Abstract
The noted disruption of thalamocortical connections and abnormalities in tactile sensory function has resulted in a new definition of cerebral palsy (CP) that recognizes the sensorimotor integration process as central to the motor impairments seen in these children. Despite this updated definition, the connection between a child's motor impairments and somatosensory processing remains almost entirely unknown. In this investigation, we explored the relationship between the magnitude of neural activity within the somatosensory cortices, the strength of the ankle plantarflexors, and the gait spatiotemporal kinematics of a group of children with CP and a typically developing matched cohort. Our results revealed that the magnitude of somatosensory cortical activity in children with CP had a strong positive relationship with the ankle strength, step length, and walking speed. These results suggest that stronger activity within the somatosensory cortices in response to foot somatosensations was related to enhanced ankle plantarflexor strength and improved mobility in the children with CP. These results provide further support for the notion that children with CP exhibit, not only musculoskeletal deficits, but also somatosensory deficits that potentially contribute to their overall functional mobility and strength limitations.
Keywords: magnetoencephalography, sensory, strength, gait, walking
cerebral palsy (CP) is one of the most prevalent and costly pediatric neurological impairments diagnosed in the United States (Christensen et al. 2014). A major therapeutic goal of the parents and children with CP is to improve and sustain mobility (Botos and Gericke 2003). To this end, a considerable amount of research funds that has been expended toward cataloging the various lower-extremity joint kinematic and kinetic deviations that are seen in the walking patterns of these children (Bell et al. 2002; Lin et al. 2000; Onley et al. 1990; Ounpuu et al. 1996; Riad et al. 2008). These efforts have largely assumed that the mobility deficiencies seen in children with CP primarily reside in the performance of the musculoskeletal machinery. Unfortunately, the outcomes of the treatment strategies that have followed this line of reasoning (i.e., surgery and flexibility and strength training) have been mixed and thus not clearly successful in improving mobility (Blumetti et al. 2012; Dreher et al. 2012; Pin et al. 2006; Taylor et al. 2013).
The revised definition of CP recognizes that the motor impairments seen in these children are at least partially a product of aberrant sensations and ability to interpret sensory information (Rosenbaum et al. 2007). This reclassification is supported by the numerous clinical reports of proprioception, stereognosis, and tactile discrimination deficits seen in children with CP (Auld et al. 2012; Clayton et al. 2003; Cooper et al. 1995; Goble et al. 2009; Robert et al. 2013; Sanger and Kukke 2007; Wingert et al. 2008). Several studies have begun to interrogate the potential relationship between the motor impairments and the sensory-processing deficits seen in children with CP. These studies have reported that the hand sensory discrimination deficits are linked with the child's upper-extremity motor impairments (Krumlinde-Sundholm and Eliasson 2002; Sakzewski et al. 2010), prediction of grip forces (Gordon and Duff 1999; Gordon et al. 1999), and the likelihood that the child will learn a new upper-extremity motor skill (Robert et al. 2013). These outcomes clearly support the notion that the processing of somatosensations is an influential component of the motor performance of children with CP. Despite these novel insights, very few studies have examined the leg somatosensations in children with CP (Damiano et al. 2013; Wingert et al. 2009). These few investigations have shown that proprioceptive somatosensory deficits also persist for the lower extremities in children with CP. Furthermore, a recent study has shown that the hip joint proprioception deficits seen in children with CP that have a hemiplegic presentation may be related to their selection of a slower walking speed (Damiano et al. 2013). Further investigation of the lower-extremity somatosensations has the potential to shed light on how these aberrant somatosensations impact the mobility of children with CP.
Outcomes from diffusion tensor imaging (DTI) studies have identified that the poor somatosensations seen in children with CP are likely related to structural damage along the thalamocortical pathways (Hoon et al. 2009; Rose et al. 2007; Trivedi et al. 2008, 2010). These studies have also reported that the extent of the damage is correlated with the degree of impairment in the child's upper-extremity somatosensation, overall muscular weakness, Gross Motor Function Classification Score (GMFCS), and deviations in the gait biomechanics (Hoon et al. 2009; Rose et al. 2007; Trivedi et al. 2008, 2010). These relationships imply that the structural damage along the thalamocortical tracks may result in faulty processing by the somatosensory networks. Several magnetoencephalography (MEG) and electroencephalography (EEG) studies have further explored this possibility and have shown that the somatosensory-evoked potentials/fields for the hand, foot, and lips are diminished and in some cases latent in children with CP (Kulak et al. 2005, 2006; Kurz and Wilson 2011; Kurz et al. 2012; Riquelme and Montoya 2010; Teflioudi et al. 2011). In addition, it has been noted that these aberrant event-related potentials are correlated with the two-point tactile discrimination deficits seen in children with CP (Maitre et al. 2012). Altogether, these results support the notion that children with CP likely have uncharacteristic activity within the somatosensory cortices. Nevertheless, it remains relatively unknown whether the mobility impairments seen in children with CP might be related to uncharacteristic activation within the somatosensory cortices.
Our prior MEG experimental work has begun to address this knowledge gap. Initially, we applied tactile stimulation to the bottom of the foot and showed that the strength of the activity within the sensorimotor cortices was related to the precision of the ankle plantarflexion force production of children with CP (Kurz et al. 2014). Recognizing that the ankle plantarflexors play a major role in the control of gait (Winter 1983), we extended our prior experimental work to evaluate whether the abnormal neural activity within the somatosensory cortices is related to the strength of the ankle plantarflexors and the mobility impairments seen in children with CP.
MATERIALS AND METHODS
Participants.
Eleven children with a diagnosis of either spastic diplegic or hemiplegia CP (age = 14.5 ± 0.7 yr) and a GMFCS score between I and III participated in this investigation. An additional 11 age-matched typically developing (TD) children (age = 14.1 ± 0.7 yr) served as a control group. The Institutional Review Board at the University of Nebraska Medical Center reviewed and approved this investigation. Informed consent was acquired from the parents, and the children assented to participate in the experiment.
Motor performance experimental methods.
An isokinetic dynamometer (Biodex, Shirley, NY) was used to measure the maximum isometric torque generated by the ankle plantarflexors. The children were situated in an adjustable chair with their foot position on a pedal that was interfaced with the torque motor. The back of the chair was in an upright position, the knee was extended, and the ankle was in a neutral position. The voltage output from the torque motor was read by custom LabVIEW (National Instruments, Austin, TX) software and sampled at 1 kHz by a 14-bit National Instruments analog-to-digital converter. The amount of torque generated was graphically displayed on a large monitor as a single box that moved vertically depending on the amount of torque the child applied to the foot pedal apparatus. The largest isometric torque generated from two maximum plantarflexion contractions was used to establish the child's maximum voluntary torque (MVT). The MVT was normalized by the child's body mass for analysis.
Mobility was quantified by having the children walk at their preferred and fast-as-possible walking speeds across a mat that registered their digital footprints (GaitRITE, Sparta, NJ). The data collected from the mat were used to calculate the child's walking velocity, step length, and cadence. Each child completed two trials at the respective speeds. The average preferred and fast-as-possible walking speeds were used in the analysis.
MEG experimental paradigm.
The children were seated with their head positioned within the helmet of the whole-head 306-sensor Elekta MEG system (Helsinki, Finland), while a unilateral tactile stimulation was applied to the bottom of the foot at the first metatarsal using a small airbladder. Neuromagnetic responses were continuously sampled at 1 kHz. The data analysis epochs were a total duration of 1.2 s (−0.5 s to +0.7 s), with the onset of the mechanical stimulation defined as time 0.0 s, and the baseline defined as −0.5 s to 0.0 s. Artifact-free epochs were imaged using a linearly constrained minimum variance vector beamformer, which employs spatial filters in the time-frequency domain to calculate 3D images of the local power of neuronal current (Gross et al. 2001; Hillebrand et al. 2005; van Veen et al. 1997). The single images were derived from the cross-spectral densities of all combinations of MEG sensors and averaged over the 4–14-Hz time-frequency range. In principle, the beamformer operator generates a spatial filter that passes signals without attenuation from a region of interest, while suppressing activity in all other brain regions. The filter properties arise from the forward solution (lead field) for each location on a volumetric grid specified by input voxel space and from the MEG covariance matrix. Basically, for each voxel, a set of beamformer weights is determined, which amounts to each MEG sensor being allocated a sensitivity weighting for activity in the specific voxel. This set of beamformer weights is the spatial filter unique to the given voxel, and this procedure was iterated until such a filter was computed for each voxel in the brain. Activity in each voxel was then determined independently and sequentially to produce a 4.0 × 4.0 × 4.0-mm resolution volumetric map of electrical source activity for each of the ∼135 artifact-free trials per participant. Following convention, noise-normalized maps of source strength were derived from these volumetric output images by dividing, on a voxel-by-voxel basis, the projected source power by the estimated amount of uncorrelated noise power projected through the beamformer weights (Hillebrand et al. 2005; van Veen et al. 1997). The functional images were transformed into a standardized space (Talairach and Tournoux 1988), and the amplitude of the peak voxel in the group-difference statistical parametric map (SPM) was extracted for each child. Complete details of the MEG experimental methods employed in this study are described in our recent paper (Kurz et al. 2014). As a secondary analysis, we extracted a virtual sensor time series representing the 4–14-Hz frequency range in each child from the peak voxel in the group-difference SPM and focused on the amplitude of the peak latency for each child within the initial 200 ms of the time series. We chose this window because it included the average peak latency in each group.
Statistical analysis.
Spearman rho rank-order correlations were used to determine the relationship between the amplitude of the peak voxel in the group-difference SPM, which was located in the medial wall of the contralateral postcentral gyrus and the respective behavioral measures. Spearman rho rank-order correlations were also used to determine the relationship between the virtual sensor peak amplitude, extracted from the peak voxel in the group-difference SPM, and the respective behavioral measures. The respective rank-order correlations were initially evaluated using the data collected from the entire group of participants. This was followed up by a separate analysis of the relationships that existed within the respective children with CP and TD control groups. Lastly, separate Wilcoxon matched pairs were used to determine whether there were differences between the children with CP and the TD children for the behavioral measures and the virtual sensor amplitude. All statistical analyses were performed with IBM SPSS Statistics Version 22, with α = 0.05.
RESULTS
MEG results.
As reported in our companion manuscript (Kurz et al. 2014), the children with CP exhibited less 4–14-Hz activity in the medial wall of the contralateral postcentral gyrus (P < 0.05, cluster corrected), whereas TD children had increased neuronal discharges in this same brain area (P < 0.05, cluster corrected). This pattern of responses gave rise to a significant group effect (P < 0.05, cluster corrected) in this same region of the medial postcentral gyrus. These results indicated that the responsiveness of primary somatosensory cortices to the external afferent feedback was weaker and aberrant in the children with CP.
The virtual sensor extracted from the peak voxel within the medial postcentral gyrus cluster corroborated these results (Fig. 1). Critically, the peak amplitude of activity in this voxel (0–200 ms) was reduced by 43% in the children with CP and significantly differed from the TD children (CP = 0.07 ± 0.04 nAm; TD = 0.12 ± 0.05 nAm; P = 0.02). There was no significant difference in the latency of the peak amplitude (CP = 173 ± 26 ms; TD = 177 ± 23 ms; P = 0.72).
Fig. 1.
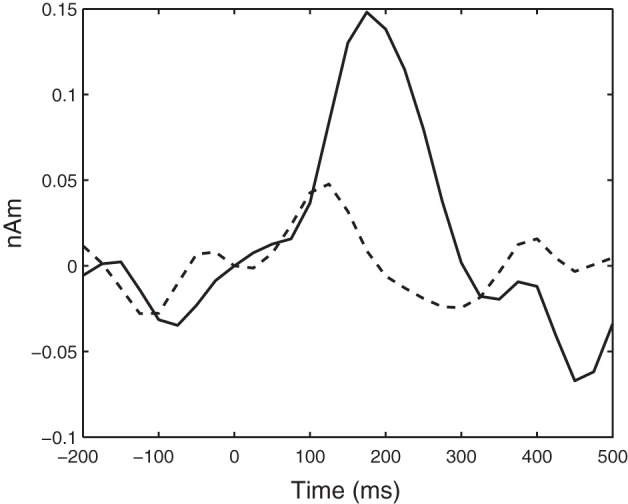
Group-averaged 4–14-Hz virtual sensor time series extracted from the peak voxel in the group-difference statistical parametric map. The virtual sensor for the typically developing children is represented by a solid line, whereas that for the children with cerebral palsy is shown with a dashed line. The application of the tactile stimulation to the bottom of the foot occurs at 0 ms. The average time series clearly shows that the initial 200 ms of the time series was diminished in children with cerebral palsy compared with the typically developing children.
Behavioral results.
The children with CP generated a lower maximum torque with the ankle plantarflexors compared with the TD children (Fig. 2; P = 0.001). For the measured gait variables, the children with CP had slower preferred (P = 0.004) and fast-as-possible walking speeds (P = 0.0001) compared with the TD children (Fig. 3A). Furthermore, the TD children used a longer step length than the children with CP while walking at their preferred (P = 0.03) and fast-as-possible speeds (Fig. 3B; P = 0.0001). There were no differences in the cadence used at the respective walking speeds (Fig. 3C).
Fig. 2.
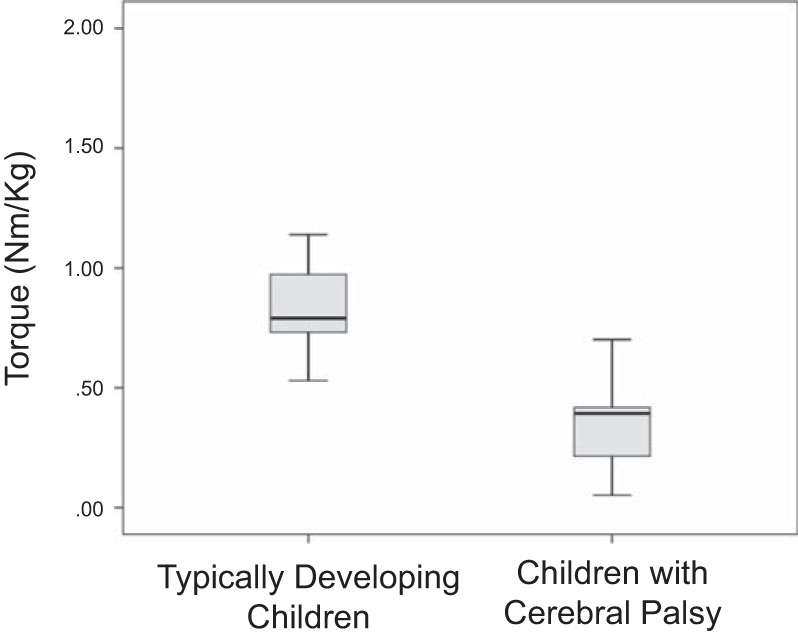
Box plots of the ankle plantarflexion torques measured for the typically developing children and the children with cerebral palsy. The data are presented in quartiles, and the bold line on each box plot represents the median.
Fig. 3.
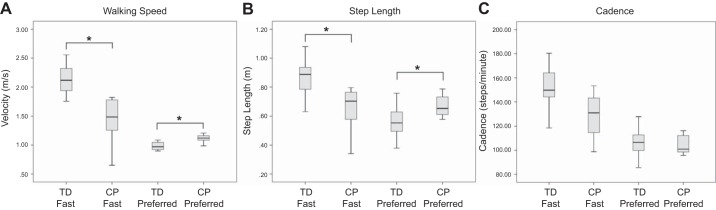
Box plots of the walking speed (A), step length (B), and cadence (C) for the typically developing children (TD) and children with cerebral palsy (CP). The data are presented in quartiles, and the bold line on each box plot represents the median. *P < 0.05.
Correlations with amplitude of peak voxel in the SPM.
For the entire group of children, there was a positive correlation between the amplitude of the peak voxel in the group-difference SPM and the torque generated by the ankle plantarflexors (rho = 0.40; P = 0.04). This implied that the participating children who had a greater amount of activity within the somatosensory cortices were likely able to generate a larger torque with the ankle plantarflexors. For the preferred walking speed, there was a positive correlation between the step length (rho = 0.38; P = 0.04) and the amplitude of the peak voxel in the group-difference SPM and a negative correlation between the cadence and the amplitude of the same peak voxel (rho = −0.38; P = 0.04). This suggests that children with a greater amount of somatosensory activity were more likely to use a longer step length and have a faster cadence while walking at their preferred pace. We also found that there was a positive correlation between the amplitude of the peak voxel in the group-difference SPM and the step length at the fast-as-possible walking speed (rho = 0.40; P = 0.03). This relationship suggests that the children who utilized a longer step length while walking fast also tended to have greater activity in the somatosensory cortices. All of the Spearman rho rank-order correlations between the amplitude of the peak voxel in the group-difference SPM and the behavioral data collected from all children are presented in Table 1.
Table 1.
Spearman rho rank-order correlations between the amplitude of the peak voxel in the group-level SPM and the respective behavioral variables
| All Children | Children with Cerebral Palsy | Typically Developing Children | |
|---|---|---|---|
| Maximum voluntary torque | 0.40* | 0.62* | 0.13 |
| Preferred walking speed | |||
| Speed | 0.28 | 0.51* | −0.38 |
| Step length | 0.38* | 0.44 | −0.01 |
| Step width | −0.21 | −0.27 | −0.03 |
| Cadence | −0.38* | 0.19 | −0.58* |
| Fast-as-possible walking speed | |||
| Speed | 0.24 | 0.68* | −0.36 |
| Step length | 0.40* | 0.63* | −0.05 |
| Step width | 0.01 | −0.31 | −0.67* |
| Cadence | 0.09 | 0.43 | −0.29 |
SPM, statistical parametric map.
P < 0.05.
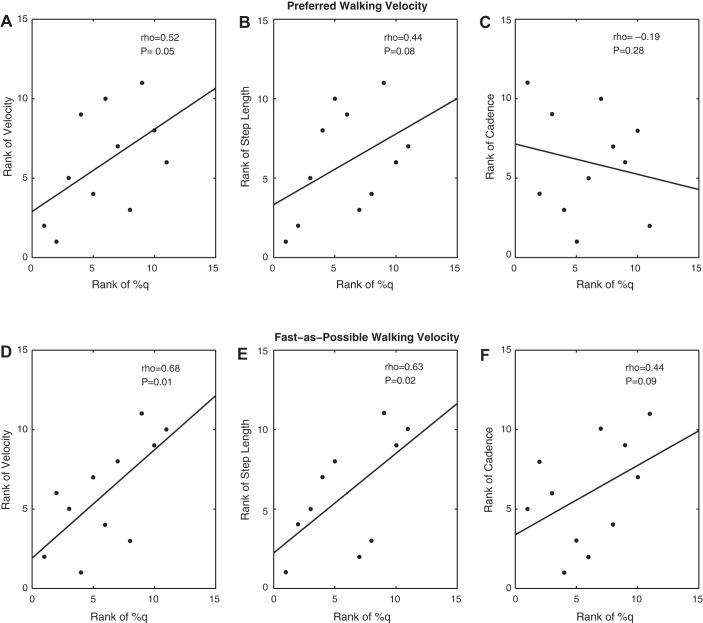
Our separate analysis of the data collected for the children with CP revealed that there was a strong positive correlation between the amplitude within the same peak voxel and the strength of the ankle plantarflexors (Fig. 4; P = 0.02). This implies that children with CP who generated larger ankle torques also tended to have greater activation within the somatosensory cortices to a peripheral tactile simulation applied to the bottom of the foot. The preferred (P = 0.05; Fig. 2A) and fast-as-possible (Fig. 5E; P = 0.03) walking speeds of the children with CP were also moderately correlated with the amplitude of the peak voxel in the group-difference SPM. In addition, the step length (Fig. 5D; P = 0.01) at the fast-as-possible walking speed was strongly correlated with the amplitude of the same peak voxel. These combined results indicate that the mobility of the participating children with CP was strongly related to the amount of activity within somatosensory cortices. All of the Spearman rho rank-order correlations between the amplitude of the peak voxel in the group-difference SPM and the behavioral data collected from the children with CP are presented in Table 1.
Fig. 4.
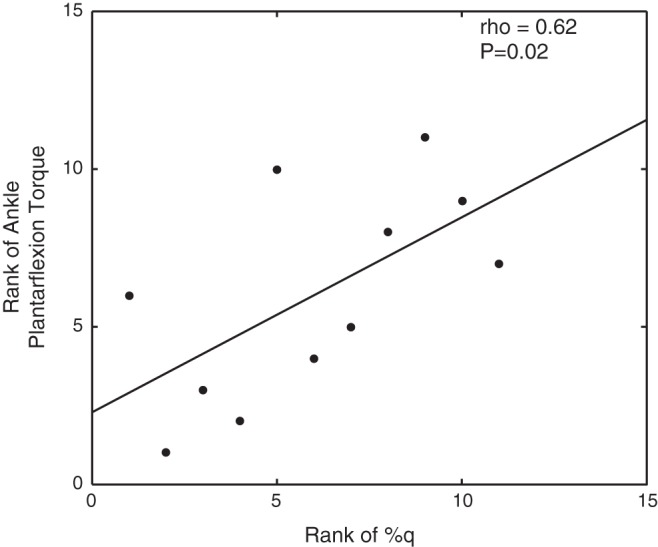
Scatter plot of the rank of the voxel with the strongest amplitude in the somatosensory cortices and the rank of the ankle plantarflexion strength for the children with cerebral palsy.
Fig. 5.
Scatter plot of the rank-order correlations between the somatosensory cortical voxel with the strongest amplitude and the respective gait spatiotemporal variables for the children with cerebral palsy. A–C: rank-order correlations for the preferred walking velocity. D–F: rank-order correlations for the fast-as-possible walking velocity.
For the data collected from the TD children, there was a moderate negative correlation between the cadence at the preferred walking speed and the amplitude of the peak voxel in the group-difference SPM (rho = −0.58; P = 0.04). This suggested that the TD children who utilized a faster cadence also tended to have greater activation within the somatosensory cortices. In addition, we found a strong negative correlation between the step width at the fast-as-possible walking speed and the amplitude of the same peak voxel (rho = −0.67; P = 0.01). This implied that the TD children who had greater activity within the somatosensory cortices also tended to use a narrower step width. None of the other variables measured were significantly correlated with the amplitude of the peak voxel in the group-difference SPM. All of the Spearman rho rank-order correlations between the amplitude of the peak voxel and the behavioral data collected from the TD children are presented in Table 1.
Correlations with source amplitude of the virtual sensor.
For the entire group of children, there was a strong positive correlation between the peak amplitude of the virtual sensor (0–200 ms), extracted from the peak voxel of the group-difference SPM, and the preferred walking velocity (rho = 0.66; P = 0.002). This implied that the participating children who had a larger somatosensory cortical response to the tactile stimulation tended to also walk at a faster speed. For the preferred walking speed, there was a positive correlation between the step length (rho = 0.47; P = 0.04) and the magnitude of the peak activity and a negative correlation between the step width and the magnitude of activity (rho = −0.61; P = 0.006). This suggests that the children with a larger somatosensory cortical response to the tactile stimulus were more likely to use a longer step length and would select a narrower step width while walking at their preferred speed. We also found that there was a negative correlation between the magnitude of peak activity and the step width for the fast-as-possible walking speed (rho = −0.46; P = 0.05). This relationship further implies that the selection of a step width during gait may be related to the amount of activity seen within somatosensory cortices after a tactile stimulation. None of the other variables were significantly correlated with the peak virtual sensor amplitude measured in the somatosensory cortices (0–200 ms) after a tactile stimulus was applied to the bottom of the foot. In addition, we were unable to find any correlations between the peak amplitude and the behavioral variables when the Spearman rho rank-order correlations were performed separately for the TD children and children with CP. All of the Spearman rho rank-order correlations between the virtual sensor peak amplitude, extracted from the peak voxel of the group-difference SPM, and the respective behavioral variables are presented in Table 2.
Table 2.
Spearman rho rank-order correlations between the source amplitude within the peak voxel of the group-level statistical image and the respective behavioral variables
| All Children | Children with Cerebral Palsy | Typically Developing Children | |
|---|---|---|---|
| Maximum voluntary torque | 0.38 | 0.12 | −0.60 |
| Preferred walking speed | |||
| Speed | 0.66* | 0.15 | 0.45 |
| Step length | 0.47* | −0.08 | 0.48 |
| Step width | −0.61* | −0.14 | −0.65 |
| Cadence | 0.13 | 0.47 | −0.08 |
| Fast-as-possible walking speed | |||
| Speed | 0.34 | −0.22 | −0.05 |
| Step length | 0.37 | −0.22 | 0.22 |
| Step width | −0.46* | −0.25 | −0.56 |
| Cadence | −0.01 | −0.18 | −0.27 |
P < 0.05.
DISCUSSION
This investigation explored the relationship between cortical activity within the somatosensory cortices and the lower-extremity motor performance of children with CP. Our results indicated that the amplitude of activity in the neural populations representing the foot in the somatosensory cortices have a diminished response to tactile stimulation in children with CP. Our results suggest that stronger activity within the somatosensory cortices in response to tactile stimulation of the foot is potentially related to enhanced ankle plantarflexor strength and improved mobility in the children with CP. These results are innovative because they show that aberrant activity within somatosensory cortices is likely related to the lower-extremity motor performance of children with CP. These results provide new support for the definition of CP that was put forth by Rosenbaum and colleagues (2007) that classified CP as having disturbances of sensation that affect movement.
The children with CP walked slower than the TD children at their preferred and fast-as-possible walking speeds. In addition, the children with CP had a shorter step length but a similar cadence as the TD children for both of the walking speeds investigated. Our experimental work suggests that these noted mobility differences may partly be a product of the strength of neural activity within the somatosensory cortices to somatosensations on the bottom of the foot. The experimental paradigm used in this investigation used a small airbladder to stimulate the foot mechanoreceptors. These receptors were chosen because they provide important sensory information about the temporal and spatial application of the pressures that are applied to the bottom of the foot during gait (Perry et al. 2001). We suggest that children with CP who display less activity within the somatosensory cortices after stimulation of these mechanoreceptors may have less certainty about the plantar pressures they experience during gait. This potential link implies that the slower walking speeds seen in the children with CP may represent a more cautious gait that is partly due to uncertainties about the peripheral somatosensations.
The children with CP that participated in our investigation generated 63% less torque with the ankle plantarflexors than the TD children. The lower strength values reported here concur with those established in the clinical literature (Elder et al. 2003; Wiley and Damiano 1998). However, our results extend this knowledge base by suggesting that less stimulation-induced neural activity within the somatosensory cortices is related to the ankle plantarflexor strength. This relationship suggests that the muscular weakness seen in children with CP may not be solely attributable to alterations in the muscle cytoskeleton architecture (Moreau et al. 2012); rather it may also be related to aberrant somatosensory neural computations about the amount of pressure that is being applied to the bottom of the foot during the plantarflexion task. Potentially, these aberrant somatosensory neural computations may impact the selective control of the ankle joint musculature. This notion is aligned with a prior study showing that the tactile discrimination deficits seen in children with CP influence their ability to predict accurate grip forces (Gordon and Duff 1999). Together these experimental results imply that the sensory deficits seen in children with CP may impact their ability to predict the muscular forces that will achieve a desired motor goal state.
On the basis of the data from all the participants, we identified that a larger response in the virtual sensor extracted from each participant's functional map, using the peak voxel of the group-difference SPM as the target, was related to a faster walking speed, a longer step length, and a narrower step width. This relationship suggests that the strength of the somatosensory cortical response to the tactile stimulus was related to the selected gait kinematics. Prior research has suggested that the selected step length is dependent on lower-level propriospinal feedback loops, whereas the selected step width involves higher-level sensory feedback integration because of the larger balance requirements in the frontal plane of gait (Bauby and Kuo 2000; O'Connor and Kuo 2009). These notions have been largely driven by experimental paradigms that have used visual perturbation and the control parameters used for walking robots (Kuo 1999). Our correlations tend to agree with the notion that the selected step width involves high-level cortical processing; however, correlations imply that some cortical computations may be involved in the selection of a step length.
Prior DTI studies have shown that damage to the thalamocortical tracts is related to the somatosensory impairments seen in children with CP (Hoon et al. 2009; Rose et al. 2007; Trivedi et al. 2008, 2010). These results suggest that the abnormal activity within somatosensory cortices reported here may have been instigated by perinatal damage to the thalamocortical tracts. Potentially, this damage may alter the signal-to-noise ratio in such a way that the threshold for activation of the somatosensory cortices becomes uncharacteristic and perhaps unresponsive to important peripheral feedback. A prior study has shown that the functional connectivity of the white matter fiber tracts can be improved in children with CP after an intensive therapeutic protocol (Trivedi et al. 2008). This suggests that the net effect of structural damage along the thalamocortical tracts may be somewhat reversible, which may improve neural activity within the somatosensory cortices.
The results of this exploratory study have provided unique information, but they fall short of showing that the strength of somatosensory activity predicts the strength and mobility problems seen in children with CP. The reason that we are cautionary in making this conjecture is because it is just as likely that the motor impairments seen in children with CP (i.e., cocontractions, spasticity, and poor selective muscular control) also played a role in the noted mobility and strength impairments. Therefore, it is more likely that the motor control problems seen in children with CP related to poor sensorimotor integration, rather than a purely sensory or motor deficiency. Additionally, we suspect that the reduced strength of somatosensory cortical activity may be related to the lack of physical activity reported in children with CP. Support for this premise is based on the plethora of experiments that have shown that somatosensory cortical activity and architecture are use dependent.
Although our experimental results are insightful, the broader clinical question is how the noted somatosensory processing deficits seen in children with CP can be overcome. This is especially salient because the outcomes from prior animal and human studies have established well that the somatosensory cortices play an integral role in the learning of new motor skills (Pavlides et al. 1993; Robert et al. 2013; Sakamoto et al. 1989; Vidoni et al. 2010). Behavioral results from a previous investigation have suggested that somatosensations are recalibrated after practicing a new motor skill (Ostry et al. 2010). On the basis of these results, we suspect that increased practice with the impaired limb may result in enhanced neural activity within the sensorimotor networks of children with CP. Similar concepts have already infiltrated the constraint-induced movement paradigms, which are being used to improve motor function of the hand (Uswatte and Taub 2013). Testing these therapeutic concepts would be laudable and may have the potential to alter the treatment strategies that are currently being used to improve the mobility and lower-extremity strength of children with CP.
GRANTS
Funding for this project was provided by the Hattie B. Munroe Foundation and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) of the National Institutes of Health (5R21HD077532).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.J.K. and T.W.W. conception and design of research; M.J.K., E.H.-G., and K.M.B. performed experiments; M.J.K., E.H.-G., K.M.B., and T.W.W. analyzed data; M.J.K. and T.W.W. interpreted results of experiments; M.J.K. prepared figures; M.J.K. and T.W.W. drafted manuscript; M.J.K., E.H.-G., K.M.B., and T.W.W. edited and revised manuscript; M.J.K., E.H.-G., K.M.B., and T.W.W. approved final version of manuscript.
REFERENCES
- Auld ML, Boyd R, Moseley L, Ware RS, Johnston LM. Impact of tactile dysfunction on upper-limb motor performance in children with unilateral cerebral palsy. Arch Phys Med Rehabil 93: 693–702, 2012. [DOI] [PubMed] [Google Scholar]
- Bauby CE, Kuo AD. Active control of lateral balance in human walking. J Biomech 33: 1433–1440, 2000. [DOI] [PubMed] [Google Scholar]
- Bell KJ, Ounpuu S, DeLuca PA, Romness MJ. Natural progression of gait in children with cerebral palsy. J Pediatr Orthop 22: 677–682, 2002. [PubMed] [Google Scholar]
- Blumetti FC, Wu JCN, Bau KV, Martin B, Hobson SA, Axt MW, Selber P. Orthopedic surgery and mobility goals for children with cerebral palsy GMFCS level IV: what are we setting out to achieve? J Child Orthop 6: 485–490, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottos M, Gericke C. Ambulatory capacity in cerebral palsy: prognostic criteria and consequences for intervention. Dev Med Child Neurol 45: 786–790, 2003. [DOI] [PubMed] [Google Scholar]
- Christensen D, Van Naarden BK, Doernberg NS, Maenner MJ, Arneson CL, Durkin MS, Benedict RE, Kirby RS, Wingate MS, Fitzgerald R, Yeargin-Allsopp M. Prevalence of cerebral palsy, co-occurring autism spectrum disorders, and motor functioning - Autism and Developmental Disabilities Monitoring Network, USA, 2008. Dev Med Child Neurol 56: 59–65, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano DL, Wingert JR, Stanley CJ, Curatalo L. Contribution of hip proprioception to static and dynamic balance in cerebral palsy: a case control study. J Neuroeng Rehabil 10: 57, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher T, Wolf SI, Maier M, Hagmann S, Vegvari D, Gantz S, Heitzmann D, Wenz W, Braatz F. Long-term results after distal rectus femoris transfer as part of multilevel surgery for the correction of stiff-knee gait in spastic diplegic cerebral palsy. J Bone Joint Surg Am 94: 1–10, 2012. [DOI] [PubMed] [Google Scholar]
- Elder GC, Kirk J, Stewart G, Cook K, Weir D, Marshall A, Leahey L. Contributing factors to muscle weakness in children with cerebral palsy. Dev Med Child Neurol 45: 542–550, 2003. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Duff SV. Relationship between clinical measures and fine manipulative control in children with hemiplegic cerebral palsy. Dev Med Child Neurol 41: 586–591, 1999. [DOI] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R. Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci USA 98: 694–699, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand A, Singh KD, Holliday IE, Furlong PL, Barnes GR. A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapp 25: 199–211, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoon AH, Stashinko EE, Nagae LM, Lin DD, Keller J, Bastian A, Campbell M, Levey E, Mori S, Johnston MV. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol 51: 697–704, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulak W, Sobaniec W, Solowiej E, Bockowski L. Somatosensory and MRI findings in children with cerebral palsy: correlations and discrepancies with clinical practice. J Pediatr Neurol 3: 77–78, 2005. [DOI] [PubMed] [Google Scholar]
- Kulak W, Sobaniec W, Solowiej E, Bockowski L. Somatosensory and visual evoked potentials in children with cerebral palsy: correlations and discrepancies with MRI findings and clinical picture. Pediatr Rehabil 9: 201–209, 2006. [DOI] [PubMed] [Google Scholar]
- Kuo AD. Stabilization of lateral motion in passive dynamic walking. Int J Robot Res 18: 917–930, 1999. [Google Scholar]
- Kurz MJ, Wilson TW. Neuromagnetic activity in the somatosensory cortices of children with cerebral palsy. Neurosci Lett 490: 1–5, 2011. [DOI] [PubMed] [Google Scholar]
- Kurz MJ, Wilson TW, Corr B, Volkman KG. Neuromagnetic activity of the somatosensory cortices associated with body weight-supported treadmill training in children with cerebral palsy. J Neurol Phys Ther 36: 16–172, 2012. [DOI] [PubMed] [Google Scholar]
- Kurz MJ, Heinrichs-Graham E, Arpin DJ, Becker KM, Wilson TW. Aberrant synchrony in the somatosensory cortices predicts motor performance errors in children with cerebral palsy. J Neurophysiol 111: 573–579, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CJ, Guo LY, Su FC, Chou YL, Cherng RJ. Common abnormal kinetic patterns of the knee in gait in spastic diplegia of cerebral palsy. Gait Posture 11: 224–232, 2000. [DOI] [PubMed] [Google Scholar]
- Maitre NL, Barnett ZP, Key APF. Novel assessment of cortical response to somatosensory stimuli in children with hemiparetic cerebral palsy. J Child Neurol 27: 1276–1283, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau NG, Falvo M, Damiano DL. Rapid force generation is impaired in cerebral palsy and is related to decreased muscle size and functional mobility. Gait Posture 35: 154–158, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor SM, Kuo AD. Directional-dependent control of balance during walking and standing. J Neurophysiol 102: 1411–1419, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onley SJ, MacPhail HA, Hedden DM, Boyce WF. Work and power in hemiplegic cerebral palsy gait. Phys Ther 70: 431–438, 1990. [DOI] [PubMed] [Google Scholar]
- Ostry DJ, Darainy M, Mattar AAG, Wong J, Gribble PL. Somatosensory plasticity and motor learning. J Neurosci 30: 5384–5393, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ounpuu S, Davis RB, DeLuca PA. Joint kinetics: methods, interpretation and treatment decision-making in children with cerebral palsy and myelomenigocele. Gait Posture 4: 62–78, 1996. [Google Scholar]
- Pin T, Dyke P, Chan M. The effectiveness of passive stretching in children with cerebral palsy. Dev Med Child Neurol 48: 855–862, 2006. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Miyashita E, Asanuma H. Projection from the sensory to the motor cortex is important in learning motor skills in the monkey. J Neurophysiol 70: 733–741, 1993. [DOI] [PubMed] [Google Scholar]
- Perry SD, Santos LC, Patla AE. Contribution of vision and cutaneous sensation to the control of centre of mass (COM) during gait termination. Brain Res 913: 27–34, 2001. [DOI] [PubMed] [Google Scholar]
- Riad J, Haglund-Akerlind Y, Miller F. Power generation in children with spastic hemiplegic cerebral palsy. Gait Posture 27: 641–647, 2008. [DOI] [PubMed] [Google Scholar]
- Riquelme I, Montoya P. Developmental changes in somatosensory processing in cerebral palsy and healthy individuals. Clin Neurol 121: 1314–1320, 2010. [DOI] [PubMed] [Google Scholar]
- Riquelme I, Zamorano A, Montoya P. Reduction of pain sensitivity after somatosensory therapy in adults with cerebral palsy. Front Hum Neurosci 7: 1–7, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert MT, Guberek R, Sveistrup H, Levin MF. Motor learning in children with hemiplegic cerebral palsy and the role of sensation in short-term motor training of goal-directed reaching. Dev Med Child Neurol 55: 1121–1128, 2013. [DOI] [PubMed] [Google Scholar]
- Rose J, Mirmiran M, Butler EE, Lin CY, Barnes PD, Kermoian R, Stevenson DK. Neonatal microstructural development of the internal capsule on diffusion tensor imaging correlates with severity of gait and motor deficits. Dev Med Child Neurol 49: 745–750, 2007. [DOI] [PubMed] [Google Scholar]
- Rosenbaum P, Paneth N, Leviton A, Goldstein N, Bax M, Damiano D, Dan B, Jacobsson B. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl 109: 8–14, 2007. [PubMed] [Google Scholar]
- Sakamoto T, Arissian K, Asanuma H. Functional role of the sensory cortex in learning motor skill in cats. Brain Res 503: 258–264, 1989. [DOI] [PubMed] [Google Scholar]
- Talairach G, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York, NY: Thieme, 1998. [Google Scholar]
- Taylor NR, Dodd KJ, Baker RJ, Willoughby K, Thomason P, Graham HK. Progressive resistance training and mobility-related function in young people with cerebral palsy: a randomized controlled trial. Dev Med Child Neurol 55: 806–812, 2013. [DOI] [PubMed] [Google Scholar]
- Teflioudi EP, Zafeiriou DI, Vargiami E, Kontopoulos E, Tsikoulas I. Somatosensory evoked potentials in children with bilateral spastic cerebral palsy. Pediatr Neurol 44: 177–182, 2011. [DOI] [PubMed] [Google Scholar]
- Trivedi R, Gupta RK, Shah V, Tripathi M, Rathore RKS, Kumar M, Pandey CM, Narayana PA. Treatment-induced plasticity in cerebral palsy: a diffusion tensor imaging study. Pediatr Neurol 39: 341–349, 2008. [DOI] [PubMed] [Google Scholar]
- Trivedi R, Agarwal S, Shah V, Goyel P, Paliwal VK, Rathore RKS, Gupta RK. Correlation of quantitative sensorimotor tractography with clinical grade of cerebral palsy. Neuroradiology 52: 759–765, 2010. [DOI] [PubMed] [Google Scholar]
- Uswatte G, Taub E. Neuroplasticity to treat motor disorders. Prog Brain Res 207: 379–401, 2013. [DOI] [PubMed] [Google Scholar]
- van Veen BD, van Drongelen W, Yuchtman M, Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng 44: 867–880, 1997. [DOI] [PubMed] [Google Scholar]
- Vidoni ED, Acerra NE, Dao E, Meehan SK, Boyd LA. Role of the primary somatosensory cortex in motor learning: An rTMS study. Neurobiol Learn Mem 93: 532–539, 2010. [DOI] [PubMed] [Google Scholar]
- Wiley ME, Damiano DL. Lower-extremity strength profiles in spastic cerebral palsy. Dev Med Child Neurol 40: 100–107, 1998. [DOI] [PubMed] [Google Scholar]
- Wingert JR, Burton H, Sinclair RJ, Brunstrom JE, Damiano DL. Joint-position sense and kinesthesia in cerebral palsy. Arch Phys Med Rehabil 90: 447–453, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter DA. Biomechanical motor patterns in normal walking. J Mot Behav 15: 302–330, 1983. [DOI] [PubMed] [Google Scholar]