Abstract
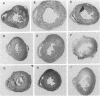
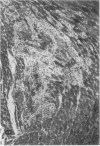
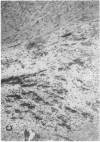
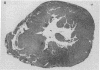
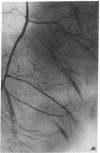
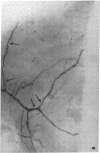
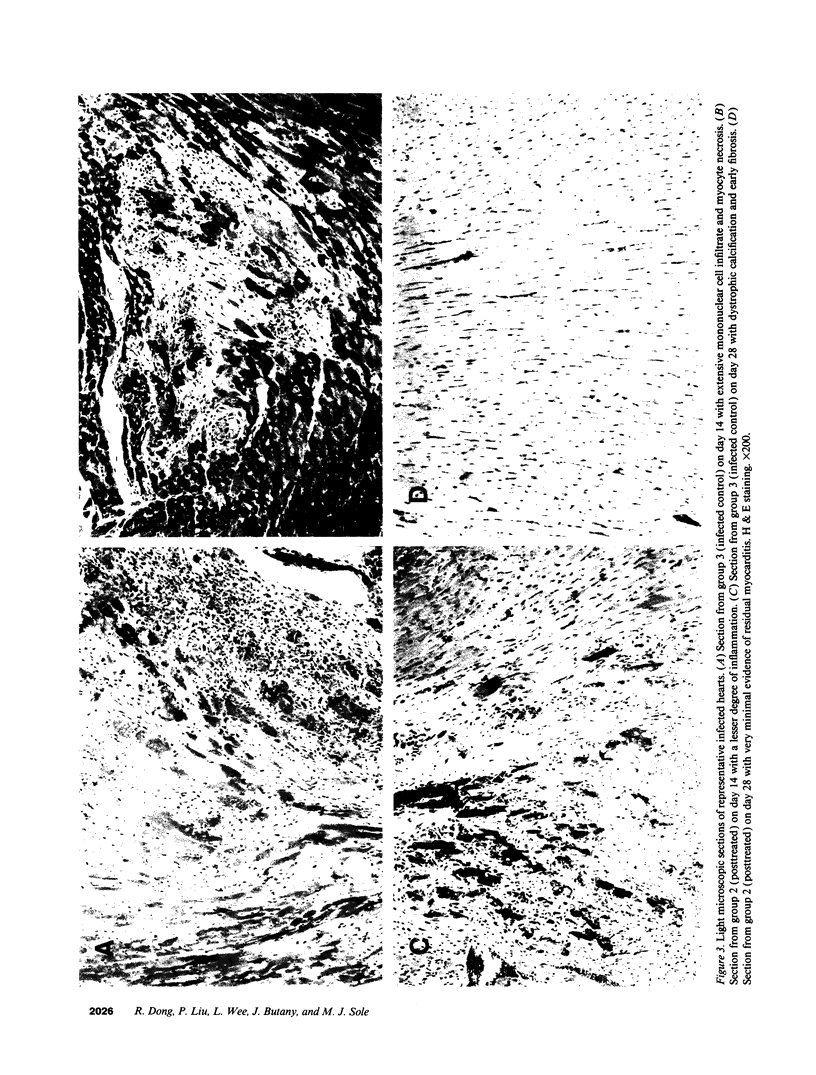
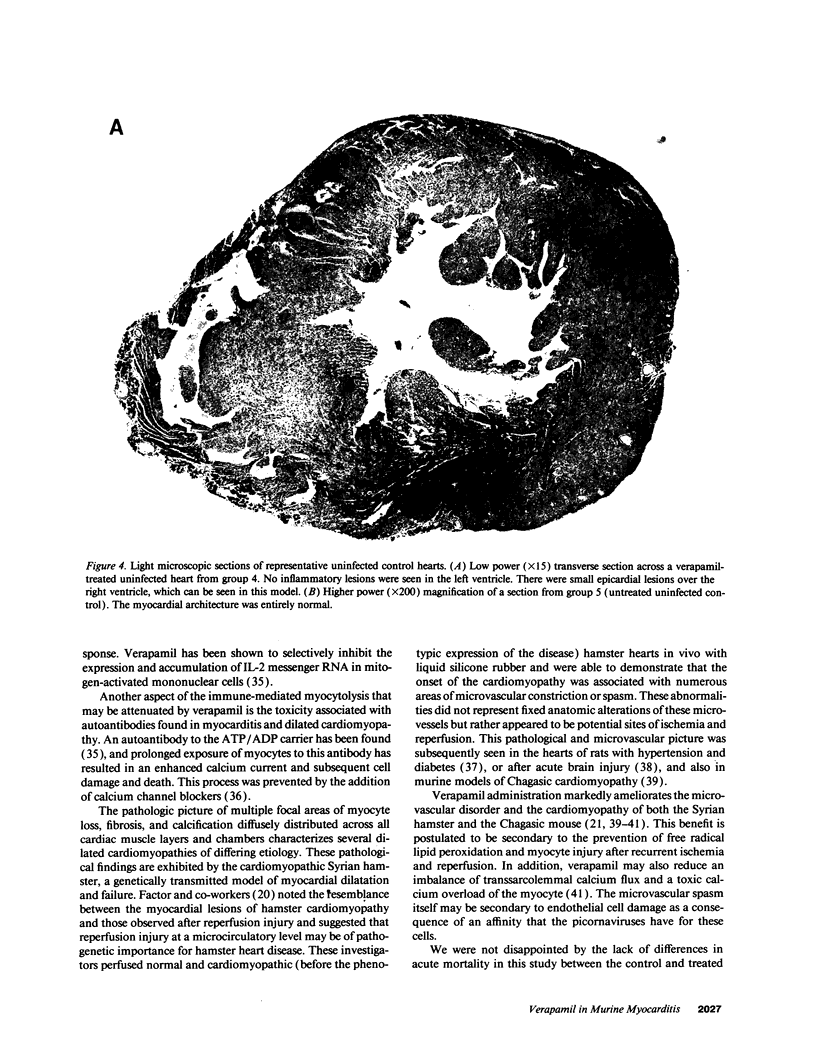
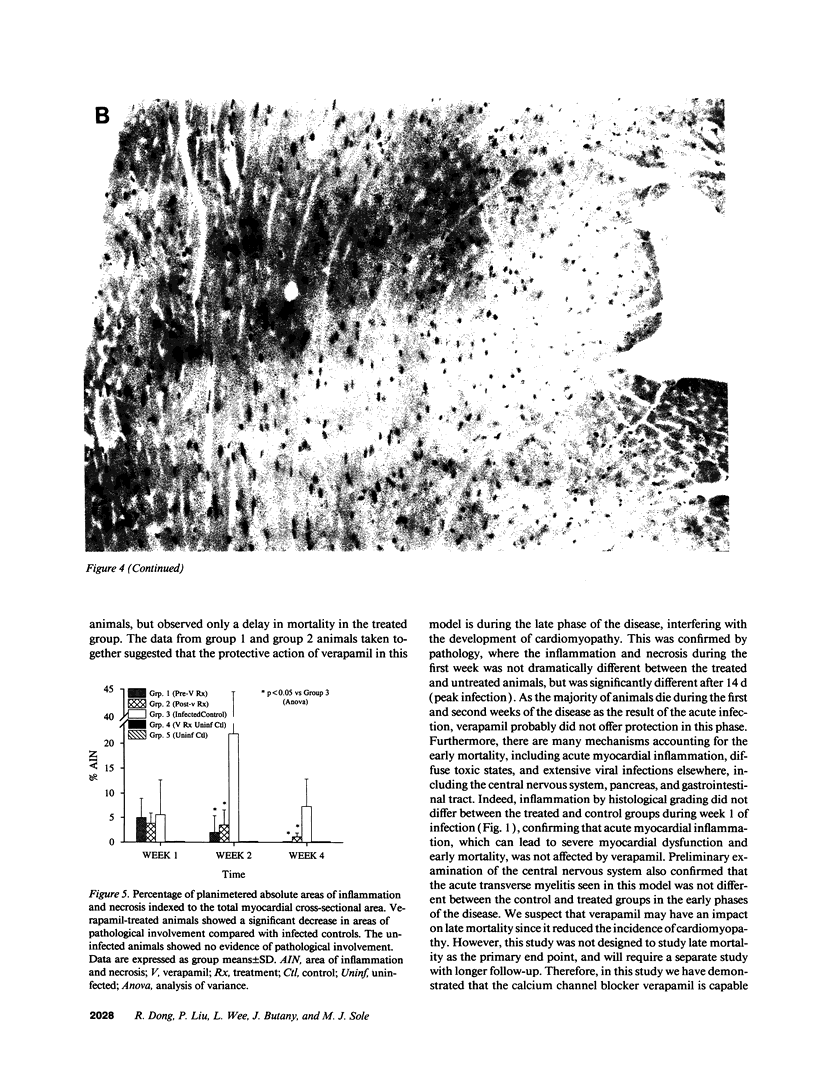
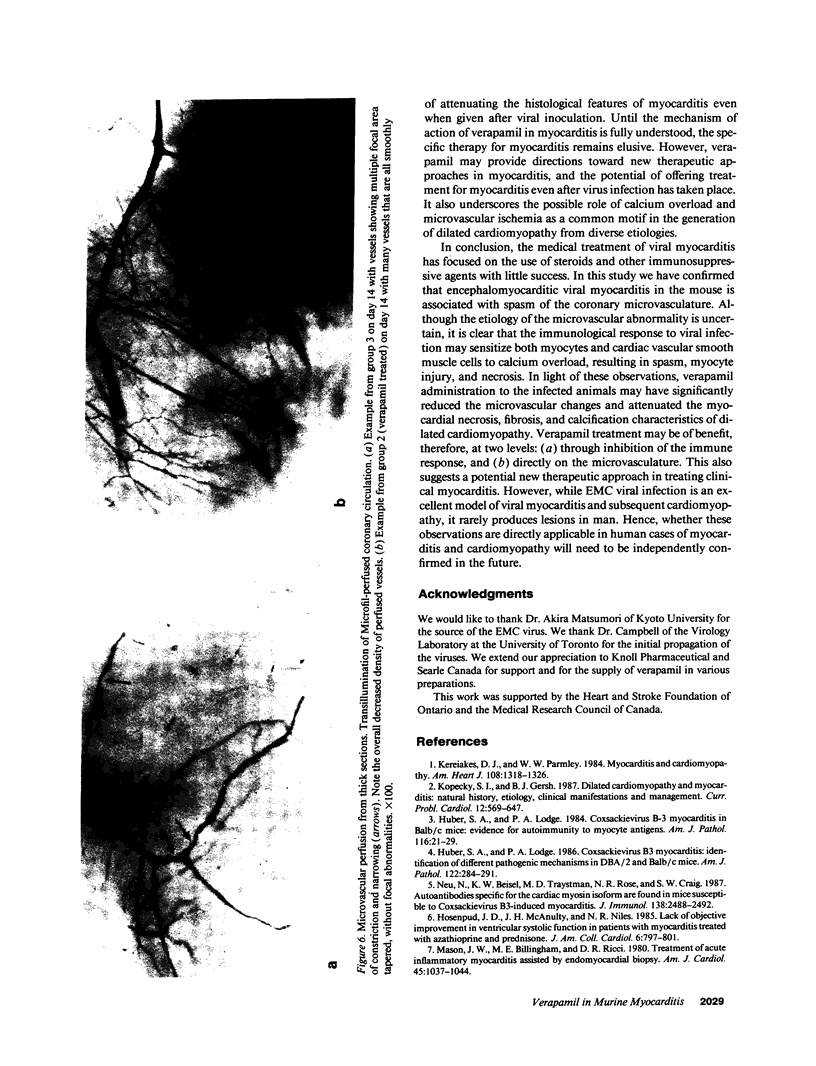
The effects of the calcium channel blocking agent, verapamil, were studied in a murine model of viral myocarditis. Three groups of 8-wk-old DBA/2 mice (n = 25 each) were inoculated with 10 plaque-forming units of encephalomyocarditis virus and randomized to three treatment regimens. Group 1 mice received verapamil intraperitoneally (5 mg/kg per d) for 7 d before infection, followed by verapamil orally (mean dose of 3.5 mg/mouse per d) in drinking water during infection. Group 2 mice received only verapamil orally starting on day 4 after infection, coincident with peak viremia. Group 3 (infected control) received no verapamil in regular drinking water after viral inoculation. Additional control animals were studied in group 4 (n = 21), consisting of uninfected control animals receiving intraperitoneal and oral verapamil at doses identical to group 1, and in group 5 (n = 21), consisting of uninfected and untreated controls. Animals were randomly killed from each group (n = 7) at 7, 14, and 28 d after infection. Routine histology was performed blindly on an apical slice of each heart and semi-quantitatively graded for inflammation, necrosis, calcification, and fibrosis on a scale of 0-4. Digital planimetry was performed to measure the absolute and relative areas of inflammation and necrosis. The pretreated animals in group 1 showed marked reduction in inflammation and necrosis (score of 3.7 +/- 1.4 vs. 8.7 +/- 2.0 in group 3 on day 14, P < 0.05) and were indistinguishable from the posttreated group 2 mice (score of 4.0 +/- 1.5 vs. 8.7 +/- 2.0 in group 3 on day 14, P < 0.05). All the uninfected control animals (groups 4 and 5) showed no myocardial lesions whether treated with verapamil or not. Quantitative planimetry confirmed decreased inflammation and necrosis (2.0 +/- 3.3% in group 1 and 3.5 +/- 3.1% in group 2 vs. 21.9 +/- 22.6% in group 3 on day 14). Untreated infected hearts injected with liquid silicone rubber exhibited extensive areas of focal microvascular constriction and microaneurysm formation; verapamil treatment in either group 1 or 2 completely abolished these abnormalities, resembling uninfected controls in groups 4 or 5. We conclude that verapamil, whether given before infection or after peak viremia in an encephalomyocarditis model of murine myocarditis, significantly reduces the microvascular changes and myocardial necrosis, fibrosis, and calcification leading to cardiomyopathy. This suggests the potentially important role of calcium and microvascular spasm in the pathogenesis of viral myocarditis leading to dilated cardiomyopathy, and may have future therapeutic implications.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birx D. L., Berger M., Fleisher T. A. The interference of T cell activation by calcium channel blocking agents. J Immunol. 1984 Dec;133(6):2904–2909. [PubMed] [Google Scholar]
- Bowles N. E., Richardson P. J., Olsen E. G., Archard L. C. Detection of Coxsackie-B-virus-specific RNA sequences in myocardial biopsy samples from patients with myocarditis and dilated cardiomyopathy. Lancet. 1986 May 17;1(8490):1120–1123. doi: 10.1016/s0140-6736(86)91837-4. [DOI] [PubMed] [Google Scholar]
- Chow L. H., Beisel K. W., McManus B. M. Enteroviral infection of mice with severe combined immunodeficiency. Evidence for direct viral pathogenesis of myocardial injury. Lab Invest. 1992 Jan;66(1):24–31. [PubMed] [Google Scholar]
- Chow L. H., Gauntt C. J., McManus B. M. Differential effects of myocarditic variants of Coxsackievirus B3 in inbred mice. A pathologic characterization of heart tissue damage. Lab Invest. 1991 Jan;64(1):55–64. [PubMed] [Google Scholar]
- Costanzo-Nordin M. R., Reap E. A., O'Connell J. B., Robinson J. A., Scanlon P. J. A nonsteroid anti-inflammatory drug exacerbates Coxsackie B3 murine myocarditis. J Am Coll Cardiol. 1985 Nov;6(5):1078–1082. doi: 10.1016/s0735-1097(85)80312-0. [DOI] [PubMed] [Google Scholar]
- Factor S. M., Cho S., Wittner M., Tanowitz H. Abnormalities of the coronary microcirculation in acute murine Chagas' disease. Am J Trop Med Hyg. 1985 Mar;34(2):246–253. doi: 10.4269/ajtmh.1985.34.246. [DOI] [PubMed] [Google Scholar]
- Factor S. M., Minase T., Cho S., Dominitz R., Sonnenblick E. H. Microvascular spasm in the cardiomyopathic Syrian hamster: a preventable cause of focal myocardial necrosis. Circulation. 1982 Aug;66(2):342–354. doi: 10.1161/01.cir.66.2.342. [DOI] [PubMed] [Google Scholar]
- Factor S. M., Minase T., Cho S., Fein F., Capasso J. M., Sonnenblick E. H. Coronary microvascular abnormalities in the hypertensive-diabetic rat. A primary cause of cardiomyopathy? Am J Pathol. 1984 Jul;116(1):9–20. [PMC free article] [PubMed] [Google Scholar]
- Gelfand E. W., Cheung R. K., Grinstein S., Mills G. B. Characterization of the role for calcium influx in mitogen-induced triggering of human T cells. Identification of calcium-dependent and calcium-independent signals. Eur J Immunol. 1986 Aug;16(8):907–912. doi: 10.1002/eji.1830160806. [DOI] [PubMed] [Google Scholar]
- Hashimoto I., Komatsu T. Myocardial changes after infection with Coxsackie virus B3 in nude mice. Br J Exp Pathol. 1978 Feb;59(1):13–20. [PMC free article] [PubMed] [Google Scholar]
- Hassin D., Fixler R., Bank H., Klein A. S., Hasin Y. Cytotoxic T lymphocytes and natural killer cell activity in the course of mengo virus infection of mice. Immunology. 1985 Dec;56(4):701–705. [PMC free article] [PubMed] [Google Scholar]
- Hosenpud J. D., McAnulty J. H., Niles N. R. Lack of objective improvement in ventricular systolic function in patients with myocarditis treated with azathioprine and prednisone. J Am Coll Cardiol. 1985 Oct;6(4):797–801. doi: 10.1016/s0735-1097(85)80485-x. [DOI] [PubMed] [Google Scholar]
- Huber S. A., Job L. P., Woodruff J. F. Lysis of infected myofibers by coxsackievirus B-3-immune T lymphocytes. Am J Pathol. 1980 Mar;98(3):681–694. [PMC free article] [PubMed] [Google Scholar]
- Huber S. A., Lodge P. A. Coxsackievirus B-3 myocarditis in Balb/c mice. Evidence for autoimmunity to myocyte antigens. Am J Pathol. 1984 Jul;116(1):21–29. [PMC free article] [PubMed] [Google Scholar]
- Huber S. A., Lodge P. A. Coxsackievirus B-3 myocarditis. Identification of different pathogenic mechanisms in DBA/2 and Balb/c mice. Am J Pathol. 1986 Feb;122(2):284–291. [PMC free article] [PubMed] [Google Scholar]
- Jin O., Sole M. J., Butany J. W., Chia W. K., McLaughlin P. R., Liu P., Liew C. C. Detection of enterovirus RNA in myocardial biopsies from patients with myocarditis and cardiomyopathy using gene amplification by polymerase chain reaction. Circulation. 1990 Jul;82(1):8–16. doi: 10.1161/01.cir.82.1.8. [DOI] [PubMed] [Google Scholar]
- Kandolf R., Ameis D., Kirschner P., Canu A., Hofschneider P. H. In situ detection of enteroviral genomes in myocardial cells by nucleic acid hybridization: an approach to the diagnosis of viral heart disease. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6272–6276. doi: 10.1073/pnas.84.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kereiakes D. J., Parmley W. W. Myocarditis and cardiomyopathy. Am Heart J. 1984 Nov;108(5):1318–1326. doi: 10.1016/0002-8703(84)90760-9. [DOI] [PubMed] [Google Scholar]
- Kishimoto C., Kuribayashi K., Fukuma K., Masuda T., Tomioka N., Abelmann W. H., Kawai C. Immunologic identification of lymphocyte subsets in experimental murine myocarditis with encephalomyocarditis virus. Different kinetics of lymphocyte subsets between the heart and the peripheral blood, and significance of Thy 1.2+ (pan T) and Lyt 1+, 23+ (immature T) subsets in the development of myocarditis. Circ Res. 1987 Nov;61(5):715–725. doi: 10.1161/01.res.61.5.715. [DOI] [PubMed] [Google Scholar]
- Kishimoto C., Kuribayashi K., Masuda T., Tomioka N., Kawai C. Immunologic behavior of lymphocytes in experimental viral myocarditis: significance of T lymphocytes in the severity of myocarditis and silent myocarditis in BALB/c-nu/nu mice. Circulation. 1985 Jun;71(6):1247–1254. doi: 10.1161/01.cir.71.6.1247. [DOI] [PubMed] [Google Scholar]
- Kishimoto C., Thorp K. A., Abelmann W. H. Immunosuppression with high doses of cyclophosphamide reduces the severity of myocarditis but increases the mortality in murine Coxsackievirus B3 myocarditis. Circulation. 1990 Sep;82(3):982–989. doi: 10.1161/01.cir.82.3.982. [DOI] [PubMed] [Google Scholar]
- Kobayashi A., Yamashita T., Kaneko M., Nishiyama T., Hayashi H., Yamazaki N. Effects of verapamil on experimental cardiomyopathy in the Bio 14.6 Syrian hamster. J Am Coll Cardiol. 1987 Nov;10(5):1128–1138. doi: 10.1016/s0735-1097(87)80356-x. [DOI] [PubMed] [Google Scholar]
- Kopecky S. L., Gersh B. J. Dilated cardiomyopathy and myocarditis: natural history, etiology, clinical manifestations, and management. Curr Probl Cardiol. 1987 Oct;12(10):569–647. doi: 10.1016/0146-2806(87)90002-8. [DOI] [PubMed] [Google Scholar]
- Mason J. W., Billingham M. E., Ricci D. R. Treatment of acute inflammatory myocarditis assisted by endomyocardial biopsy. Am J Cardiol. 1980 May;45(5):1037–1044. doi: 10.1016/0002-9149(80)90174-5. [DOI] [PubMed] [Google Scholar]
- Matsumori A., Kawai C. An animal model of congestive (dilated) cardiomyopathy: dilatation and hypertrophy of the heart in the chronic stage in DBA/2 mice with myocarditis caused by encephalomyocarditis virus. Circulation. 1982 Aug;66(2):355–360. doi: 10.1161/01.cir.66.2.355. [DOI] [PubMed] [Google Scholar]
- Mills G. B., Cheung R. K., Grinstein S., Gelfand E. W. Increase in cytosolic free calcium concentration is an intracellular messenger for the production of interleukin 2 but not for expression of the interleukin 2 receptor. J Immunol. 1985 Mar;134(3):1640–1643. [PubMed] [Google Scholar]
- Monrad E. S., Matsumori A., Murphy J. C., Fox J. G., Crumpacker C. S., Abelmann W. H. Therapy with cyclosporine in experimental murine myocarditis with encephalomyocarditis virus. Circulation. 1986 May;73(5):1058–1064. doi: 10.1161/01.cir.73.5.1058. [DOI] [PubMed] [Google Scholar]
- Morris S. A., Weiss L. M., Factor S., Bilezikian J. P., Tanowitz H., Wittner M. Verapamil ameliorates clinical, pathologic and biochemical manifestations of experimental chagasic cardiomyopathy in mice. J Am Coll Cardiol. 1989 Sep;14(3):782–789. doi: 10.1016/0735-1097(89)90126-5. [DOI] [PubMed] [Google Scholar]
- Neu N., Beisel K. W., Traystman M. D., Rose N. R., Craig S. W. Autoantibodies specific for the cardiac myosin isoform are found in mice susceptible to Coxsackievirus B3-induced myocarditis. J Immunol. 1987 Apr 15;138(8):2488–2492. [PubMed] [Google Scholar]
- O'Connell J. B., Reap E. A., Robinson J. A. The effects of cyclosporine on acute murine Coxsackie B3 myocarditis. Circulation. 1986 Feb;73(2):353–359. doi: 10.1161/01.cir.73.2.353. [DOI] [PubMed] [Google Scholar]
- Rezkalla S., Khatib G., Khatib R. Coxsackievirus B3 murine myocarditis: deleterious effects of nonsteroidal anti-inflammatory agents. J Lab Clin Med. 1986 Apr;107(4):393–395. [PubMed] [Google Scholar]
- Rouleau J. L., Chuck L. H., Hollosi G., Kidd P., Sievers R. E., Wikman-Coffelt J., Parmley W. W. Verapamil preserves myocardial contractility in the hereditary cardiomyopathy of the Syrian hamster. Circ Res. 1982 Mar;50(3):405–412. doi: 10.1161/01.res.50.3.405. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., Cahill D. Verapamil and chlorpromazine inhibit the budding of Sindbis and vesicular stomatitis viruses from infected chicken embryo fibroblasts. Virology. 1989 Jan;168(1):187–190. doi: 10.1016/0042-6822(89)90421-2. [DOI] [PubMed] [Google Scholar]
- Schultheiss H. P., Schulze K., Kühl U., Ulrich G., Klingenberg M. The ADP/ATP carrier as a mitochondrial auto-antigen--facts and perspectives. Ann N Y Acad Sci. 1986;488:44–64. doi: 10.1111/j.1749-6632.1986.tb46547.x. [DOI] [PubMed] [Google Scholar]
- Schulze K., Becker B. F., Schultheiss H. P. Antibodies to the ADP/ATP carrier, an autoantigen in myocarditis and dilated cardiomyopathy, penetrate into myocardial cells and disturb energy metabolism in vivo. Circ Res. 1989 Feb;64(2):179–192. doi: 10.1161/01.res.64.2.179. [DOI] [PubMed] [Google Scholar]
- Shanlin R. J., Sole M. J., Rahimifar M., Tator C. H., Factor S. M. Increased intracranial pressure elicits hypertension, increased sympathetic activity, electrocardiographic abnormalities and myocardial damage in rats. J Am Coll Cardiol. 1988 Sep;12(3):727–736. doi: 10.1016/s0735-1097(88)80065-2. [DOI] [PubMed] [Google Scholar]
- Silver M. A., Kowalczyk D. Coronary microvascular narrowing in acute murine coxsackie B3 myocarditis. Am Heart J. 1989 Jul;118(1):173–174. doi: 10.1016/0002-8703(89)90089-6. [DOI] [PubMed] [Google Scholar]
- Tomioka N., Kishimoto C., Matsumori A., Kawai C. Effects of prednisolone on acute viral myocarditis in mice. J Am Coll Cardiol. 1986 Apr;7(4):868–872. doi: 10.1016/s0735-1097(86)80349-7. [DOI] [PubMed] [Google Scholar]
- Tracy S., Wiegand V., McManus B., Gauntt C., Pallansch M., Beck M., Chapman N. Molecular approaches to enteroviral diagnosis in idiopathic cardiomyopathy and myocarditis. J Am Coll Cardiol. 1990 Jun;15(7):1688–1694. doi: 10.1016/0735-1097(90)92846-t. [DOI] [PubMed] [Google Scholar]
- Wright B., Zeidman I., Greig R., Poste G. Inhibition of macrophage activation by calcium channel blockers and calmodulin antagonists. Cell Immunol. 1985 Oct 1;95(1):46–53. doi: 10.1016/0008-8749(85)90293-x. [DOI] [PubMed] [Google Scholar]