tumor markers in basal and parabasal cells of oral epithelial dysplasia.
Figure 4.
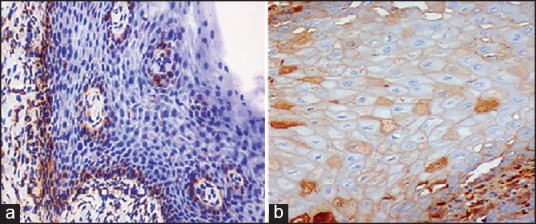
Immonohistochemical view (×20) (a) BCL-2 and (b) BAX. Showing cytoplasmic high intensity brown staining of tumor markers in basal and parabasal cells of oral epithelial dysplasia.
Table 1.
Scoring system for staining intensity (Nakagawa et al.[19])

Statistical analysis
Statistical analysis was performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) for windows. Pair-wise comparison between the markers and the groups was done using Mann-Whitney U-test. P < 0.05 was considered to be statistically significant.
RESULTS
This study was undertaken to evaluate and compare the tumor suppressive, proliferative and apoptotic activity of cells in normal subjects, OLD and subjects with OED using immunohistochemical markers; Ki-67, p53, BCL-2, and BAX. Both males and females were found to be equally distributed in all the groups.
Table 2 shows the comparison of P values between the three groups for p53, Ki-67, BCL-2, and BAX. Group 1 was statistically significant with Groups 2 and 3 for p53, Ki-67 and BCL-2. None of the groups were found to be statistically significant for BAX proteins.
Table 2.
Two-by-two comparison between the three studied groups for p53, Ki-67, BCL-2 and BAX
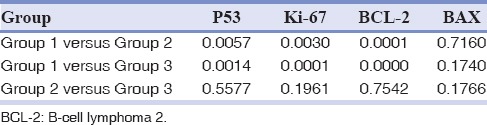
Table 3 shows the intensity of staining for all markers among three groups. In Group 1, only 20% have shown positivity for p53, which were mildly stained. In relation to Ki-67, majority of the staining was of mild type (13.3%) in both Groups 2 and 3 cases. It was totally negative among controls. Intensity of staining for BCL-2 marker was predominantly moderate in Group 2 (36.7%) and 3 (66.7%) cases. For BAX marker staining was mostly negative. Severe staining was found to be more in Group 3 (20%) than Group 2 (10%) cases.
Table 3.
Distribution of staining intensity for P53, Ki-67, BCL-2 and BAX in three groups according to the criteria of Nakagawa et al.[19]
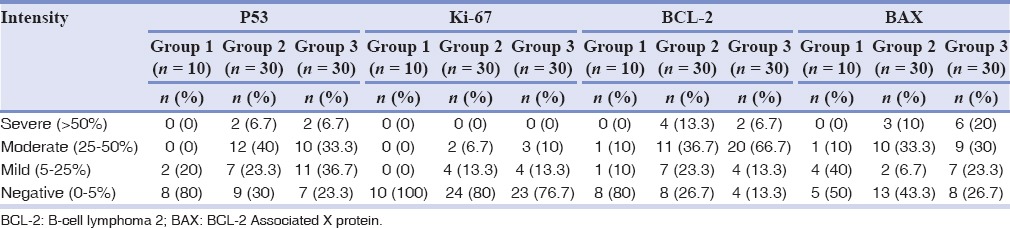
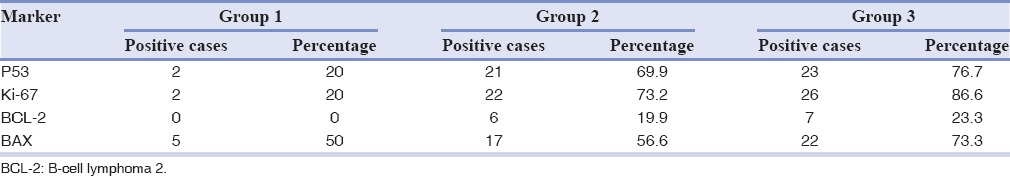
Table 4 shows the percentage positivity of all markers among the three groups. In Group 1, only 2 (20%) have shown positivity for p53 and Ki-67 protein, no subject for BCL-2 and 5 (50%) for BAX proteins. In Group 3, 23 (76.6%), 26 (86.58%), 7 (23.31%), and 22 (73.26%) have shown positivity for respective markers.
Table 4.
Percentage distribution of positive cases in three groups and four markers
DISCUSSION
The present study focused on evaluation of malignant potential with IHC in OED and OLP by comparing the frequency of positively stained proteins (p53, Ki-67, BCL-2, and BAX) depending on their intensity of staining. The age range of patients diagnosed in Group 2 was 30-52 years with mean age of 40.1 years with 46.6% males and 53.3% females, which was similar to Sugerman et al.[20] who have reported that females were more affected than men with OLP (1.4:1). In Group 3, the age ranged between 31 and 54 years with mean age of 41.1 years and males (53.3%) were more affected when compared to females (46.6%). Our results were similar to Brothwell et al.[2] who have shown 38 males (59.4%) and 26 females (40.6%) with ages ranging from 20 to 86 years.
The normal p53 protein has a very short half-life; therefore, can be hard to detect in normal tissues. However, the protein can remain in the tissues longer due to mutations, or a defect in the degradation pathway or by binding to other proteins. The physiological function of p53 protein is that of preventing accumulation of genetic damage in cells either by allowing for repair of the damage before cell division or by causing death of the cell. The mutant p53 protein is normally not active, thus leading to the loss of the tumor suppressor function of the protein. The alterations of p53 impair the ability of the cells to repair and undergo apoptosis in response to DNA damage, which will lead to uncontrolled cell growth.[21]
In Group 1 of this study, the p53 positivity was found to be 20%, which was quite lower than Fakhrjou and Toutounchi study (40%).[22] This variation may be due to small sample size and also because of excess cell damage in few benign conditions and it does not necessarily indicate mutations or malignant transformation.
The p53 positivity, in Group 2, was 69.9% in this study which is almost similar to Taniguchi et al. (64%) study.[23] In contrast to our study, substantially higher p53 positivity was present in Fakhrjou and Toutounchi study (86.7%)[22] and Acay et al. (90.9%).[24] They explained that p53 protein (Clone DO-7) reacts only with its mutant form, thus revealing the presence of mutation in the protein and hence OLP has potential malignant transformation.[22,24] In Sousa et al.[13] study, the p53 positivity (41.7%) was lesser compared to our study, which could be due to sample size.
In this study, the p53 positivity in Group 3 (76.7%) is almost similar to Raju et al. (79%) study.[25] They explained that the tumor suppressor gene p53 plays a central role in controlling the progression of cell cycle from G1- phase to the S phase and alterations in this gene may provide cancer cells with a growth advantage leading to uncontrolled proliferation as well as increased p53 levels.
In contrast to our study, lower p53 positivity was present in Sousa et al. (41.67%)[13] and Angiero et al. (64.3%)[26] studies and this might be due to substantial differences in detection techniques as well as the varied habits practiced in different geographical regions and races. The expression of p53 positivity in Groups 2 and 3, in the present study, is a relevant finding. The p53 is a nuclear protein whose mutation is strongly associated to several cancer types. It has been shown that alterations in the expression of p53 is essential for carcinogenesis and can indicate an important step in transformation of normal to neoplastic epithelium.[27,28] According to Stoll et al.[28] the loss of p53 function is found in at least half of oral cancer cases. Therefore, the similar expression of p53 in Groups 2 and 3 can be an important indicator of malignant transformation potential of these lesions.
Ki-67 is a cell cycle associated human nuclear protein present in perichromosomal region, the expression of which is strictly associated with cell proliferation and is widely used in pathology as a proliferation marker to measure the growth fraction of cells in human tumors. The estimated half-life of Ki-67 antigen is 60-90 min. The Ki-67 antigen starts to be expressed in the S phase, progressively increasing through S and G2 phases and reaching a plateau at mitosis. After cell division, the cells return to G1 with a stock of Ki-67 antigen, whose level decreases rapidly during this phase.[21]
Ki-67 positivity was 20% in Group 1 which is similar to Fakhrjou and Toutounchi study[22] (20%) who explained that Ki-67 is a nuclear protein that can be detected in all phases of cell cycle except go phase and plays a pivotal role in maintaining cell proliferation. In Group 2, the Ki-67 positivity was found to be 73.2% which is lower than Fakhrjou and Toutounchi (86.7%)[22] and Acay et al. studies (95.4%).[24] The authors explained that this high positivity of Ki-67 could be due to an increased cell proliferation, secondary to repeated break down of cycling cells leading to increased state of proliferation.[24,29]
The Ki-67 positivity in Group 3 was found to be 86.6% in the present study with almost similar value observed in a study by Raju et al.[25] (93%). The increase in the proliferative activity could be due to alteration in p53 activity. The percentage positivity for Ki-67 in Group 2 is lower than that of Group 3 and this difference is in the expected direction in that dysplastic lesions have a higher growth fraction.[30]
In this study, based on the IHC evaluation, both p53 and Ki-67 were significantly more prevalent in specimens in Groups 2 and 3 compared with Group 1. These findings indicate that there might be a potential tendency for malignancy in Groups 2 and 3 and both Ki-67 and p53 might be used as relevant prognostic markers.[25,31]
The BCL-2 proto-oncogene blocks a distal step in an evolutionary conserved pathway of apoptosis. Its abnormal expression, usually in terms of over expression in genetically modified cells such as tumor cells, contributes to the expansion of the damaged cell clone by preventing cell turnover due to programmed cell death, leading to cellular immortalization. By promoting cell survival, BCL-2 facilitates the permanent acquisition of mutations and malignant transformation. Moreover, increased BCL-2 expression in cancer cells possibly reflects tumor cell resistance to apoptosis and may have implications for their responsiveness to treatments.[32]
The BCL-2 positivity in Group 1 was (0%) almost similar to Leyva-Huerta et al.[33] study where mild positivity in the cytoplasm was present. In Group 2, BCL-2 positivity was found to be 19.9%, which was similar to Sousa et al.[13] study in which it was found to be 16.7%. In Tanda et al.[34] study, the BCL-2 positivity was 10%, which was slightly lower than our study and suggested that the low rate of apoptosis in OLP is a consequence of the antiapoptotic action exerted by BCL-2. In contrast to our study, Leyva-Huerta et al.[33] study found no BCL-2 immunoexpression in OLP group and the authors suggested that this protein does not seem to be involved in OLP epithelial changes and the loss of BCL-2 antiapoptotic control is associated with a concomitant loss of other prosurvivor molecules or an increase in the proapoptotic molecules.
In Group 3, BCL-2 positivity was found to be 23.3% in our study, which was slightly lower than Sousa et al. (25%)[13] and Tanda et al. (30%)[34] studies. This might be due to subtle inherent genetic differences among different populations, gender and age of the population and anatomical location of the studied lesions. In the various studies, it is observed that BCL-2 is present from initial stages of carcinogenesis up to appearance of metastasis in oral cancer.[27,28] Therefore, the high immune expression of BCL-2 in Groups 2 and 3 compared with Group 1 may indicate premalignant potential of these two groups. Alterations in BCL-2 expression play a role in cellular differentiation and development of tumors and this oncoprotein has a role in early stages of tumor progression as it is up regulated in sequentially progressing OED.[35]
BAX is a BCL-2-related protein that promotes apoptosis. It plays a tumor suppressor role in human malignancies and high BAX expression is associated with favorable prognosis in several cancer types.[36] According to Zhan et al.,[37] BAX expression is selectively induced in apoptosis — proficient cells; therefore, OSCC cells with elevated BAX expression could be those that are primed for apoptosis due to the inherent genetic instability associated with malignant transformation.
In this study, the positivity rate for BAX in Group 1 (50%), Group 2 (56.61%) and Group 3 (73.26%) had no statistical significant difference and was almost similar to Sousa et al.[13] study showing 50% in Group 2 and 83.33% in Group 3. Though BAX is a pro apoptotic protein, it is not used much as a marker to assess malignant transformation in Groups 2 and 3. Although BAX and BCL-2 are strongly associated in apoptosis, no correlation between these proteins was observed in this study, which can be explained by the existence of different mechanisms of apoptosis regulation. The expression of BAX was lower in Group 2 compared to Group 3 possibly preventing the death of genetically damaged cells and consequently increasing the malignant transformation risk.
Markers are useful in predicting severity of malignant transformation risk. A potential advantage of the use of immunohistochemical markers is their application to routinely processed surgical specimens. However, technical pitfalls of IHC technique should also be considered as a limitation, because differences in antigen retrieval procedure and diverse antibody clones are sources for various interpretations of protein expression. Therefore, there is a need for identification of any alterations that can indicate a possible malignant transformation. Larger prospective studies are needed to confirm these findings.
CONCLUSION
Groups 2 and 3 established a potential tendency for malignancy compared to Group 1 and Ki-67, p53 and BCL-2 can be considered as reliable prognostic markers for malignancy. Though BAX is a proapoptotic protein, it appears that it is not of much use as a marker to assess malignant transformation in these groups. Alterations in expression of these proteins play a role in cellular differentiation and development and progression of tumor as these proteins participate actively in oral carcinogenesis.
Footnotes
Source of Support: Nil.
Conflict of Interest: The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
REFERENCES
- 1.Warnakulasuriya S, Johnson NW, van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med. 2007;36:575–80. doi: 10.1111/j.1600-0714.2007.00582.x. [DOI] [PubMed] [Google Scholar]
- 2.Brothwell DJ, Lewis DW, Bradley G, Leong I, Jordan RC, Mock D, et al. Observer agreement in the grading of oral epithelial dysplasia. Community Dent Oral Epidemiol. 2003;31:300–5. doi: 10.1034/j.1600-0528.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- 3.Silverman S, Jr, Gorsky M, Lozada F. Oral leukoplakia and malignant transformation. A follow-up study of 257 patients. Cancer. 1984;53:563–8. doi: 10.1002/1097-0142(19840201)53:3<563::aid-cncr2820530332>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 4.van der Waal I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral Oncol. 2009;45:317–23. doi: 10.1016/j.oraloncology.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 5.van der Meij EH, Mast H, van der Waal I. The possible premalignant character of oral lichen planus and oral lichenoid lesions: A prospective five-year follow-up study of 192 patients. Oral Oncol. 2007;43:742–8. doi: 10.1016/j.oraloncology.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Moles MA, Scully C, Gil-Montoya JA. Oral lichen planus: Controversies surrounding malignant transformation. Oral Dis. 2008;14:229–43. doi: 10.1111/j.1601-0825.2008.01441.x. [DOI] [PubMed] [Google Scholar]
- 7.Speight PM. Update on oral epithelial dysplasia and progression to cancer. Head Neck Pathol. 2007;1:61–6. doi: 10.1007/s12105-007-0014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmstrup P. The malignant potential of oral lichen planus. Oral Dis. 2010;16:509–10. [Google Scholar]
- 9.Scully C, Beyli M, Ferreiro MC, Ficarra G, Gill Y, Griffiths M, et al. Update on oral lichen planus: Etiopathogenesis and management. Crit Rev Oral Biol Med. 1998;9:86–122. doi: 10.1177/10454411980090010501. [DOI] [PubMed] [Google Scholar]
- 10.Sugerman PB, Savage NW. Oral lichen planus: Causes, diagnosis and management. Aust Dent J. 2002;47:290–7. doi: 10.1111/j.1834-7819.2002.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 11.Laeijendecker R, Van Joost T, Tank B, Oranje AP, Neumann HA. Oral lichen planus in childhood. Pediatr Dermatol. 2005;22:299–304. doi: 10.1111/j.1525-1470.2005.22403.x. [DOI] [PubMed] [Google Scholar]
- 12.Aminzadeh A, Jahanshahi G, Ahmadi M. A retrospective comparative study on clinico-pathologic features of oral lichen planus and oral lichenoid lesions. Dent Res J (Isfahan) 2013;10:168–72. doi: 10.4103/1735-3327.113328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sousa FA, Paradella TC, Carvalho YR, Rosa LE. Immunohistochemical expression of PCNA, p53, bax and bcl-2 in oral lichen planus and epithelial dysplasia. J Oral Sci. 2009;51:117–21. doi: 10.2334/josnusd.51.117. [DOI] [PubMed] [Google Scholar]
- 14.Bloor BK, Malik FK, Odell EW, Morgan PR. Quantitative assessment of apoptosis in oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:187–95. doi: 10.1016/s1079-2104(99)70116-2. [DOI] [PubMed] [Google Scholar]
- 15.Dragomir LP, Simionescu C, Mărgăritescu C, Stepan A, Dragomir IM, Popescu MR. p53, p16 and Ki67 immunoexpression in oral squamous carcinomas. Rom J Morphol Embryol. 2012;53:89–93. [PubMed] [Google Scholar]
- 16.Jordan RC, Daniels TE, Greenspan JS, Regezi JA. Advanced diagnostic methods in oral and maxillofacial pathology. Part II: Immunohistochemical and immunofluorescent methods. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:56–74. doi: 10.1067/moe.2002.119567. [DOI] [PubMed] [Google Scholar]
- 17.Loro LL, Johannessen AC, Vintermyr OK. Loss of BCL-2 in the progression of oral cancer is not attributable to mutations. J Clin Pathol. 2005;58:1157–62. doi: 10.1136/jcp.2004.021709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dekker NP, Lozada-Nur F, Lagenaur LA, MacPhail LA, Bloom CY, Regezi JA. Apoptosis-associated markers in oral lichen planus. J Oral Pathol Med. 1997;26:170–5. doi: 10.1111/j.1600-0714.1997.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa K, Yamamura K, Maeda S, Ichihashi M. bcl-2 expression in epidermal keratinocytic diseases. Cancer. 1994;74:1720–4. doi: 10.1002/1097-0142(19940915)74:6<1720::aid-cncr2820740613>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 20.Sugerman PB, Savage NW, Zhou X, Walsh LJ, Bigby M. Oral lichen planus. Clin Dermatol. 2000;18:533–9. doi: 10.1016/s0738-081x(00)00142-5. [DOI] [PubMed] [Google Scholar]
- 21.Humayun S, Prasad VR. Expression of p53 protein and ki-67 antigen in oral premalignant lesions and oral squamous cell carcinomas: An immunohistochemical study. Natl J Maxillofac Surg. 2011;2:38–46. doi: 10.4103/0975-5950.85852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fakhrjou A, Toutounchi SJ. Morphologic evaluation of p53 apoptotic signaling responses and proliferative activity of Ki-67 in oral lichen planus, oral squamous cell carcinoma and normal specimens. J Med Sci. 2012;12:51–6. [Google Scholar]
- 23.Taniguchi Y, Nagao T, Maeda H, Kameyama Y, Warnakulasuriya KA. Epithelial cell proliferation in oral lichen planus. Cell Prolif. 2002;35 Suppl 1:103–9. doi: 10.1046/j.1365-2184.35.s1.11.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Acay RR, Felizzola CR, de Araújo N, de Sousa SO. Evaluation of proliferative potential in oral lichen planus and oral lichenoid lesions using immunohistochemical expression of p53 and Ki67. Oral Oncol. 2006;42:475–80. doi: 10.1016/j.oraloncology.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Raju B, Mehrotra R, Oijordsbakken G, Al-Sharabi AK, Vasstrand EN, Ibrahim SO. Expression of p53, cyclin D1 and Ki-67 in pre-malignant and malignant oral lesions: Association with clinicopathological parameters. Anticancer Res. 2005;25:4699–706. [PubMed] [Google Scholar]
- 26.Angiero F, Berenzi A, Benetti A, Rossi E, Del Sordo R, Sidoni A, et al. Expression of p16, p53 and Ki-67 proteins in the progression of epithelial dysplasia of the oral cavity. Anticancer Res. 2008;28:2535–9. [PubMed] [Google Scholar]
- 27.Kannan K, Latha PN, Shanmugam G. Expression of bcl-2 oncoprotein in Indian oral squamous cell carcinomas. Oral Oncol. 1998;34:373–6. doi: 10.1016/s1368-8375(98)00037-2. [DOI] [PubMed] [Google Scholar]
- 28.Stoll C, Baretton G, Ahrens C, Löhrs U. Prognostic significance of apoptosis and associated factors in oral squamous cell carcinoma. Virchows Arch. 2000;436:102–8. doi: 10.1007/pl00008207. [DOI] [PubMed] [Google Scholar]
- 29.Basu A, Haldar S. The relationship between BcI2, Bax and p53: Consequences for cell cycle progression and cell death. Mol Hum Reprod. 1998;4:1099–109. doi: 10.1093/molehr/4.12.1099. [DOI] [PubMed] [Google Scholar]
- 30.Zhao J, Guo B, Ma SC, Zhou XD. Expression of p53 and Ki-67 genes in epithelial dysplasia from old oral mucosa and clinical significance. Sichuan Da Xue Xue Bao Yi Xue Ban. 2005;36:689–91. [PubMed] [Google Scholar]
- 31.Agha-Hosseini F, Khalili M, Rohani B. Immunohistochemistry analysis of p53 and Ki-67 proteins in oral lichen planus and normal mucosa. Iran J Public Health. 2009;38:37–43. [Google Scholar]
- 32.Suri C. The immunohistochemical evaluation of the expression of BCL-2 in different histological grades of squamous cell carcinoma. J Clin Diagn Res. 2009;3:1891–9. [Google Scholar]
- 33.Leyva-Huerta ER, Ledesma-Montes C, Rojo-Botello RE, Vega-Memije E. p53 and bcl-2 immunoexpression in patients with oral lichen planus and oral squamous cell carcinoma. Med Oral Patol Oral Cir Bucal. 2012;17:e745–50. doi: 10.4317/medoral.18013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanda N, Mori S, Saito K, Ikawa K, Sakamoto S. Expression of apoptotic signaling proteins in leukoplakia and oral lichen planus: Quantitative and topographical studies. J Oral Pathol Med. 2000;29:385–93. doi: 10.1034/j.1600-0714.2000.290804.x. [DOI] [PubMed] [Google Scholar]
- 35.Nair RG, Shameena PM, Varghese I, Sudha S. Immunohistochemical evaluation of BCL-2 oncoprotein in oral dysplasia and carcinoma. Oral Maxillofac Pathol J. 2011;2:83–8. [Google Scholar]
- 36.Bose P, Klimowicz AC, Kornaga E, Petrillo SK, Matthews TW, Chandarana S, et al. Bax expression measured by AQUanalysis is an independent prognostic marker in oral squamous cell carcinoma. BMC Cancer. 2012;12:332. doi: 10.1186/1471-2407-12-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhan Q, Fan S, Bae I, Guillouf C, Liebermann DA, O’Connor PM, et al. Induction of bax by genotoxic stress in human cells correlates with normal p53 status and apoptosis. Oncogene. 1994;9:3743–51. [PubMed] [Google Scholar]


