Abstract
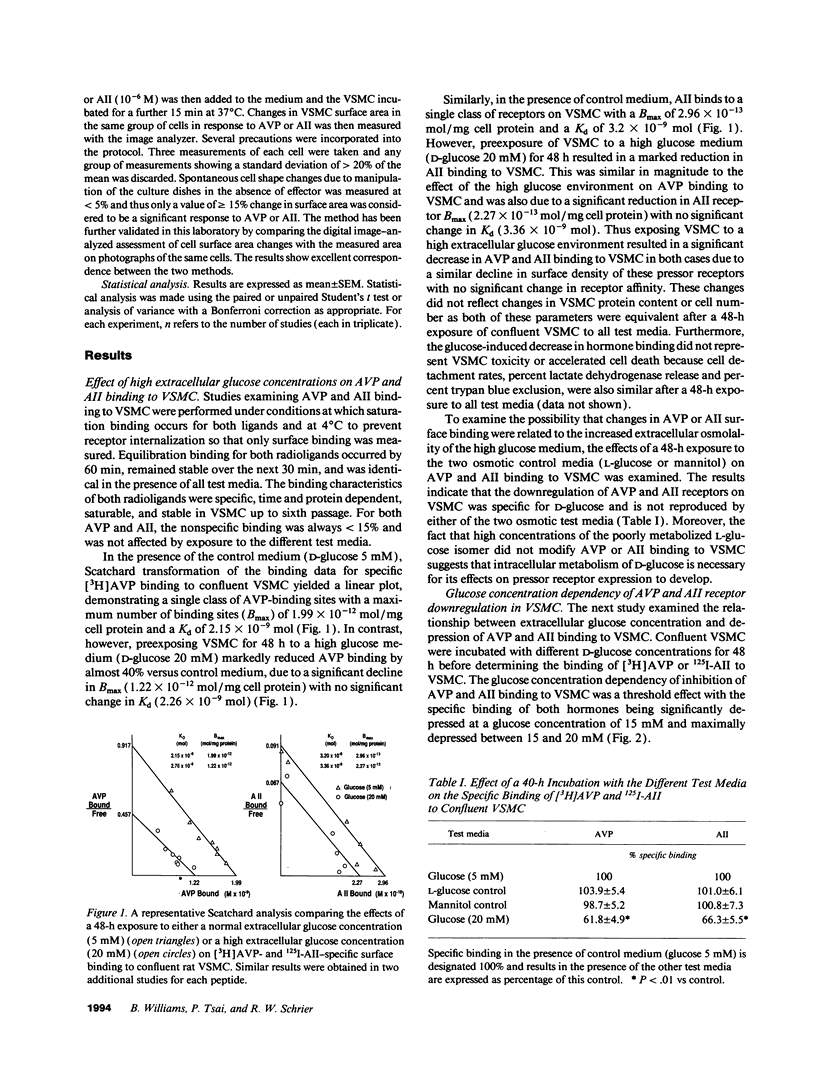
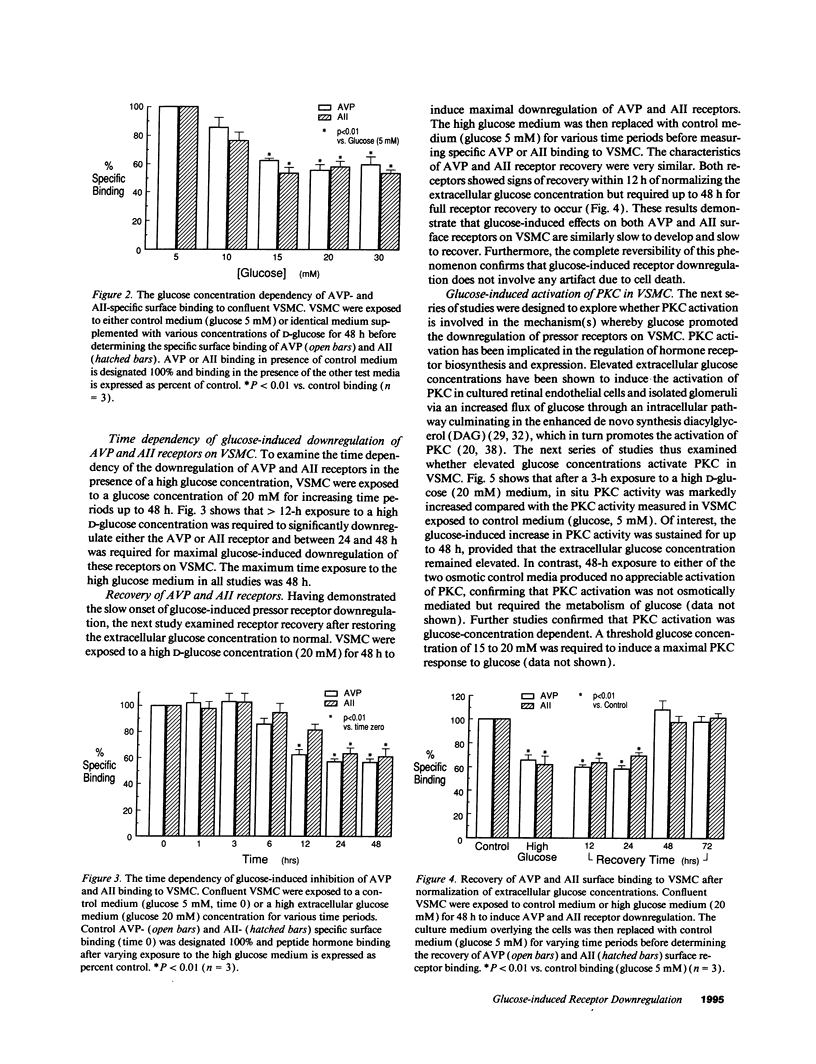
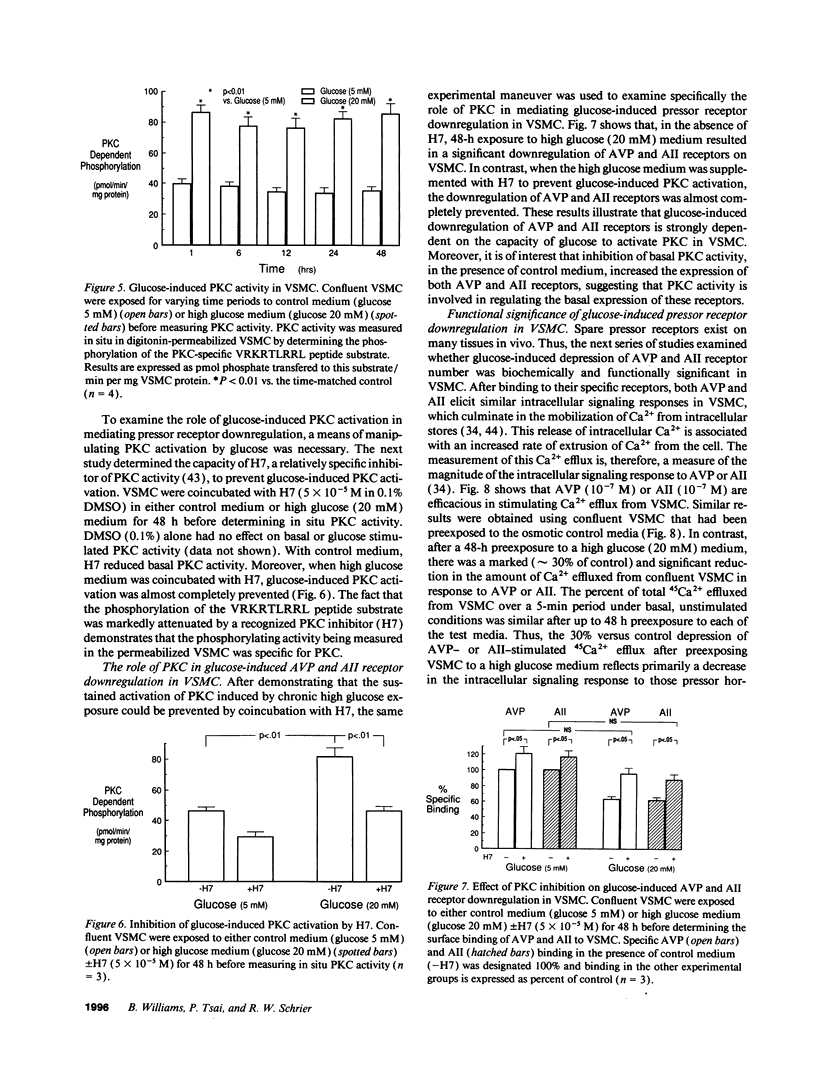
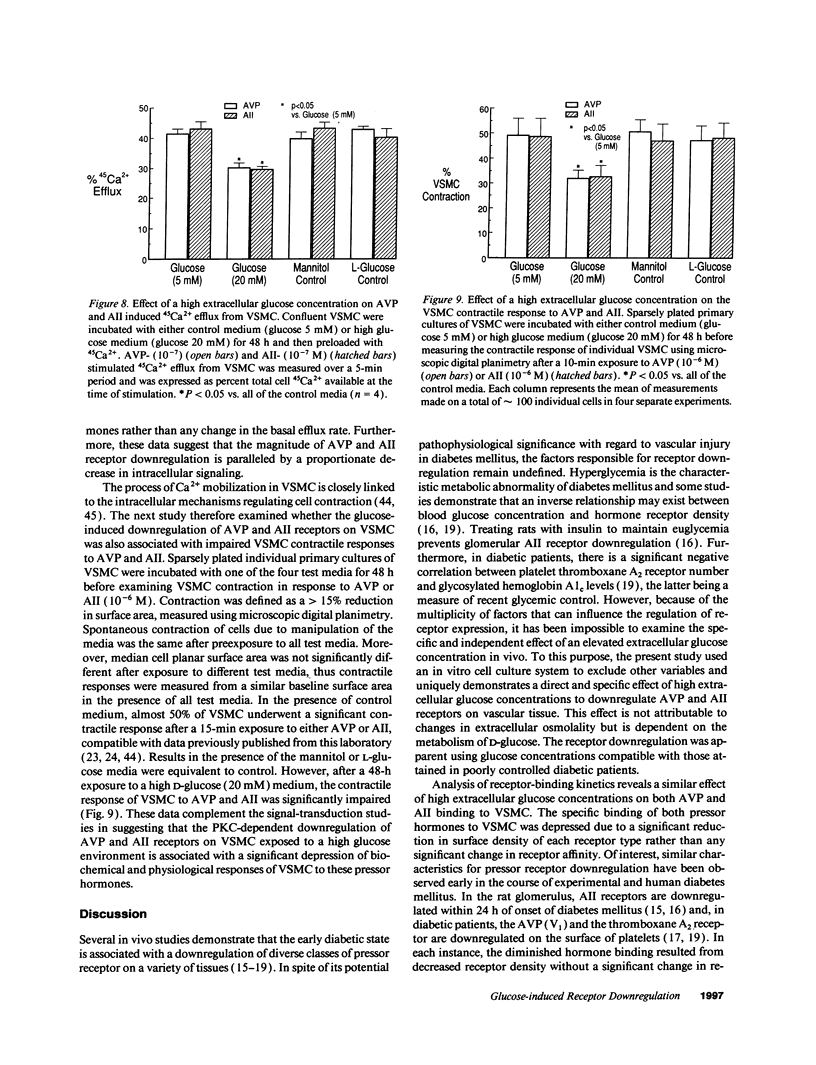
Early diabetes mellitus is characterized by impaired responses to pressor hormones and pressor receptor downregulation. The present study examined the effect of elevated extracellular glucose concentrations on angiotensin II (AII) and arginine vasopressin (AVP) receptor kinetics in cultured rat vascular smooth muscle cells (VSMC). Scatchard analysis of [3H]AVP and 125I-AII binding to confluent VSMC showed that high glucose concentrations (20 mM) similarly depressed AVP and AII surface receptor Bmax but did not influence receptor Kd. This receptor downregulation was not reproduced by osmotic control media containing either L-glucose or mannitol. Receptor downregulation was maximal at a glucose concentration of 15-20 mM and required 24-48 h for a maximum effect. Normalization of the extracellular glucose concentration allowed complete recovery of AVP and AII binding within 48 h. Receptor downregulation was associated with depressed AVP and AII-stimulated intracellular signaling and cell contraction. High glucose concentrations induced a sustained activation of protein kinase C (PKC) in VSMC, which was prevented by coincubation with H-7. H-7 also markedly attenuated glucose-induced downregulation of AVP and AII receptors on VSMC. This study demonstrates a novel cellular mechanism whereby high extracellular glucose concentrations directly and independently downregulate pressor hormone receptors and their function on vascular tissue via glucose-stimulated PKC activation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Hathaway D. R. Role of calcium and cyclic adenosine 3':5' monophosphate in regulating smooth muscle contraction. Mechanisms of excitation-contraction coupling in smooth muscle. Am J Cardiol. 1979 Oct 22;44(5):783–787. doi: 10.1016/0002-9149(79)90197-8. [DOI] [PubMed] [Google Scholar]
- Atherton A., Hill D. W., Keen H., Young S., Edwards E. J. The effect of acute hyperglycaemia on the retinal circulation of the normal cat. Diabetologia. 1980 Mar;18(3):233–237. doi: 10.1007/BF00251922. [DOI] [PubMed] [Google Scholar]
- Bell R. M. Protein kinase C activation by diacylglycerol second messengers. Cell. 1986 Jun 6;45(5):631–632. doi: 10.1016/0092-8674(86)90774-9. [DOI] [PubMed] [Google Scholar]
- Bentsen N., Larsen B., Lassen N. A. Chronically impaired autoregulation of cerebral blood flow in long-term diabetics. Stroke. 1975 Sep-Oct;6(5):497–502. doi: 10.1161/01.str.6.5.497. [DOI] [PubMed] [Google Scholar]
- Caramelo C., Okada K., Tsai P., Linas S. L., Schrier R. W. Interaction of arginine vasopressin and angiotensin II on Ca2+ in vascular smooth muscle cells. Kidney Int. 1990 Jul;38(1):47–54. doi: 10.1038/ki.1990.165. [DOI] [PubMed] [Google Scholar]
- Caramelo C., Okada K., Tsai P., Schrier R. W. Mechanisms of the vascular effect of pressor hormones. Am J Cardiol. 1988 Oct 5;62(11):47G–53G. doi: 10.1016/0002-9149(88)90032-x. [DOI] [PubMed] [Google Scholar]
- Caramelo C., Okada K., Tsai P., Schrier R. W. Phorbol esters and arginine vasopressin in vascular smooth muscle cell activation. Am J Physiol. 1989 May;256(5 Pt 2):F875–F881. doi: 10.1152/ajprenal.1989.256.5.F875. [DOI] [PubMed] [Google Scholar]
- Caramelo C., Tsai P., Okada K., Briner V. A., Schrier R. W. Mechanisms of rapid desensitization to arginine vasopressin in vascular smooth muscle cells. Am J Physiol. 1991 Jan;260(1 Pt 2):F46–F52. doi: 10.1152/ajprenal.1991.260.1.F46. [DOI] [PubMed] [Google Scholar]
- Caramelo C., Tsai P., Schrier R. W. Mechanism of cellular effect of phorbol esters on action of arginine vasopressin and angiotensin II on rat vascular smooth muscle cells in culture. Biochem J. 1988 Sep 15;254(3):625–629. doi: 10.1042/bj2540625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamley-Campbell J., Campbell G. R., Ross R. The smooth muscle cell in culture. Physiol Rev. 1979 Jan;59(1):1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- Craven P. A., Davidson C. M., DeRubertis F. R. Increase in diacylglycerol mass in isolated glomeruli by glucose from de novo synthesis of glycerolipids. Diabetes. 1990 Jun;39(6):667–674. doi: 10.2337/diab.39.6.667. [DOI] [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Protein kinase C is activated in glomeruli from streptozotocin diabetic rats. Possible mediation by glucose. J Clin Invest. 1989 May;83(5):1667–1675. doi: 10.1172/JCI114066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. J., Czech M. P. Tumor-promoting phorbol diesters cause the phosphorylation of epidermal growth factor receptors in normal human fibroblasts at threonine-654. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1974–1978. doi: 10.1073/pnas.82.7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar H., Zisman Y., Ullrich A., Livneh E. Overexpression of protein kinase C alpha-subtype in Swiss/3T3 fibroblasts causes loss of both high and low affinity receptor numbers for epidermal growth factor. J Biol Chem. 1990 Aug 5;265(22):13290–13296. [PubMed] [Google Scholar]
- Faris I., Vagn Nielsen H., Henriksen O., Parving H. H., Lassen N. A. Impaired autoregulation of blood flow in skeletal muscle and subcutaneous tissue in long-term Type 1 (insulin-dependent) diabetic patients with microangiopathy. Diabetologia. 1983 Dec;25(6):486–488. doi: 10.1007/BF00284456. [DOI] [PubMed] [Google Scholar]
- Gonzales R. A., Greger P. H., Jr, Baker S. P., Ganz N. I., Bolden C., Raizada M. K., Crews F. T. Phorbol esters inhibit agonist-stimulated phosphoinositide hydrolysis in neuronal primary cultures. Brain Res. 1987 Dec 15;465(1-2):59–66. doi: 10.1016/0165-3806(87)90228-8. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Ideura T., Yoshimura A., Koshikawa S. Autoregulation of renal blood flow in streptozocin-induced diabetic rats. Diabetes. 1989 Sep;38(9):1109–1113. doi: 10.2337/diab.38.9.1109. [DOI] [PubMed] [Google Scholar]
- Heasley L. E., Johnson G. L. Detection of nerve growth factor and epidermal growth factor-regulated protein kinases in PC12 cells with synthetic peptide substrates. Mol Pharmacol. 1989 Mar;35(3):331–338. [PubMed] [Google Scholar]
- Heasley L. E., Johnson G. L. Regulation of protein kinase C by nerve growth factor, epidermal growth factor, and phorbol esters in PC12 pheochromocytoma cells. J Biol Chem. 1989 May 25;264(15):8646–8652. [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hise M. K., Mehta P. S. Characterization and localization of calcium/phospholipid-dependent protein kinase-C during diabetic renal growth. Endocrinology. 1988 Sep;123(3):1553–1558. doi: 10.1210/endo-123-3-1553. [DOI] [PubMed] [Google Scholar]
- Hostetter T. H., Troy J. L., Brenner B. M. Glomerular hemodynamics in experimental diabetes mellitus. Kidney Int. 1981 Mar;19(3):410–415. doi: 10.1038/ki.1981.33. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Sahyoun N. E., Saltiel A. R., Cuatrecasas P. Phorbol esters stimulate the phosphorylation of receptors for insulin and somatomedin C. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6211–6213. doi: 10.1073/pnas.80.20.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa U., Kishimoto A., Nishizuka Y. The protein kinase C family: heterogeneity and its implications. Annu Rev Biochem. 1989;58:31–44. doi: 10.1146/annurev.bi.58.070189.000335. [DOI] [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- Lee T. S., MacGregor L. C., Fluharty S. J., King G. L. Differential regulation of protein kinase C and (Na,K)-adenosine triphosphatase activities by elevated glucose levels in retinal capillary endothelial cells. J Clin Invest. 1989 Jan;83(1):90–94. doi: 10.1172/JCI113889. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Leeb-Lundberg L. M., Cotecchia S., Lomasney J. W., DeBernardis J. F., Lefkowitz R. J., Caron M. G. Phorbol esters promote alpha 1-adrenergic receptor phosphorylation and receptor uncoupling from inositol phospholipid metabolism. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5651–5655. doi: 10.1073/pnas.82.17.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer S. M., Brown D. M., Steffes M. W., Azar S. Studies of renal autoregulation in pancreatectomized and streptozotocin diabetic rats. Kidney Int. 1990 Mar;37(3):909–917. doi: 10.1038/ki.1990.65. [DOI] [PubMed] [Google Scholar]
- Meyer-Lehnert H., Caramelo C., Tsai P., Schrier R. W. Interaction of atriopeptin III and vasopressin on calcium kinetics and contraction of aortic smooth muscle cells. J Clin Invest. 1988 Oct;82(4):1407–1414. doi: 10.1172/JCI113745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modesti P. A., Abbate R., Gensini G. F., Colella A., Neri Serneri G. G. Platelet thromboxane A2 receptors in type I diabetes. Clin Sci (Lond) 1991 Feb;80(2):101–105. doi: 10.1042/cs0800101. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Okada K., Tsai P., Briner V. A., Caramelo C., Schrier R. W. Effects of extra- and intracellular pH on vascular action of arginine vasopressin. Am J Physiol. 1991 Jan;260(1 Pt 2):F39–F45. doi: 10.1152/ajprenal.1991.260.1.F39. [DOI] [PubMed] [Google Scholar]
- Okumura K., Akiyama N., Hashimoto H., Ogawa K., Satake T. Alteration of 1,2-diacylglycerol content in myocardium from diabetic rats. Diabetes. 1988 Sep;37(9):1168–1172. doi: 10.2337/diab.37.9.1168. [DOI] [PubMed] [Google Scholar]
- Parving H. H., Kastrup H., Smidt U. M., Andersen A. R., Feldt-Rasmussen B., Christiansen J. S. Impaired autoregulation of glomerular filtration rate in type 1 (insulin-dependent) diabetic patients with nephropathy. Diabetologia. 1984 Dec;27(6):547–552. doi: 10.1007/BF00276965. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J. Protein kinase C from rat renal mesangial cells: its role in homologous desensitization of angiotensin II-induced polyphosphoinositide hydrolysis. Biochim Biophys Acta. 1988 May 13;969(3):263–270. doi: 10.1016/0167-4889(88)90061-4. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J. Tumour promotor 12-O-tetradecanoylphorbol 13-acetate inhibits angiotensin II-induced inositol phosphate production and cytosolic Ca2+ rise in rat renal mesangial cells. FEBS Lett. 1986 Jul 28;203(2):262–266. doi: 10.1016/0014-5793(86)80755-4. [DOI] [PubMed] [Google Scholar]
- Schrier R. W., Holzgreve H. Hemodynamic factors in the pathogenesis of diabetic nephropathy. Klin Wochenschr. 1988 Apr 15;66(8):325–331. doi: 10.1007/BF01735788. [DOI] [PubMed] [Google Scholar]
- Schrier R. W. Pathogenesis of sodium and water retention in high-output and low-output cardiac failure, nephrotic syndrome, cirrhosis, and pregnancy (1) N Engl J Med. 1988 Oct 20;319(16):1065–1072. doi: 10.1056/NEJM198810203191606. [DOI] [PubMed] [Google Scholar]
- Sinclair S. H., Grunwald J. E., Riva C. E., Braunstein S. N., Nichols C. W., Schwartz S. S. Retinal vascular autoregulation in diabetes mellitus. Ophthalmology. 1982 Jul;89(7):748–750. doi: 10.1016/s0161-6420(82)34720-x. [DOI] [PubMed] [Google Scholar]
- Takayama S., White M. F., Lauris V., Kahn C. R. Phorbol esters modulate insulin receptor phosphorylation and insulin action in cultured hepatoma cells. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7797–7801. doi: 10.1073/pnas.81.24.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibonnier M., Woloschak M. Platelet aggregation and vasopressin receptors in patients with diabetes mellitus. Proc Soc Exp Biol Med. 1988 Jun;188(2):149–152. doi: 10.3181/00379727-188-42720. [DOI] [PubMed] [Google Scholar]
- Turlapaty P. D., Lum G., Altura B. M. Vascular responsiveness and serum biochemical parameters in alloxan diabetes mellitus. Am J Physiol. 1980 Dec;239(6):E412–E421. doi: 10.1152/ajpendo.1980.239.6.E412. [DOI] [PubMed] [Google Scholar]
- Ullian M. E., Linas S. L. Angiotensin II surface receptor coupling to inositol trisphosphate formation in vascular smooth muscle cells. J Biol Chem. 1990 Jan 5;265(1):195–200. [PubMed] [Google Scholar]
- Ullian M. E., Linas S. L. Role of receptor cycling in the regulation of angiotensin II surface receptor number and angiotensin II uptake in rat vascular smooth muscle cells. J Clin Invest. 1989 Sep;84(3):840–846. doi: 10.1172/JCI114244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkes B. M. Reduced glomerular angiotensin II receptor density in diabetes mellitus in the rat: time course and mechanism. Endocrinology. 1987 Apr;120(4):1291–1298. doi: 10.1210/endo-120-4-1291. [DOI] [PubMed] [Google Scholar]
- Zatz R., Brenner B. M. Pathogenesis of diabetic microangiopathy. The hemodynamic view. Am J Med. 1986 Mar;80(3):443–453. doi: 10.1016/0002-9343(86)90719-9. [DOI] [PubMed] [Google Scholar]
- Zatz R., Meyer T. W., Rennke H. G., Brenner B. M. Predominance of hemodynamic rather than metabolic factors in the pathogenesis of diabetic glomerulopathy. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5963–5967. doi: 10.1073/pnas.82.17.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]



