Abstract
Patient: Female, 67
Final Diagnosis: Dermatomyositis
Symptoms: Muscle weakness • skin rash • subcutaneous nodules
Medication: —
Clinical Procedure: Drug administration
Specialty: Dermatology
Objective:
Rare disease
Background:
Radiation-induced morphea is a rare complication of radiation therapy. The affected areas are generally restricted to the radiation field or to the nearby surrounding area.
Case Report:
A 67-year-old Japanese woman with a history of right breast cancer followed by adjuvant radiotherapy was referred our hospital because of 7-year history of symmetrical indurated erythematous plaques on her trunk. Three months after completion of irradiation, erythematous plaques developed on her right chest and gradually spread accompanied tenderness. She did not have a history of trauma to her right chest. Laboratory testing was positive for antinuclear antibody test at 1: 640 but negative for anti-SS-A/B, anti-U1-RNP, anti-DNA, anti-Sm, anticentromere, anti-topoisomerase I antibodies, and Borrelia and cytomegalovirus infection. She had no Raynaud’s phenomenon, sclerodactyly, or nail-fold bleeding. She did not have interstitial lung disease or other internal organ involvement. A biopsy specimen revealed reticular dermal fibrosis with thickened collagen bundles with superficial and deep perivascular infiltration of mononuclear cells. These findings were consistent with morphea. Furthermore, mucin deposition was present in the papillary dermis upon Alcian blue staining, which has been reported to be observed in generalized morphea. Consequently, a diagnosis of generalized morphea induced by radiotherapy was made. She had been treated with oral hydroxychloroquine sulfate, resulting in the resolution of tenderness but the erythematous plaques remained.
Conclusions:
To the best of our knowledge, this is the first report of radiation-induced generalized morphea with prominent mucin deposition. Hydroxychloroquine sulfate may be efficacious for radiation-induced morphea-associated tenderness.
MeSH Keywords: Alcian Blue; Hydroxychloroquine; Radiotherapy, Adjuvant; Scleroderma, Localized
Background
Morphea, also known as localized scleroderma, is an uncommon cutaneous disorder characterized by circumscribed sclerotic plaques with dermal fibrosis and collagen deposition [1]. Morphea is different from systemic sclerosis in that it lacks acrosclerosis, internal organ involvement, and Raynaud’s phenomenon. Generalized morphea, the most severe form, is characterized by multiple sclerotic plaques. Although the pathogenesis of morphea remains unknown, autoimmunity is one of the central features of morphea. Patients with morphea, especially generalized morphea, have various autoantibodies [2]. Furthermore, trauma and infection with Borrelia burgdorferi and cytomegalovirus have been thought to induce morphea [1]. Radiation-induced morphea is a rare complication of radiation therapy that has been estimated to occur in 1 in 500 patients [3]. The majority of cases have occurred in patients with breast cancer [4]. Its onset ranges from 1 month to 3 years, although there is 1 reported case developing 32 years after radiotherapy [3,5,6]. The affected areas have generally been restricted to the radiation field or to the nearby surrounding area in the majority of previously reported cases, whereas only a few previous cases have had skin lesions extending beyond the irradiated area [4,7–9]. We here describe a patient with radiation-induced generalized morphea with unique clinical features.
Case Report
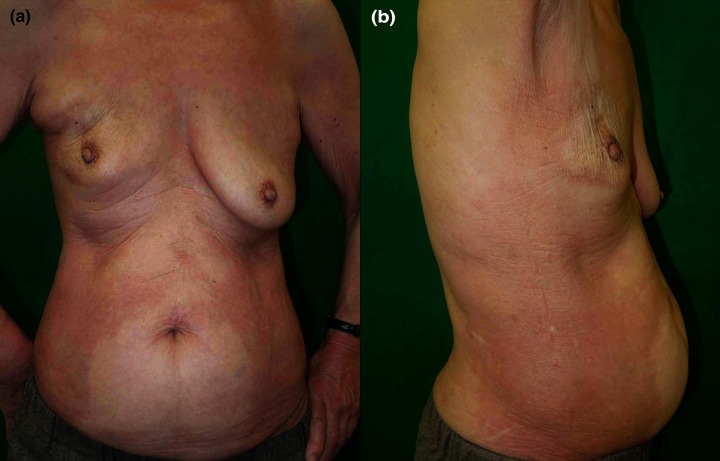
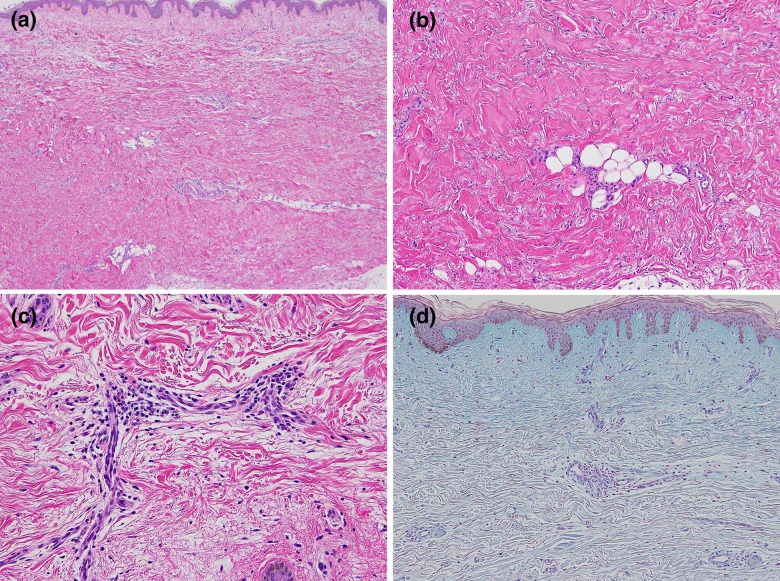
A 67-year-old Japanese woman diagnosed as having right breast cancer had undergone local excision of the right breast, followed by adjuvant radiotherapy to the right breast and axilla. Three months after completion of irradiation, erythematous plaques developed on her right chest. The lesions gradually spread and became tender. She was initially treated with topical corticosteroids, tacrolimus, and narrow-band ultraviolet B irradiation at another hospital without any improvement. Seven years later, she was referred to us with symmetrical indurated erythematous plaques on her trunk (Figure 1A, 1B). She had a family history of autoimmune diseases; 2 of her 4 sisters had systemic lupus erythematosus and 1 had rheumatoid arthritis. She had no Raynaud’s phenomenon, sclerodactyly, or nail-fold bleeding. Laboratory investigations showed positive antinuclear antibody test (1:640, speckled), but anti-SS-A/B, anti-U1-RNP, anti-DNA, anti-Sm, anticentromere, and anti-topoisomerase I antibodies were all negative. Chest computed tomography did not show interstitial lung disease or other diseases. She did no have renal or digestive diseases. A biopsy specimen obtained from the right upper abdomen histologically revealed reticular dermal fibrosis with thickened collagen bundles with superficial and deep perivascular infiltration of mononuclear cells (Figure 2A–2C). Direct immunofluorescence was negative. These findings were consistent with morphea, although mucin deposition shown by Alcian blue staining was present in the papillary dermis (Figure 2D).
Figure 1.
(A, B) Clinical features on the first visit. Symmetrical indurated erythematous plaques on the trunk.
Figure 2.
(A, B) Marked dermal fibrosis with thickened collagen bundles (hematoxylin and eosin). (C) Dermal perivascular infiltration of mononuclear cells (hematoxylin and eosin). (D) Presence of mucin deposition in the upper dermis (Alcian blue stain).
She did not have any history of trauma on her right chest. Furthermore, she had negative results for Borrelia and cytomegalovirus infection. Consequently, a diagnosis of generalized morphea induced by radiotherapy was made. She had been treated with oral hydroxychloroquine sulfate at 200 mg daily for 6 months, resulting in the resolution of tenderness but the indurated erythematous plaques remained.
Discussion
Our case is unique because the skin lesions extended far beyond the field of radiotherapy and showed symmetrical plaques, resembling systemic sclerosis rather than morphea. However, skin sclerosis normally begins from the fingers and advances towards the center of the body in systemic sclerosis, unlike our case. This is the first reported case of radiation-induced generalized morphea with symmetrical widespread lesions. Irradiated dermal fibroblasts showed increased mRNA expression of transforming growth factor-β, tissue inhibitor of metal-loprotease-1, and Smad3 [10], which are thought to play key roles in the development of morphea and systemic sclerosis.
Furthermore, patients with systemic sclerosis have been reported to develop exaggerated skin fibrosis following radio-therapy [11]. Our case had a positive antinuclear antibody with a strong family history of autoimmune diseases, suggesting that she was genetically susceptible to autoimmune diseases. Therefore, radiotherapy might have affected dermal fibroblasts, and initiated the development of skin fibrosis. Long-term follow-up is essential to determine whether our case develops typical clinical features of systemic sclerosis such as sclerodactyly or Raynaud’s phenomenon.
Prominent mucin deposition in the papillary dermis in our case is not considered a typical pathological feature of morphea. It is unclear whether mucin deposition is commonly seen in radiation-induced morphea, because none of the previous reports have mentioned the presence of mucin deposition. Interestingly, it has been reported that mucin deposition was observed in all of the 7 cases of systemic sclerosis, 3 cases of morphea, and 10 cases of generalized morphea [12]. In addition, mucin deposition was seen in the deep dermis in 18 out of these 20 cases, whereas 2 cases of generalized morphea exhibited mucin deposition in the upper dermis [12], suggesting that mucin deposition in the upper dermis may appear in some patients with generalized morphea. Moreover, our case had tenderness, which is not a typical feature of morphea. The presence of pain has been reported in several previous cases of radiation-induced morphea [5,6,8,13–16], indicating that pain may be a characteristic feature of radiation-induced morphea. It has been reported that 26% of patients with morphea experienced pain [17], although there are no other studies reporting the presence of pain in patients with morphea. Therefore, further studies are required to assess the presence of mucin deposition and pain associated with morphea, including radiation-induced morphea.
Little is known about treatment of radiation-induced morphea. Although the natural history of radiation-induced morphea remains obscure, in some cases skin lesions have resolved, without intervention, as morphea [13,18]. Topical or oral corticosteroids, topical tacrolimus, topical calcipotriol, systemic antibiotics, ultraviolet A irradiation, and methotrexate have been used with varying degrees of success [3–8,19,20]. Although narrow-band ultraviolet B irradiation has been reported to resolve both sclerosis and tenderness [16], our case showed a poor response to not only topical corticosteroids, and tacrolimus, but also to narrow-band ultraviolet B irradiation. Because oral chloroquine provided clinical improvement in 1 patient [21], she agreed to try oral hydroxychloroquine sulfate, resulting in some improvement in pain, indicating that hydroxychloroquine sulfate may be efficacious for radiation-induced morphea-associated tenderness.
Conclusions
This case was unique because the skin lesions extended far beyond the field of radiotherapy and showed symmetrical plaques, resembling systemic sclerosis.
To the best of our knowledge, this is the first report of radiation-induced generalized morphea with prominent mucin deposition.
Hydroxychloroquine sulfate may be effective for radiation-induced morphea-associated tenderness.
Footnotes
Conflict of interest
None declared.
References:
- 1.Fett N, Werth VP. Update on morphea: part I. Epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2011;64:217–28. doi: 10.1016/j.jaad.2010.05.045. quiz 29–30. [DOI] [PubMed] [Google Scholar]
- 2.Takehara K, Sato S. Localized scleroderma is an autoimmune disorder. Rheumatology (Oxford) 2005;44:274–79. doi: 10.1093/rheumatology/keh487. [DOI] [PubMed] [Google Scholar]
- 3.Davis DA, Cohen PR, McNeese MD, et al. Localized scleroderma in breast cancer patients treated with supervoltage external beam radiation: radiation port scleroderma. J Am Acad Dermatol. 1996;35:923–27. doi: 10.1016/s0190-9622(96)90116-4. [DOI] [PubMed] [Google Scholar]
- 4.Colver GB, Rodger A, Mortimer PS, et al. Post-irradiation morphoea. Br J Dermatol. 1989;120:831–35. doi: 10.1111/j.1365-2133.1989.tb01382.x. [DOI] [PubMed] [Google Scholar]
- 5.McClelland M, VanLoock JS, Patterson JW, et al. Radiation-induced morphea occurring after fluoroscopy. J Am Acad Dermatol. 2002;47:962–64. doi: 10.1067/mjd.2002.128286. [DOI] [PubMed] [Google Scholar]
- 6.Cheah NL, Wong DW, Chetiyawardana AD. Radiation-induced morphea of the breast: a case report. J Med Case Rep. 2008;2:136. doi: 10.1186/1752-1947-2-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ardern-Jones MR, Black MM. Widespread morphoea following radiotherapy for carcinoma of the breast. Clin Exp Dermatol. 2003;28:160–62. doi: 10.1046/j.1365-2230.2003.01186.x. [DOI] [PubMed] [Google Scholar]
- 8.Kushi J, Csuka ME. Generalized morphea after breast cancer radiation therapy. Case Rep Rheumatol. 2011;2011:951948. doi: 10.1155/2011/951948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akay BN, Sanli H, Heper AO. Postirradiation linear morphoea. Clin Exp Dermatol. 2010;35:e106–8. doi: 10.1111/j.1365-2230.2009.03717.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee JW, Zoumalan RA, Valenzuela CD, et al. Regulators and mediators of radiation-induced fibrosis: Gene expression profiles and a rationale for Smad3 inhibition. Otolaryngol Head Neck Surg. 2010;143:525–30. doi: 10.1016/j.otohns.2010.06.912. [DOI] [PubMed] [Google Scholar]
- 11.Varga J, Haustein UF, Creech RH, et al. Exaggerated radiation-induced fibrosis in patients with systemic sclerosis. JAMA. 1991;265:3292–95. [PubMed] [Google Scholar]
- 12.Rongioletti F, Gambini C, Micalizzi C, et al. Mucin deposits in morphea and systemic scleroderma. Dermatology. 1994;189:157–58. doi: 10.1159/000246821. [DOI] [PubMed] [Google Scholar]
- 13.Wernicke AG, Goltser Y, Trichter S, et al. Morphea as a consequence of accelerated partial breast irradiation. Clin Breast Cancer. 2011;11:67–70. doi: 10.3816/CBC.2011.n.012. [DOI] [PubMed] [Google Scholar]
- 14.Laetsch B, Hofer T, Lombriser N, et al. Irradiation-induced morphea: x-rays as triggers of autoimmunity. Dermatology. 2011;223:9–12. doi: 10.1159/000330324. [DOI] [PubMed] [Google Scholar]
- 15.Reddy SM, Pui JC, Gold LI, et al. Postirradiation morphea and subcutaneous polyarteritis nodosa: case report and literature review. Semin Arthritis Rheum. 2005;34:728–34. doi: 10.1016/j.semarthrit.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Newland K, Marshman G. Success treatment of post-irradiation morphoea with acitretin and narrowband UVB. Australas J Dermatol. 2012;53:136–38. doi: 10.1111/j.1440-0960.2011.00864.x. [DOI] [PubMed] [Google Scholar]
- 17.Kroft EB, de Jong EM, Evers AW. Physical burden of symptoms in patients with localized scleroderma and eosinophilic fasciitis. Arch Dermatol. 2008;144:1394–95. doi: 10.1001/archderm.144.10.1394. [DOI] [PubMed] [Google Scholar]
- 18.Schaffer JV, Carroll C, Dvoretsky I, et al. Postirradiation morphea of the breast presentation of two cases and review of the literature. Dermatology. 2000;200:67–71. doi: 10.1159/000018322. [DOI] [PubMed] [Google Scholar]
- 19.Lim D, Johnston S, Novakovic L, et al. Radiation-induced morphoea treated with UVA-1 phototherapy. Clin Exp Dermatol. 2014;39:612–15. doi: 10.1111/ced.12345. [DOI] [PubMed] [Google Scholar]
- 20.Alhathlool A, Hein R, Andres C, et al. Post-Irradiation Morphea: Case report and review of the literature. J Dermatol Case Rep. 2012;6:73–77. doi: 10.3315/jdcr.2012.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neill SM, Nicholl JJ, Hanham IWF, et al. Localized morphoea at site of previous radiotherapy. Br J Dermatol. 1989;119(Suppl.33):110–11. [Google Scholar]